Back to Journals » Orthopedic Research and Reviews » Volume 15
Dedifferentiated Low-Grade Osteosarcoma, Outcome with or Without Chemotherapy: A Systematic Review
Authors Pacheco M , Guzmán R, Bonilla P
Received 20 January 2023
Accepted for publication 18 April 2023
Published 28 April 2023 Volume 2023:15 Pages 79—89
DOI https://doi.org/10.2147/ORR.S404146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Marina Pacheco,1,2 Rodolfo Guzmán,3 Patricia Bonilla4
1Department of Pathology, Complejo Hospitalario Metropolitano CSS, Social Security Fund, Panama, Panama; 2Sistema Nacional de Investigación; Secretaria Nacional de Ciencia, Tecnologia e Innovación, Panama, Panama; 3Department of Pathology, Hospital San Juan de Dios CCSS, San Jose, Costa Rica; 4Department of Orthopedics, Hospital del Niño Dr. José Renán Esquivel, Panama, Panama
Correspondence: Marina Pacheco, Departamento de Patología, Complejo Hospitalario Metropolitano Dr. Arnulfo Arias Madrid CSS, Avenida José de Fábrega y Simón Bolivar, Bella Vista, Panama, Panama, Tel +507 503 6219, Email [email protected]
Abstract: The treatment of low-grade osteosarcomas is surgical resection with wide margins. In instances of dedifferentiation, a therapeutic paradigm similar to that of conventional high-grade osteosarcoma has not been adequately evaluated in these neoplasms. The main objective of this review was to define whether the addition of chemotherapy to surgical treatment has an impact on the survival of patients with dedifferentiated low-grade osteosarcomas. Secondary objectives were to observe the degree of histological response to neoadjuvant chemotherapy and to describe the percentage of de novo dedifferentiation. A systematic search of articles including dedifferentiated low-grade osteosarcomas, published between 1980 and 2022 was carried out in the PubMed, Cochrane and Scielo databases. A qualitative synthesis of the results was performed. Twenty-three articles comprising 117 patients were included. The survival of patients treated with surgery alone and surgery with chemotherapy was not statistically significant between the two groups. A good histological response was seen in 20% of specimens treated with neoadjuvant chemotherapy. De novo dedifferentiation was seen in approximately a fifth of low-grade osteosarcomas. The evidence available suggests that the addition of chemotherapy does not have an impact on the survival of patients with low-grade dedifferentiated osteosarcomas.
Keywords: dedifferentiated, osteosarcoma, parosteal osteosarcoma, low-grade central osteosarcoma
Introduction
Dedifferentiated low-grade osteosarcoma is a type of osteosarcoma in which a high-grade sarcomatous component coexists histologically, synchronously or metachronously, with a precursor low-grade osteosarcoma.
From a cytogenetic point of view, low-grade osteosarcomas are characterized by the presence of supernumerary ring chromosomes harboring amplification of chromosomal regions 12q13-q15 that contain the MDM2 and CDK4 loci, among other less prevalent genes. The chromosomal alteration translates into overexpression of MDM2 and CDK4 at the protein level, which constitutes the pathogenic and diagnostic mark that is maintained in the progression to a high-grade sarcoma.1–5
Based on their intramedullary or juxtacortical location, they are classified as low-grade central osteosarcoma (LGCOS) and parosteal osteosarcoma, respectively.6 Together they constitute approximately 6% of all osteosarcomas, being parosteal osteosarcoma twice as common as LGCOS. Low-grade osteosarcomas almost exclusively affect the metaphysis of long bones.7
Surgery is the standard treatment for low-grade osteosarcomas and obtaining wide surgical margins that guarantee adequate local control is of utmost importance because a considerable proportion of low-grade osteosarcomas undergo metachronous dedifferentiation during recurrences. Based on the information provided by retrospective studies,8–12 however, it is still not clear what should be the optimal treatment for low-grade dedifferentiated osteosarcomas. Currently, a pathology report describing high-grade areas within a low-grade osteosarcoma, even in the absence of specific guidelines, represents support for the use of chemotherapy in these neoplasms. However, from the available information it can be deduced that the survival impact associated to the addition of chemotherapy to primary surgery of low-grade dedifferentiated osteosarcoma is not entirely evident.
