Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Dapagliflozin Activates Neurons in the Central Nervous System and Regulates Cardiovascular Activity by Inhibiting SGLT-2 in Mice
Authors Nguyen T, Wen S , Gong M, Yuan X, Xu D, Wang C, Jin J, Zhou L
Received 28 April 2020
Accepted for publication 10 July 2020
Published 5 August 2020 Volume 2020:13 Pages 2781—2799
DOI https://doi.org/10.2147/DMSO.S258593
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Ming-Hui Zou
Thiquynhnga Nguyen,* Song Wen,* Min Gong,* Xinlu Yuan, Dongxiang Xu, Chaoxun Wang, Jianlan Jin, Ligang Zhou
Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai 201399, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ligang Zhou
Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai 201399, People’s Republic of China
Tel +86 13611927616
Email [email protected]
Purpose: This study investigates the possible effect and central mechanism of novel antidiabetic medication sodium glucose transporter-2 (SGLT-2i) on the cardiovascular activity.
Material and Methods: Thirty-four normal male C57BL/6 mice were randomly assigned to 2 groups to receive single Dapagliflozin (1.52mg/kg) dose via intragastric gavage or a comparable dose of saline. Glycemic level (BG), blood pressure (BP) and heart rate (HR) were measured 2 hours after administration of the respective treatments. Immunohistochemical tests were performed to determine the effect of SGLT-2i on neural localization of SGLT-2 and c-Fos, a neural activator. The distributional relationships of SGLT-2 and c-Fos were examined by immunofluorescence.
Results: Administration of SGLT-2i significantly decreased BP but did not affect the HR. There was no difference in BG between the two groups. Results showed that SGLT-2 was localized to specific regions involved in autonomic control. Expression of c-Fos was significantly higher in major critical nuclei in the aforementioned regions in groups treated with Dapagliflozin.
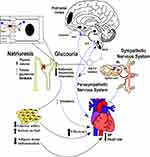
Conclusion: This study demonstrates that SGLT-2 is expressed in CNS tissues involved in autonomic control and possibly influence cardiovascular function. Dapagliflozin influences central autonomic activity via unidentified pathways by inhibiting central or peripheral SGLT-2. These results provide a new concept that sympathetic inhibition by SGLT-2i can be mediated by central autonomic system, a mechanism that explains how SGLT-2i improves the cardiovascular function.
Keywords: sodium glucose co-transporter-2, dapagliflozin, c-Fos, cardiovascular activity, brain nuclei
Introduction
Type 2 diabetes mellitus (T2DM) is a common clinical disorder characterized by hyperglycemia (fasting plasma glucose (FPG) ≥7.0mmol/l, 2-hours, postprandial plasma glucose, 2hPPG ≥11.1mmol/l) and distinct insulin resistance, which is different from the pathogenesis of type 1 diabetes mellitus (T1DM). In recent years, multiple antidiabetic drugs have been developed among which sodium-glucose co-transporter inhibitors (SGLT-2i) which exhibit distinct therapeutic mechanisms is independent of physiological insulin action. Given that it is widely agreed that the implications of T2DM have detrimental effects on the cardiovascular system as they cause macrovascular and microvascular damage,1 available guidelines strongly suggest that the treatment of T2DM should not only focus on managing blood glucose, but also preventing cardiovascular complications or postponing its progression.2 Consistent with this notion, existing evidence argue that SGLT-2i possess cardiovascular benefits.3 Conspicuously, evidence from some studies demonstrates that cardiovascular status is governed by the central nervous system (CNS) which is arguably critical for the macro- and microvascular lesions.4 The regulation of cardiovascular activity is controlled by neuro-endocrine organization depending on the function and levels of pertinent neural peptides or hormones and their receptors, relevant neural circuits or nets, essential renal renin-angiotensin-aldosterone system (RAAS) to keep blood pressure (BP) and heart rate (HR) in a relatively adaptive range.5 Importantly cardiovascular control via CNS is monitored and regulated by the autonomic activity-related nucleus.6 Therefore, an advanced understanding of the neuro-endocrine pathways for controlling cardiovascular activities by drugs such as SGLT-2i is essential.