This systematic review seeks to provide a more robust answer to the question of whether the addition of preoperative and/or postoperative chemotherapy to the surgical treatment of dedifferentiated low-grade osteosarcoma provides survival benefit to patients.
Materials and Methods
Bibliographic Search
A systematic bibliographic search was carried out in PubMed, Cochrane and Scielo databases using the following individual and combined search terms: “osteosarcoma”, “Parosteal osteosarcoma”, “Dedifferentiated parosteal”, “dedifferentiated parosteal osteosarcoma”, “Dedifferentiated osteosarcoma”, “Surface osteosarcoma”, “juxtacortical osteosarcoma”, “low -grade central osteosarcoma”, “dedifferentiated low -grade central”, “dedifferentiated central osteosarcoma” and “dedifferentiated low -grade central osteogenic sarcoma”.
The process was carried out following the recommendations of the Preferred declaration reporting items for systematic Reviews and Meta- Analysis (PRISMA).13
The search terms were kept as broad as possible and the following filters were used: species (humans), publication date (1980-August 2022), language (English and Spanish).
Eligibility Criteria
Articles that reported or compared the oncological outcome of patients with dedifferentiated low-grade osteosarcomas who met the established PICO (Population-Intervention-Comparison-Outcomes) parameters were included: patients of all ages with dedifferentiated low-grade osteosarcomas with primary and localized disease in the extremities that have received either surgical treatment with non-intralesional margins or that have received pre-operative or post-operative chemotherapy or both. Of note, a marginal surgical margin is not adequate for the treatment of conventional and dedifferentiated low-grade osteosarcomas. The reason for including the cases treated marginally was a pragmatic one to include a larger number of cases in this analysis.
The following types of publications were included in the search: case reports, retrospective case series, clinical trials, observational studies, retrospective cohort analysis. Only studies in English and Spanish and those with available abstracts were considered. The following types of articles were excluded: (1) review articles and monographs; (2) comments on published articles; (3) editorials; (4) conference abstracts or posters; (5) studies focused on animal experiments and in vitro studies with cell lines; (6) studies focused on bioinformatic discoveries; (7) molecular or in situ biomarker studies in fixed, paraffin-embedded tissue samples; (8) ultrastructural studies; (9) studies from which information needed to compare outcomes between intervention and comparison could not be extracted; (10) studies with a population diagnosed as “juxtacortical osteosarcoma” that did not provide radiological and microscopic images that would support the diagnosis of dedifferentiated parosteal osteosarcoma and (11) studies on dedifferentiated low-grade osteosarcomas secondary to Paget’s disease, radiation, or other causes.
Data Extraction
The abstracts of the relevant publications were assessed by two reviewers independently (M.P, R.G). Studies that met the inclusion criteria were selected and those titles and abstracts that implicitly contained exclusion criteria were rejected.
An evaluation form was used to screen the abstracts, which included the general information of the publications, the inclusion and exclusion criteria and the parameters defined in the PICO question. The abstracts that could not be categorically excluded at this stage were included and their full texts assessed for final decision. Discrepancies were resolved by consensus between the two authors. The information extracted from the studies was tabulated in a systematic way in a data extraction spreadsheet. The selected articles had to contain patients with the minimum essential information that would allow us achieving the primary objective of this work. For each study, the following information was extracted: first author and year of publication, country, type of study, study period, sample size of the study, number of cases in the study sample that met the inclusion criteria, type of treatment (surgery alone/surgery and chemotherapy), oncological outcomes at last follow-up: dead of disease (DOD), not evidence of disease (NED) and the follow-up duration.
Additional information not essential for the primary comparison was extracted from the selected articles and tabulated in the spreadsheet to achieve the secondary objectives. Other variables were the following: general characteristics (age and sex), type of precursor low-grade osteosarcoma, chronology of dedifferentiation (synchronous and metachronous), Enneking stage, largest dimension of the tumor, type of surgery (amputation/disarticulation or limb-sparing surgery), type of negative margin (marginal or wide/radical), timing of chemotherapy (neoadjuvant/adjuvant/both), chemotherapy regimen (drugs), histological response to neoadjuvant treatment (Huvos >90% and <90%).
While reading the complete articles, three retrospective studies were identified8,11,14 that were excluded from the analysis of the primary objective because the minimum data necessary could not be extracted from them. These articles, however, were considered for the descriptive results of the secondary objectives, which, therefore, were obtained from a total of 26 articles.
Quality Appraisal
One investigator (M.P) conducted a case report quality appraisal using a modified version of a case report quality assessment tool.15 The following criteria were used: (1) the publication presents the information of the patients’ treatment and outcome in an understandable way (2) the publication provides an unambiguous diagnosis according to updated classification (3) the publication presents histological and radiological evidence that supports the diagnosis.
Data Synthesis and Analysis
The primary outcome of this study is survival. Secondary objectives are proportion of good histologic response to chemotherapy and de novo dedifferentiation.
Data were qualitatively synthesized using descriptive statistics with means and ranges for continuous variables and percentages for dichotomous variables.
Survival was estimated for those patients within the studies in which the follow-up information was presented, using Kaplan-Meier method. Log rank test was used to compare survival curves between the two groups. All statistical tests were two-tailed and a p value <0.05 was considered significant.
Results
Selection of Studies
The systematic literature search yielded a total of 24,388 titles. Of these, 23,909 were excluded due to containing explicit exclusion criteria in the title. 479 titles were selected and, after eliminating duplications, 237 abstracts were included. Of these, 189 were excluded. Lastly, of the 48 studies selected for the complete reading of their texts, 23 manuscripts were included for data extraction. Figure 1 shows the flowchart of the selection process.
 |
Figure 1 Flowchart of the study selection process. |
Characteristics of the Studies
In total, 23 articles that together contained 117 eligible patients were included. The characteristics of the studies are summarized in Table 1. The manuscripts were published between 1984 and 2020 (1980–1990:5; 1991–2000:6; 2001–2010:7 and 2011–2022:5) and their participants were recruited from 1916 to 2018. Dedifferentiated low-grade osteosarcomas ranged from 1 to 33 in each of the selected papers. Six studies were conducted in Italy,10,12,16–19 six in the United States,20–25 four in Japan,9,26–28 one in each of the following countries: Australia,29 Austria,30 Spain,31 Netherlands,32 Indonesia,33 South Korea34 and one was a multicenter European study.35 Fourteen were retrospective series and nine were case reports.
 |
Table 1 Characteristics of the Studies Included in the Analysis |
Quality Evaluation
Most of the articles reported an adequate description of the patients’ treatments and outcomes (83%). An unambiguous diagnosis according to the current bone tumors classification and convincing histological and radiological evidence that supported the diagnosis was provided in 87% and 74% of the publications, respectively.
Results of the Objectives
Primary Objective
The clinical data of the patients that fulfilled the inclusion criteria within the studies that were selected is resumed in Table 2. Overall, the 23 articles that were reviewed contained a total of 143 dedifferentiated low-grade osteosarcomas, of which 117 were primaries, located in the extremities, non-metastatic at the time of diagnosis and had been treated surgically with negative margins [wide/radical: 97 (83%); marginal: 10 (8.5%) and unspecified negative margin: 10 (8.5%)]. Thirty-eight (32%) received surgical treatment exclusively and 79 (68%) were treated with surgery and perioperative chemotherapy. Of the latter, 56 (71%) received adjuvant chemotherapy, six (7.5%) neoadjuvant chemotherapy, and 17 (21.5%) were treated with pre- and postoperative chemotherapy.