The existing clinical trials have demonstrated the beneficial effects of SGLT-2i to the cardiovascular system in reducing the incidence of cardiovascular events, this has caused an upsurge of research in the field of endocrinology, cardiology, and nephrology.3,7 The presently accepted partial mechanisms of SGLT-2i on glucose metabolism and other benefits beyond glycemic control are due to glycosuria and natriuresis secondary to inhibiting renal glucose and sodium co-transporter of proximal tubules.8 However, due to the availability of inadequate studies elucidating the pathway of SGLT-2i action in CNS or influence the sympathetic nervous system, the rationale behind SGLT-2i causing a decrease in BP (systolic BP: 5mmHg, diastolic BP: 2mmHg on average)9 without a change in the heart rate remains unknown.10–12 Nonetheless, in the past few years, researchers asserted that besides its role in glucose reabsorption, SGLT may exhibit other distinct functions in CNS.13–15 Unfortunately, the definite localization of the SGLT family and their functions in CNS, specifically SGLT-2 have not been comprehensively established. As such, this study sought to establish SGLT roles in CNS through immunohistochemistry methodology combined with cardiovascular activity detection. Firstly, we examined the change in blood glucose (BG), BP, HR after drug administration. Thereafter, we localized the distribution of the central SGLT-2 protein expression. Through c-Fos immunohistochemistry, we further investigated the possible pathway of SGLT-2i in regulating cardiovascular activity, at the same time, we unmasked the distributional relationships between these proteins. Our results uncover the scientific mystery of how SGLT-2i influences the sympathetic system. Also, they can potentially confirm the clinical findings that unlike diuretics, SGLT-2i has a role in improving the functional status of cardiovascular activities, although some reports suggest that diuretics seemingly elicit a more powerful effect on the reducing the fluid retention.
Materials and Methods
Animals
The male C57BL/6 mice (5 weeks old, 14–15 g) were purchased from Shanghai SIPPR-BK Laboratory Animal Co., Ltd. Mice were housed in separate cages and provided with food (standard laboratory chow) and water ad libitum. They were allowed to adapt to the animal house environment until their body weight reached 20g. The mice room was controlled to a 12:12-h dark-light cycle and an ambient temperature of 21.5–22.5°C. All animal experiments were carried out in accordance with pertinent Experimental Animals Act or Regulation of China. The study was approved by the Institutional Animal Care and Utilization Committee of Fudan University Pudong Animal Experimental Center.
Drug Administration
The doses of Dapagliflozin (1.52 mg/kg, Astra Zenica Pharmaceutical Solutions LLC) were comparable to those used clinically for diabetes (10 mg daily). Each tablet was ground and dissolved in 1mL DMSO solution, and diluted in 65mL saline before use (DMSO: 1.52%).
Blood Glucose, Blood Pressure, and Heart Rate Measuring
The mice were randomly divided into 2 groups (7 weeks old, 20–22g) in accordance with their body weight distribution ensuring homogeneity. The treatments were handled by skilled professional staff and investigators ensuring that drug administration, measuring of BG, BP, HR, and perfusion in each mouse were standard, homogenous, and do not induce gavage stress. The experiment groups received a single intragastric gavage (0.1mL/10g) with Dapagliflozin (n = 17, 30.4ug/20g) solution (DMSO: 3.04ul/20g) whereas the control group (n = 17) received comparable doses of saline. Using blood glucose meter (ACCU-CHEK Performa Nano, Roche), we measured BG levels of mice tails before the intragastric gavage, every 15 min of the first 1h, and 2 hours after intragastric gavage. At the same time, the other 14 male mice were randomly divided into two groups and received the aforementioned treatments, BP, HR measured at 0, 1h, 2h after gavage using a non-invasive monitor device (Kent scientific, CODA-MNTR). Each mouse received a 15 cycle of measuring via volume pressure sensing.
Neurohistochemical Analysis
Tissue Preparation
After taking measurements, the 34 mice were anesthetized with 3% sodium pentobarbital and perfused transcardially with 0.01 mol/L phosphate-buffered saline (PBS; pH 7.4) and then perfused with 10% neutral buffered formalin (Sigma). The specimens were stored and experienced post-fixation for 4 h. After 4 hours, we carefully extracted the fixed tissues including the brain. Afterward, we immersed the brains in a 20% sucrose solution at 4°C. Brain samples prepared to be furtherly processed were stored until sunk. The retrieved brain samples were embedded with reagent optimal cutting temperature (O.C.T) with the use of dry ice and sectioned coronally on a freezing-sliding microtome (Leica) at a thickness of 30 μm, and slices were collected in 12 equal series to cryogenic vials filled with 30% glycerol sucrose buffer.
Immunohistochemistry for SGLT-2 and c-Fos
Notably, c-Fos belongs to the Fos family.16 A variety of stimuli, especially strong stimuli including shock, harmful stimuli, and drugs have been demonstrated to induce spontaneous expression of c-Fos before other mRNA or protein.17,18 For long, expressed c-Fos has been considered as a marker of neuronal activity whereby neurons were firstly stimulated, then detection of the c-Fos expression particularly its maximum expression in 2 hrs. This has been adopted by multiple neurological studies. The single dose of 1.52mg/kg of Dapagliflozin in the present study can be considered as an effective stimulus. Thus in this work, via activation of certain neurons by SGLT-2i, we chose to detect the neural c-Fos expression within a 2 hr duration to search for the possible neural pathway.