 |
Table 2 Resume of Clinical Data |
Seventeen articles included patients who had received some scheme of chemotherapy. Of those, eleven articles, which included patients recruited between 1965 and 2016, specified the therapeutic regimen in a variably detailed manner. In six articles, the details of the chemotherapy regimen were unspecified (Table 3).
 |
Table 3 Chemotherapy Schemes |
Of 38 patients treated with surgery alone, 29 (76%) had no evidence of disease and six (16%) had died of disease at last follow-up. Of the remaining three, two (5%) were demised of unrelated causes and one (3%) had no follow-up.
Of 79 patients treated with surgery and chemotherapy, 57 (72%) had no evidence of disease and 19 (24%) had died of disease at last follow-up. Of the remaining three, one (1.5%) was alive with disease and two (2.5%) had not been followed up.
The median follow-up- based on 17 articles containing a total of 78 patients with available information- was 73.5 months (Range 4–440; IQR: 24–137). The time interval between surgery and outcome was specified in 21 patients out of 25 that died of disease during the follow up period. The negative outcomes occurred at a median of 15 months (range: 6–36) in patients treated with surgery and at a median of 17.5 months (5–108) in those treated with perioperative chemotherapy and surgery. For patients alive at the time of last follow-up, continuous disease-free survival was prolonged. There were no significant differences in survival between those patients who were treated exclusively with surgery and those who received perioperative chemotherapy [p= 0.51 (p < 0.05)] (Figure 2 and Supplementary Material).
 |
Figure 2 Kaplan-Meier curve comparing survival by type of treatment. |
Forty percent of the patients in whom marginal margins were obtained and 68% in whom the margins were wide or radical received chemotherapy. There was no difference regarding the indication for chemotherapy according to the type of negative margins obtained [X2 (1, N =107)] = 3151; p=0.0759 (p< 0.01).
Within the articles, most of the tumor dimensions were depicted in summary statistics. Twelve patients (10%) had the tumoral size specified.9,20,22,25,26,32 The median largest tumor dimension was 10.7 cm. (range 6.5–11.5) Six and five patients with tumors smaller and larger than the median, respectively, received chemotherapy and one patient with a tumor larger than 10.7 cm. did not receive chemotherapy. The indication for the use of chemotherapy seems to have been irrespective of tumor size at least in the small subset of patients with information available.
Secondary Objective
Table 4 summarizes some of the characteristics of the dedifferentiated low-grade osteosarcomas extracted from the 26 articles that were analyzed to achieve the secondary objectives.
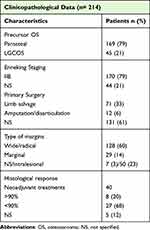 |
Table 4 Characteristics of the Dedifferentiated Low-Grade Osteosarcomas Analyzed in Secondary Objectives |
All the tumors in this review are high grade and M0, so they all correspond to Enneking II. Undifferentiated low-grade central osteosarcomas were considered IIB when the stage was explicit in the original article or when it could be inferred from the information provided in the gross or histological description. Undifferentiated parosteal osteosarcomas were considered IIB as they are extracompartmental by definition. Of 214 high-grade dedifferentiated osteosarcomas, 170 (79%) were stage IIB and in the remaining 44, the extent was neither specified nor could be inferred.
Forty patients received neoadjuvant or combined pre and postoperative chemotherapy. Of their resection specimens, 27 (68%) had histologic responses of <90%, eight (20%) had responses of >90%, and in five (12%) the degree of response was not specified.