On the first day, slices of the brain were taken out of cryogenic vials and 3 times rinsed with 0.01 mol/L PBS (pH 7.4), each time for 5 min. We pre-treated the rinsed slices for 20 min with 0.3% H2O2 to eliminate endogenous catalase, then similarly rinsed with 0.01 mol/L PBS 3 times with each time for 5 min. After rinsing, brain slices were transferred to a blocking solution containing PBX (0.01 mol/L PBS, 0.3% Triton X-100) and 1% donkey serum for 30 min. Eventually, we incubated the brain slices with PBX, 1% donkey serum, and primary mouse anti-c-Fos antibody (1:1 0000, ab208942, Abcam) or rabbit anti-SGLT-2 antibody (1:1000, ab85626, Abcam) at 4°C for overnight.
After overnight incubation, the brain slices were rinsed 3 times with PBS and then transferred to a solution containing PBX, 1% donkey serum, and secondary generic antibody (1:10, G1210-2-A, Service Bio) and incubated for 1 h thereafter rinsed once for 5 min. The slices were stained by reagent 3, 3’-diaminobenzidine (DAB, G1210-2-B, G1210-2-C Service Bio) for 8–10 min. Later, stained brain slices were carefully mounted on gelatin-coated glass slides. To closely attach the brain slices, we dehydrated using graded ethanol, cleared with turpentine oil (TO), and finally coverslipped with neutral balsam.
Nissle Staining to Distinguish the Brain Area and Structure
In this step, brain slices of mice were retrieved from cryogenic vials and rinsed with 0.01M PBS (PH 7.4) 3 times for 5 minutes each time. Afterward, slices were carefully mounted on gelatin-coated glass slides and left to dry. Then slices were rehydrated with graded ethanol with a descending level of 100%, 100%, 95%, 80%, 70%, for 30 seconds each. Thereafter, the slices were rinsed with water for 30 seconds, nissle dye (service bio) added, and stained for 5–6 minutes. After staining, slices were rinsed with water for 2 minutes and following differentiated by 1% hydrochloric alcohol. Again, we rinsed with water and used the microscope to check if staining was satisfactory, if not, the above-stipulated steps will be repeated. Finally, the slices were dehydrated using grade ethanol with ascendant level for 10–15 seconds, cleared with Turpentine oil (TO) and coverslipped with neutral balsam. The Nissle staining distinguishes various parts or regions in the brain to understand the c-Fos expression.
Dual Immunofluorescent Staining of Brain
On the first day, (1) Brain specimens were retrieved from cryopreserved tubes, and washed it in a Petri dish containing 0.01M PBS for 3 times 5 minutes each time. (2) The slices were pretreated with 3 mL PBS containing 0.3% hydrogen peroxide solution in a six-well plate for 20 minutes to inhibit the endogenous peroxidase. After 20 minutes, the slices were washed three times with 0.01M PBS, 5 minutes each time. (3) Tissues sections were put in a six-well plate containing 3 mL of blocking fluid for 30 minutes. The fluid contained 0.01M PBS, 1% Donkey Serum and 0.3% Triton X-100. (4) After blocking, the solution containing 3 mL 1:10000 c-Fos antibody and 1:1000 SGLT-2 antibody was selected, and tissue was incubated overnight at 4°C. The solution contained 0.01M PBS, 1% donkey serum, 0.3% Triton X-100, 1:10000 mouse anti-c-Fos antibody and 1:1000 rabbit anti-SGLT-2 antibody.
On the second day, (5) The sections were washed 3 times in 0.01M PBS from the antibody incubator for 5 minutes each time, (6) The cleaned sections were incubated in a 6-well plate of 3mL PBX containing 1:1000 biotin-conjugated donkey anti-mouse antibody for 1 hour. The solution contains 0.01M PBS, 1% donkey serum, 0.3% Triton X-100 and 1:1000 biotin-conjugated donkey anti-mouse antibody. (7) After incubation, the tissue was rinsed once with 0.01M PBS for 5 minutes. (8) It was transferred to a six-well plate containing 0.01M PBS, 1% donkey serum, 0.3% Triton X-100, and incubated with 1:1000 fluorescent dye 594-conjugated Strepto-avidin and 1:300 fluorescent dye 488-conjugated donkey anti-rabbit antibody for 1 hour. (9) Afterward, the reaction was terminated. The slices were mounted on glass slides, followed by addition of anti-fluorescence quenching solution to the cover slides. The slides were fixed and then transferred to the darkroom to avoid light, (10) Finally, we observed the slices under a fluorescence microscope.
Data Collection and Statistical Analyses
Stained sections were analyzed under an optical microscope (Leica DM2500, Leica Microsystems, Switzerland) equipped with a DFC550 digital camera (Leica Microsystems, Switzerland). Images were captured using Leica application suite software V4.4 (Leica Microsystems). Further, quantitative analysis of Fos immunoreactivity (Fos-IR) induction was conducted blinded by a professional staff, and based on the Allen mouse brain atlas. Through two-way ANOVA and Sidak multiple comparison tests, we analyzed the comparison of treatment-induced changes in Fos-IR expression in the brain. Additionally, data on blood glucose were counted and compared via repeated-measures analysis of variance (RM ANOVA) by Sidak multiple comparison tests, whereas data on heart rate and blood pressure were counted and compared via repeated-measures analysis of variance (RM ANOVA) by Tukey multiple comparison test. For statistical analysis, we used SPSS (IBM, version 23.0) and Prism (Graphpad, version 7.0), where P ≤ 0.05 was considered statistically significant.
Results
Glycemic Dynamic and Change of BP and HR After Intragastric Gavage with SGLT-2i
Generally, our results showed no significant differences in blood glucose levels between the experimental group and the control group in 2 hours after administration (Mean ± SEM: Dapagliflozin: 9.5±0.163mmol/l; Control group: 9.6±0.163 mmol/l; P > 0.05) (Figure 1A). Although the blood glucose level of SGLT-2 group became lower compared to that of the control group 2 hours after administration, the difference was statistically insignificant (Mean ± SEM: Dapagliflozin: 1 hour: 8.9±0.331mmol/l, 2 hours: 7.9±0.229mmol/l; Control: 1 hour: 9.1±0.331 mmol/l, 2 hours: 8.5±0.229 mmol/l; P > 0.05).
For the 14 mice whose BP and HR were measured before, 1h, and 2h after intragastric gavage, both systolic and diastolic BP were significantly lower in mice of SGLT-2i group than control group 2h after gavage (n=14, P<0.05 Tables 1 and 2, Figure 1B–D); however, the HR was not significantly different between the two groups (P>0.05) (Table 3, Figure 1B–D).
 |
Table 1 Changes in Systolic BP in Control Group vs SGLT-2i Treatment Group |
 |
Table 2 Changes in Diastolic BP in Control Group vs SGLT-2i Treatment Group |
 |
Table 3 The Change of HR in Control Group vs SGLT-2i Treatment Group |
SGLT-2 Distribution in the Brain
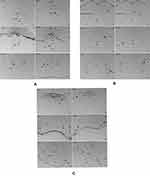
The results from immunohistochemical analysis showed that SGLT-2 was abundantly expressed in the brain, from the forebrain to brainstem, precisely in the hypothalamus, amygdala, periaqueductal gray (PAG), and the nucleus of the solitary tract (NTS) related to autonomic control (Figure 2).
c-Fos Expression Distribution in the Brain After Administration with SGLT-2i
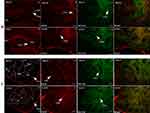
Autonomic regions from telencephalon to caudal brainstem extensively showed c-Fos expression. In particular, high c-Fos expression occurred hypothalamus, amygdala and periaqueductal gray, and significantly higher when compared to the control group (Table 4). Among these areas, PO, MPO, MPA of the forebrain, Me, Ce, BMA, BLA, Pirl of amygdala nucleus, CA3 of the hippocampus, PVN, VMH, LH of the hypothalamus, SC, PAG, DR, VTA, Pn of the midbrain, NTS, LC, CG, PO, LPB, Rt of brainstem have the largest differences in c-FOS counts. Areas in brainstem such as NTS, Rt, LC correlated to sympathetic and parasympathetic outflow exhibited significant c-Fos expression, besides, Me, VMH which according to previous studies showed a relationship with glucose sensing, demonstrated increased c-Fos expression when compared to control group. However, compared to the above-mentioned regions, sparse specific distribution was witnessed in the cerebellar cortex, thalamic area (except for PVA), cerebellum cortex, and basal ganglion (Table 5, Figures 3 and 4). In addition, immunofluorescence results showed increased c-Fos expression in multiple areas expressing SGLT-2 (Figure 5). The comparison of SGLT-2 distribution and c-Fos expression is presented in Table 5
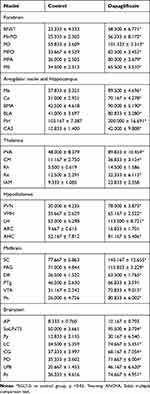 |
Table 4 Quantitative Measure of Fos-Immunoreactivity in Control Group vs SGLT-2i Treatment Group |
 |
Table 5 Analysis of SGLT-2 and c-Fos Expression in Mice Brain |
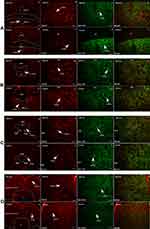 |
Figure 5 Continued. |
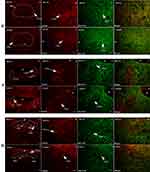 |
Figure 5 Continued. |
Discussion