To have an idea of the proportion of synchronous dedifferentiation in the group of low-grade osteosarcomas, we obtained the proportion of low-grade osteosarcomas with de novo dedifferentiation included in series containing both, low-grade osteosarcomas and osteosarcomas with concomitant dedifferentiation. Of a total of 17 studies, 14 included both conventional low-grade osteosarcomas and low-grade osteosarcomas with high-grade progression. Of a total of 900 low-grade osteosarcomas included in these studies, 186 contained areas of dedifferentiation. Of these, 151 at diagnosis (synchronous) and 35 during recurrences. The proportion of de novo dedifferentiation in conventional low-grade osteosarcomas was approximately 17% (Table 4).
Discussion
This paper reviews the existing literature regarding the survival of patients with dedifferentiated low-grade osteosarcomas treated either with surgery with negative margins or surgery and chemotherapy for localized disease of the extremities. Twenty-three studies with 117 patients were included for the descriptive analysis of the primary objective. All the studies have a level of evidence III.
The median follow-up of the patients in the studies was over 6 years, which we consider adequate since both, local and distant recurrences, are late phenomena in dedifferentiated low-grade osteosarcomas.
The information from the available literature indicates that the addition of cytotoxic perioperative chemotherapy to the surgical treatment of low-grade dedifferentiated osteosarcoma does not affect patient survival. All the information gathered derives from small retrospective series and case reports that comprise heterogenous treatment regimens ranging from intraarterial applications and less effective drugs that have been abandoned to schemes which are comparable to the current standard for perioperative osteosarcoma management, thus for a contextual interpretation of these results, the above information should be considered.
In three of the largest retrospective case series of dedifferentiated low-grade osteosarcomas with direct comparisons of overall survival between patients treated with and without adjuvant chemotherapy8,10–12 – the meaning herein used to refer to any combination of agents before or after curative surgery- a survival advantage of perioperative chemotherapy could not be demonstrated.8,10,11
Approximately 70% of the patients in this review that were treated with preoperative chemotherapy showed a rate of induced tumor necrosis of <90%. It was not the objective of this review to correlate the percentage of histological response with the oncological result, however, in the study of Toki et al,dedifferentiated low-grade osteosarcomas showed a poorer histological response than the control cohort of conventional high-grade osteosarcomas, yet the clinical outcome of the former was not markedly different. The foregoing observations suggest that as a group, dedifferentiated low-grade osteosarcomas exhibit a rather disappointing histological response to neoadjuvant cytotoxic chemotherapy and, above all, the level of response is unrelated to the oncological outcome of the patients.
Patients with dedifferentiated low-grade osteosarcomas have a prolonged clinical presentation that can last from several months to even 10 years.10,19,21 In this review, 17% of tumors were dedifferentiated at the time of diagnosis, indicating that synchronous dedifferentiation in low-grade osteosarcoma may occur early in approximately one-fifth of patients in the context of a localized disease.
Dedifferentiated low-grade osteosarcomas, although high-grade, are osteosarcomas that affect an older age group and exhibit a biological behavior that is dissimilar to conventional osteosarcoma in terms of late local recurrences, lower rate of distant metastases and longer disease-free survival.8,23 Likewise, the cytogenetic basis of dedifferentiated low-grade osteosarcomas, characterized by co-amplifications of MDM2 and CDK4 in the 12q13-15 region, is different from the aneuploid and complex karyotypes of conventional high-grade osteosarcomas. MDM2 is an E3 ligase involved in transporting TP53 to the cytoplasm for degradation36 and CDK4 is a cyclin-dependent kinase involved in the G1 to S cell cycle transition.37 In low-grade osteosarcomas, levels of mRNA expression of MDM2 and CDK4 can reach levels of up to 30 times and 7 times more than basal levels, respectively5 translating into protein overexpression.4 The effects of MDM2 and CDK4 amplification on TP53 regulation and cell cycle progression, respectively, suggest that overexpression of both genes is necessary for the growth of low-grade osteosarcomas. This makes these tumors potential candidates for targeted therapies with inhibitors of MDM2, CDK4, and selective inhibitors of nuclear transport. However, the progression of low-grade osteosarcomas towards a more aggressive phenotype most likely requires the acquisition of other genetic abnormalities, that are yet to be dilucidated to direct research towards the discovery of useful biomarkers. Despite limited data in this regard, the proportion of high-grade component of the tumor has been inquired as a predictive biomarker for benefiting from adjuvant chemotherapy. Righi et al interestingly investigated the impact of the percentage of dedifferentiation on the survival of patients with low-grade central osteosarcomas. In their work,12 patients with dedifferentiation rates of <50% were found to have high survival rates regardless of whether they received chemotherapy or did not, while patients with resection specimens of tumors with areas of dedifferentiation > 50% had a more aggressive behavior and a lower probability of survival. A single additional study in this review quantifies the proportion of the dedifferentiated component, thus the proportion of dedifferentiation within the tumor as a rationale for the addition of postoperative chemotherapy would be worth exploring further.
This systemic review has several limitations rooted inherently in the very low incidence of dedifferentiated low-grade osteosarcomas resulting in availability of scanty and suboptimal literature. Other limitations are the exclusion of the largest published series due to the unfeasibility of extracting data in line with the pre-established inclusion criteria of our study, the lack of microscopic and radiological evidence supporting the diagnosis of low-grade dedifferentiated osteosarcoma in approximately a quarter of the publications, the unavailability of important information such as tumor size, stage and histological response in an important proportion of the studies, which hampers the comparison by background factors; the inclusion of cases from many dispersed centers in different continents that received heterogeneous treatments during a recruitment period spanning over 100 years inexorably subject this review to chronological and treatment bias.
Conclusion
We conclude that the available evidence does not support the notion that the addition of chemotherapy to surgical treatment has a positive impact on the survival of patients with adequately resected dedifferentiated low-grade osteosarcomas of the extremities. Despite the limitations of this review, we aim that this exhaustive systematic bibliographic search and patient selection work contributes positively to the current debate on the topic raised in the objectives and help as a foundation stone to the search of prognostic and predictive biomarkers in this disease.
Acknowledgments
M.P was supported by the Sistema Nacional de Investigadores de Panamá (S.N.I) of the Secretaria Nacional de Ciencia y Tecnología (SENACYT). We would like to thank Dr. Irene Barrientos Ruiz from Hospital Universitario La Paz (Spain), Dr. Ronald Badilla from Hospital Dr. Rafael Ángel Calderón Guardia (Costa Rica) and Dr. Amador Goodridge from Instituto de Investigaciones Científicas y Servicios de Alta Tecnología INDICASAT-AIP (Panama) for providing valuable comments and suggestions, and Dr. Aaron O’Dea for help producing the figures.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Szymanska J, Mandahl N, Mertens F, Tarkkanen M, Karaharju E, Knuutila S. Ring chromosomes in parosteal osteosarcoma contain sequences from 12q13-15: a combined cytogenetic and comparative genomic hybridization study. Genes Chromosomes Cancer. 1996;16:31–34. doi:10.1002/(SICI)1098-2264(199605)16:1<31::AID-GCC4>3.0.CO;2-4
2. Duhamel LA, Ye H, Halai D, et al. Frequency of Mouse Double Minute 2 (MDM2) and Mouse Double Minute 4 (MDM4) amplification in parosteal and conventional osteosarcoma subtypes. Histopathology. 2012;60:357–359. doi:10.1111/j.1365-2559.2011.04023.x
3. Dujardin F, Binh MB, Bouvier C, et al. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2011;24:624–637.
4. Yoshida A, Ushiku T, Motoi T, et al. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low -grade osteosarcoma from benign mimics. ModPathol. 2010;23:1279–1288.