In this work, we measured the acute blood glucose (BG) variation, blood pressure (BP) and heart rate (HR) change after intragastric gavage, and revealed that as an antidiabetic medication, Dapagliflozin could significantly decrease the BP in normal C57BL/6 mice. This was enabled through a special mechanism independent of the BG regulation that causes an insignificant change in the HR. Using immunohistochemistry, we localized the substantial distributions of SGLT-2 in CNS, which provide direct confirmation and basis for the role of SGLT-2 in autonomic regulation. Importantly, this is arguably a maiden study to simultaneously perform immunohistochemistry assay to analyze the activation signals of brain nuclei associated with autonomic control by dapagliflozin, and discovered remarkably increased signals in multiple nuclei compared with the control group. Furthermore, our study suggested that paraventricular nucleus (PVN) is a crucial target by dapagliflozin in cardiovascular regulation. Besides, our results demonstrated that Dapagliflozin reduces blood volume via a unique mechanism rather than diuretics, which is largely through central autonomic control especially associated with cardiovascular regulation. This contributes to the advancement of prognosis of the chronic heart failure in patients with T2DM. Our findings corroborate with the results proposed by multiple clinical trials.19,20
Effects of SGLT-2i on the BG, BP, HR
As an effective hypoglycemic drug, Dapagliflozin did not significantly change BG in 2 hours but demonstrated a significant change in BP. One possibility is that the blood glucose concentration-depends on the hypoglycemic effect of SGLT-2i meaning it can effectively lower blood glucose at higher levels, but attenuates lowering at low levels, and exerts a moderate protective effect on the target organs of glucose metabolism in the body. Moreover, we speculate that this may be attributed to the pharmacokinetic and pharmacodynamics of dapagliflozin. Of note, the normal absorption into the circulation time range of Dapagliflozin is within 1–2 hours after administration. During 1–2 hours after administration, we also found that the blood glucose of the experimental group was lower than that of the control group at the beginning, but the difference was statistically insignificant. Another key speculation is that the regulation of BG in mice was more stable than BP. Despite not monitoring the urine volume, we discovered that BP can easily be influenced by the dapagliflozin compared to BG; hence, a strong evidence that the ability of SGLT-2i to cut down the BP is not entirely dependent on the circulation volume depletion in such short duration. Again, this reminds us of the subsequent observations on c-Fos expression not caused by blood glucose fluctuations or hypoglycemic stimulation, thus can reliably reflect the changes in cardiovascular activity that we observed concurrently. In contrast, we found the average HR of mice administered with dapagliflozin showing negligible disparity, in agreement with studies that SGLT-2i reduces BP but does not elevate or slow down the HR, and therefore this effect may attribute to the inhibition of sympathetic nervous system by SGLT-2i.21,22 Interestingly, when compared with the control group, BG in the dapagliflozin administered group seems minor lower at 2 hours after administration; however, it did not attain statistical significance (P > 0.05). Similar results were reported by some studies that the blood glucose concentration of the dapagliflozin treated group peaked in 2 hours,23,24 and it may involve the mechanisms of SGLT-2i reducing the blood glucose level distinctly from conventional drugs that stimulate the release of insulin or promote the uptake by the target tissue of insulin. Therefore, we provided evidence that Dapagliflozin may acutely reduce the BP without noticeably influencing on the HR, and the mechanism is partially independent of glycemic change.
Localization of SGLT-2 in the Brain of Mice
Next, we performed histochemistry of localization of SGLT-2 in CNS, which may provide the histological basis for our aforementioned findings on BP, HR. Although the previous studies had not disclosed the exact location of SGLT-2,14,25,26 we found that SGLT-2 protein expressed in multiple brain areas particularly those associated with autonomic control including hypothalamus, midbrain, and brainstem. Besides, we found expression data in some database (GEO, Allen brain atlas, Gene cards, etc.) consistent with our research findings including, ISH data, array expression, and mRNA sequence data. Compared with the kidney, SGLT2 there expressed in low levels in the brain. However, although SGLT-2 expression is not abundant in intracellular space, there could be some functions of central SGLT-2 related to neuronal metabolism, signal transmission, and other neural functions, among others.8,27
Neurons require a constant supply of glucose for optimal functioning. Glucose is transported to the CNS tissues by specific transporters including sodium-independent glucose transporters (GLUTs) and sodium-glucose co-transporters (SGLTs).12,13,27 However, SGLT-2 plays a role in other functions in CNS besides glucose transport. A recent study found the SGLT-2 accumulates in the seizing focus using radioactive tracing. They also discovered that Dapagliflozin can prevent the seizure activities by inhibiting SGLT-2 implying that SGLT-2 regulates neural electrophysiology.28 Elsewhere, it was reported that SGLT-2i can induce remission of inflammation in CNS which is a new landmark of metabolic syndrome29–31 In the present study, we found that SGLT-2 expression was confined to the forebrain in the brainstem of mice, suggesting the existence of possibilities of the central inhibition of SGLT-2.