5. Mejia-Guerrero S, Quejada M, Gokgoz N, et al. Characterization of the 12q15 MDM2 and 12q13-14 CDK4 amplicons and clinical correlations in osteosarcoma. Genes Chromosomes Cancer. 2010;49:518–525. doi:10.1002/gcc.20761
6. WHO Classification of Tumors Editorial Board Eds. World Health Organization Classification of Tumors of Soft Tissue and Bone.
7. Picci P, Manfrini M, Donati DM, et al. Diagnosis of Musculoskeletal Tumors and Tumor-Like Conditions 2nd Edition. Switzerland: Springer Nature; 2020.
8. Laitinen M, Parry M, Albergo JI, et al. The prognostic and therapeutic factors which influence the oncological outcome of parosteal osteosarcoma. Bone Joint J. 2015;97:1698–1703. doi:10.1302/0301-620X.97B12.35749
9. Toki S, Kobayashi E, Yoshida A, et al. A clinical comparison between dedifferentiated low-grade osteosarcoma and conventional osteosarcoma. Bone Joint J. 2019;101:745–752. doi:10.1302/0301-620X.101B6.BJJ-2018-1207.R1
10. Bertoni F, Bacchini P, Staals EL, Davidovitz P. Dedifferentiated parosteal osteosarcoma: the experience of the Rizzoli Institute. Cancer. 2005;103:2373–2382. doi:10.1002/cncr.21039
11. Ruengwanichayakun P, Gambarotti M, Frisoni T, et al. Parosteal osteosarcoma: a monocentric retrospective analysis of 195 patients. HumPathol. 2019;91:11–18.
12. Righi A, Paioli A, Dei Tos AP, et al. High-grade focal areas in low - grade central osteosarcoma: high - grade or still low -grade osteosarcoma? Clin Sarcoma Res. 2015;5:23. doi:10.1186/s13569-015-0038-7
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi:10.1136/bmj.b2700
14. Okada K, Frassica FJ, Sim FH, Beabout JW, Bond JR, Unni KK. Parosteal osteosarcoma. A clinicopathological study. J Bone Joint Surg Am. 1994;76:366–378. doi:10.2106/00004623-199403000-00007
15. Abdel-Wahab N, Lopez-Olivo MA, Pinto-Patarroyo GP, Suarez-Almazor ME. Systematic review of case reports of antiphospholipid syndrome after infection. Lupus. 2016;25:1520–1531. doi:10.1177/0961203316640912
16. Bertoni F, Present D, Hudson T, Enneking WF. The meaning of radiolucencies in parosteal osteosarcoma. J Bone Joint Surg Am. 1985;67:901–910. doi:10.2106/00004623-198567060-00012
17. Azura M, Vanel D, Alberghini M, Picci P, Staals E, Mercuri M. Parosteal osteosarcoma dedifferentiating into telangiectatic osteosarcoma: importance of lytic changes and fluid cavities at imaging. skeleton radiol. 2009;38:685–690. doi:10.1007/s00256-009-0672-3
18. Franchi A, Bacchini P, Della Rocca C, Bertoni F. Central low-grade osteosarcoma with pagetoid bone formation: a potential diagnostic pitfall. ModPathol. 2004;17:288–291.
19. Campanacci M, Picci P, Gherlinzoni F, Guerra A, Bertoni F, Neff JR. Parosteal osteosarcoma. J Bone Joint Surg Br. 1984;66:313–321. doi:10.1302/0301-620X.66B3.6586725
20. Cardona DM, Knapik JA, Reith JD. Dedifferentiated parosteal osteosarcoma with giant cell tumor component. skeleton radiol. 2008;37:367–371. doi:10.1007/s00256-007-0440-1
21. Sheth DS, Yasko AW, Raymond AK, et al. Conventional and dedifferentiated parosteal osteosarcoma. Diagnosis, treatment, and outcome. Cancer. 1996;78:2136–2145.
22. Kenan S, Ginat DT, Steiner GC. Dedifferentiated high-grade osteosarcoma originating from low-grade central osteosarcoma of the fibula. Skeleton Radiol. 2007;36:347–351. doi:10.1007/s00256-006-0123-3
23. Schwab JH, Antonescu CR, Athanasian EA, Boland PJ, Healey JH, Morris CD. A comparison of intramedullary and juxtacortical low-grade osteogenic sarcoma. Clinic Orthop Relat Res. 2008;466:1318–1322. doi:10.1007/s11999-008-0251-2
24. Lindell MM, Shirkhoda A, Raymond AK, Murray JA, Harle TS. Parosteal osteosarcoma: radiologic-pathologic correlation with emphasis on CT. AJR Am J Roentgenol. 1987;148:323–328. doi:10.2214/ajr.148.2.323
25. Pintado SO, Lane J, Huvos AG. Parosteal osteogenic sarcoma of bone with coexisting low- and high-grade sarcomatous components. HumPathol. 1989;20:488–491.
26. Futani H, Okayama A, Maruo S, Kinoshita G, Ishikura R. The role of imaging modalities in the diagnosis of primary dedifferentiated parosteal osteosarcoma. J Orthop Sci. 2001;6:290–294. doi:10.1007/s007760100050
27. Ogose A, Hotta T, Emura I, Imaizumi S, Takeda M, Yamamura S. Repeated dedifferentiation of low-grade intraosseous osteosarcoma. HumPathol. 2000;31:615–618.
28. Iemoto Y, Ushigome S, Fukunaga M, Nikaido T, Asanuma K. Case report 679. Central low-grade osteosarcoma with foci of dedifferentiation. Skeleton radiol. 1991;20:379–382. doi:10.1007/BF01267668
29. Wines A, Bonar F, Lam P, McCarthy S, Stalley P. Telangiectatic dedifferentiation of a parosteal osteosarcoma. skeleton radiol. 2000;29:597–600. doi:10.1007/s002560000247
30. Funovics PT, Bucher F, Toma CD, Kotz RI, Dominkus M. Treatment and outcome of parosteal osteosarcoma: biological versus endoprosthetic reconstruction. J Surg Oncol. 2011;103:782–789. doi:10.1002/jso.21859
31. Encinas-Ullán CA, Ortiz-Cruz EJ, Barrientos-Ruiz I, Valencia-Mora M, González-López JM. Parosteal osteosarcomas: unusual results [Parosteal osteosarcomas: unusual findings]. Rev Esp Circle Ortop Traumatol. 2012;56:281–285.
32. van Oven MW, Molenaar WM, Freling NJ, et al. Dedifferentiated parosteal osteosarcoma of the femur with aneuploidy and lung metastases. Cancer. 1989;63:807–811. doi:10.1002/1097-0142(19890215)63:4<807::AID-CNCR2820630434>3.0.CO;2-B
33. Prabowo Y, Kamal AF, Kodrat E, Prasetyo M, Maruanaya S, Efar TS. Parosteal osteosarcoma: a benign-looking tumour, amenable to a variety of surgical reconstruction. Int J Surg Oncol. 2020;2020:4807612. doi:10.1155/2020/4807612
34. Lin HY, Hondar Wu HT, Wu PK, et al. Can imaging distinguish between low-grade and dedifferentiated parosteal osteosarcoma? J Chin Med Assoc. 2018;81:912–919. doi:10.1016/j.jcma.2018.01.014
35. Ritschl P, Wurnig C, Lechner G, Roessner A. Parosteal osteosarcoma. 2-23-year follow-up of 33 patients. Acta Orthop Scand. 1991;62:195–200. doi:10.3109/17453679108993592
36. Deb SP. Cell cycle regulatory functions of the human oncoprotein MDM2. Mol Cancer Res. 2003;1:1009–1016.
37. Lee MH, Yang HY. Regulators of G1 cyclin-dependent kinases and cancers. Cancer Metastasis Rev. 2003;22:435–449. doi:10.1023/A:1023785332315
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