Similarly, we found that SGLT-2 was expressed in the aforementioned regions and affected cardiovascular function via regulating autonomic control.4,6,32 These regions have distinct autonomic function among them, (1) the central nucleus of the amygdala (CEA), the bed nucleus of stria terminalis (BNST), which are linked to the autonomous emotional state control, (2) the hypothalamus, which has various functions including, autonomic nervous activity, neuroendocrine, behavioral and stress response regulates BP circadian, (3) the periaqueductal gray matter (PAG) of the midbrain, which coordinates the autonomic nerve, pain control, pressure-related, aggressive and reproductive related behaviors, and (4) the nucleus of the solitary tract (NTS) is the site of visceral sensory afferent which initiates multiple cardiovascular, respiratory and other bulbar reflexes and transmits to other areas related to autonomic control.
Dapagliflozin Activates Neurons in Nuclei Related to Cardiovascular Activity
Several studies have been conducted to explore the activities of the neural circuits associated with the regulation of an autonomic or cardiovascular activity, which is intended to explore the pathogenesis of neural hypertension or metabolic syndrome.
Evidence from previous studies demonstrated that various stimuli, including serum, growth factors, tumor promoters, cytokines, and ultraviolet light, can induce the expression of c-Fos.33–35 Because they can be expressed within 15 minutes of being stimulated by a strong ex-stimuli, the mRNA and protein of c-fos are one of the earliest expressed molecules, identified as “immediate early genes”. Additionally, due to its participation in the polarizing of the neuronal electrical activity, it can be used as a marker of neuronal functional activity. After being stimulated, its c-Fos mRNA peaks within 30 minutes, while the protein peaks within 60–90 minutes and last for about 2–5 hours. Because the protein exists longer than the mRNA after being stimulated, it can be argued that the pertinent neuron is activated by the related stimulation, and disappears at least 30–45 minutes after the conversion stimulation, which can facilitate the researchers to quickly dispose of the animal before anesthesia, nor need to consider treatment such as perfusion to cause non-specific c-Fos and related gene expression.
Herein, we selected c-Fos marker of direct neuronal activation and found significantly distinct expression fashion from the control group in neural areas of mice treated with SGLT-2i, including BNST, amygdala, PVN, NTS, etc., which have been established and reported by various literature to participate in regulating BP, HR and other autonomic activities in CNS.32,36,37 This suggests that Dapagliflozin, as a highly selective SGLT-2 inhibitor,38 may have the ability to influence the function of central autonomic control that results in neural activation related to BP and HR regulation.
First, we found that significantly increased expressions of c-Fos in the cortex of piriform and cingulated gyrus, which is a senior autonomic region may be associated with visceral activities. Previously, multiple studies have reported the role of piriform and cingulated gyrus in controlling the cardiovascular system and pathogenesis or as treatment targets of cardiovascular disorders such as hypertension.39–42 Additionally, we established that there was a significant expression of c-Fos in the CA3 region of the Hippocampus. Also, there are studies reported the role of the hippocampus in cardiovascular regulation and related disorders.43,44
Concurrently, we witnessed an increased c-fos expression in nuclei within the hypothalamus. Of note, the hypothalamus possesses multiple autonomic functions including feeding,45 thermal regulation,46 circadian,47 reproduction,48 cardiovascular control.49 Many literature reports have revealed the association of hypothalamus with hypertension and other cardiovascular disorders.49 Notably, over 50% of hypertension cases in the clinic can be attributed to neurogenic essential hypertension.49 The paraventricular nucleus of the hypothalamus (PVN) is a crucial link, and release CRH of the parvocellular part may influence the HPA axis and project to brainstem to control autonomic efferent.50,51 We particularly reported significant c-Fos expression in this region, which suggested SGLT-2i may influence cardiovascular activity via the PVN. However, more exact methodologies are essential to further investigate the functional alterations of different parts and related functions of PVN. Besides, we reported significantly increased c-Fos expression in MnPO which is near the circumventricular organs and can be bound by angiotensin II (AngII), proposing that SGLT-2 may influence the MnPO.52 Other nuclei such as MPO, LHA, VMH, and DMH, showed significant c-Fos expression, which was also revealed to possess SGLT-2. Some nuclei play role in energy regulation; however, a clear-cute technology, such as neuronal potential change or peptides expressions detection, is needed to reveal whether activating the neurons here indicates the relationship between central SGLT-2 and metabolic syndrome.53 Massive clinical trials have demonstrated that the injury of hypothalamus is associated with hypertension.6 Therefore, more deliberated investigations on the reflexes or circuitry within the hypothalamus associated to autonomic control will potentially advance the treatments of novel targets against hypertension and other cardiovascular diseases.
In the midbrain, we noted that the c-Fos expression in the PAG of the experimental group was significantly higher than that of the control group. PAG is an area that some studies found to regulate BP and vessel dilation, stimulating different parts to cause either hypertension or hypotension, respectively.54 Because of the reported expression of SGLT-2 in PAG, we speculate that the significant expression of c-Fos in this region indicates that SGLT-2i may affect the activities of PAG. Again, a precise methodology is needed to expose the function of this nucleus in central SGLT-2 inhibition and cardiovascular activities.
Through electrical stimulation or other tracer studies, much has been determined on the role of brainstem involvement in cardiovascular control.6,55,56 Also, we detected significantly high c-Fos expression in LC, PBL, NTS, Rt, among others. The generalized links of these nuclei are that afferent sensory signals relay to the neurons of NTS and then activate interneurons to send the signals from peripheral to either other brainstem nuclei such as the nucleus ambiguous, dorsal motor nucleus to modulate parasympathetic outflow, or via the rostral ventrolateral medulla inhibitions through GABAergic mechanisms by caudal ventrolateral medulla.57–59 Consistently, we found that c-Fos expression was upregulated in NTS and Rt implying that the effects of Dapagliflozin were mediated by the autonomic efferent flow. Evidence from recent studies show that inflammation in the CNS tissues enhances sympathetic outflow, increases BP and SGLT-2 expression in kidney, causing hypertension or metabolic syndrome.60,61 Several studies have reported that SGLT-2i can prevent inflammation in CNS and peripheral nerve tissues thereby improve BG, BP, TC, and bodyweight.30,31 However, we still need to detect more indices and more precise in-depth research to reveal whether the above-mentioned neural circuit is related to these metabolism alterations, and their relationships with cardiovascular control. Presently, studies have reported SGLT-2i can regulate the sympathetic system which can be found to be especially over-activated in heart failure,62,63 and SGLT-2i has a role of improving the multi-functional status of cardiovascular activities, although the diuretics seem to elicit a more powerful effect on reducing fluid retention.64,65 Thiazide and thiazide-like diuretics may be associated with deleterious biological effects, such as metabolic disturbances (insulin resistance, lipid abnormalities), hypokalemia (which may increase the risk of arrhythmias) and hyperuricemia, as well as clinically detrimental effects such as increased heart rate, all factors that can negatively influence CV outcomes and possibly depress the positive effects of BP reduction.65 However, SGLT-2i did not significantly increase heart rate or creatinine. It modulated both metabolism and cardiovascular activity, decreased arterial stiffness or vascular resistance, thereby improving the cardiovascular function. In a nutshell, our study provides possible evidence on how Dapagliflozin modulates neuroendocrine activities and changes the cardiovascular condition (Figure 6).
In this study, we did not explore the dynamics of SGLT-2i in the brain due to technical limitations. We hope to adopt more precise techniques like radiography or Fluro-labeling in our future studies. It is likely that other neural pathways exist through which dapagliflozin activates neurons in the autonomic area. Here, we recorded inconsistent variation patterns of c-Fos expression and distinct expression areas between two groups, indicating that c-Fos change could not merely be influenced by peripheral blood pressure change. The existence of direct or indirect mechanisms is a possibility requiring further investigations.
Conclusion
Dysregulation of the autonomic nervous system causes cardiovascular disarrangements with detrimental consequences such as severe cardiovascular events. In summary, we localized central SGLT-2 distribution and discovered the expression of c-Fos in multiple nuclei that co-express the SGLT-2, suggesting that the role of SGLT-2i in regulating cardiovascular activity may depend on the neural circuits between these nuclei. The activation of this loop reveals that drugs such as Dapagliflozin can regulate cardiovascular activity and metabolic change through the CNS. In the future, we will focus on the associated neural pathways and molecular mechanisms. The current study presents new explanations on how SGLT-2i influences the sympathetic system and why it outperforms diuretics in improving cardiovascular function. Our findings provide theoretical and practical data for developing new antidiabetic drugs that benefit the cardiovascular system.
Acknowledgment
We thank the staff and investigators at the Science and Innovation Center of Pudong Hospital for their technical assistance.
Disclosure
The drug tablets were provided by Astra Zeneca. The authors declare that they have no conflicts of interest for this work.
References
1. Sobel BE, Schneider DJ. Cardiovascular complications in diabetes mellitus. Curr Opin Pharmacol. 2005;5:143–148. doi:10.1016/j.coph.2005.01.002
2. Valensi P, Prevost G. CVOTs: what did the endocrinologist learn? Diabetes Res Clin Pract. 2019;107947. doi:10.1016/j.diabres.2019.107947
3. Dewan P, Solomon SD, Jhund PS, et al. Efficacy and safety of sodium–glucose co-transporter2 inhibition according to left ventricular ejection fraction in DAPA-HF. Eur J Heart Fail. 2020. doi:10.1002/ejhf.1867
4. Salman IM. Major autonomic neuroregulatory pathways underlying short- and long-term control of cardiovascular function. Curr Hypertens Rep. 2016;18(18). doi:10.1007/s11906-016-0625-x
5. de Kloet AD, Liu M, Rodriguez V, Krause EG, Sumners C. Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am J Physiol Regul Integr Comp Physiol. 2015;309:R444–458. doi:10.1152/ajpregu.00078.2015
6. Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clinic Proceedings. 1993;68:988–1001. doi:10.1016/s0025-6196(12)62272-1
7. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi:10.1161/CIRCULATIONAHA.116.021887
8. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi:10.1152/physrev.00055.2009
9. Baker WL, Smyth LR, Riche DM, et al. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262–275 e269. doi:10.1016/j.jash.2014.01.007
10. Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–476. doi:10.1016/j.jjcc.2017.12.004
11. Sawada Y, Izumida Y, Takeuchi Y, et al. Effect of sodium-glucose cotransporter 2 (SGLT2) inhibition on weight loss is partly mediated by liver-brain-adipose neurocircuitry. Biochem Biophys Res Commun. 2017;493:40–45. doi:10.1016/j.bbrc.2017.09.081
12. Chiba Y, Yamada T, Tsukita S, et al. Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, acutely reduces energy expenditure in BAT via neural signals in mice. PLoS One. 2016;11:e0150756. doi:10.1371/journal.pone.0150756
13. Szablewski L. Glucose transporters in brain: in health and in Alzheimer’s disease. J Alzheimers Dis. 2017;55:1307–1320. doi:10.3233/JAD-160841
14. Koekkoek LL, Mul JD, la Fleur SE. Glucose-sensing in the reward system. Front Neurosci. 2017;11:716. doi:10.3389/fnins.2017.00716
15. Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB. Transport of sugars. Annu Rev Biochem. 2015;84:865–894. doi:10.1146/annurev-biochem-060614-033904
16. Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41:2449–2461. doi:10.1016/j.ejca.2005.08.008
17. Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi:10.1126/science.3131879
18. Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi:10.1016/0166-2236(89)90096-9
19. Patel DK, Strong J. The Pleiotropic effects of sodium-glucose cotransporter-2 inhibitors: beyond the glycemic benefit. Diabetes Ther. 2019;10:1771–1792. doi:10.1007/s13300-019-00686-z
20. Chilton RJ. Effects of sodium-glucose cotransporter-2 inhibitors on the cardiovascular and renal complications of type 2 diabetes. Diabetes Obes Metab. 2020;22:16–29. doi:10.1111/dom.13854
21. Matthews VB, Elliot RH, Rudnicka C, et al. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35:2059–2068. doi:10.1097/HJH.0000000000001434
22. Jordan J, Tank J, Heusser K, et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J Am Soc Hypertens. 2017;11:604–612. doi:10.1016/j.jash.2017.07.005
23. Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85(5):520–526. doi:10.1038/clpt.2008.251
24. Kasichayanula S, Liu X, Lacreta F, Griffen SC, Boulton DW. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet. 2014;53:17–27. doi:10.1007/s40262-013-0104-3
25. Sabolic I, Vrhovac I, Eror DB, et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol. 2012;302:C1174–C1188. doi:10.1152/ajpcell.00450.2011
26. Yu AS, Hirayama BA, Timbol G, et al. Regional distribution of SGLT activity in rat brain in vivo. Am J Physiol Cell Physiol. 2013;304:C240–C247. doi:10.1152/ajpcell.00317.2012
27. Vemula S, Roder KE, Yang T, et al. A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J Pharmacol Exp Ther. 2009;328:487–495. doi:10.1124/jpet.108.146589
28. Erdogan MA, Yusuf D, Christy J, et al. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol. 2018;18:81. doi:10.1186/s12883-018-1086-4
29. Gong M, Wen S, Nguyen T, et al. Converging relationships of obesity and hyperuricemia with special reference to metabolic disorders and plausible therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:943–962. doi:10.2147/dmso.s232377
30. Naznin F, Sakoda H, Okada T, et al. Canagliflozin, a sodium glucose cotransporter 2 inhibitor, attenuates obesity-induced inflammation in the nodose ganglion, hypothalamus, and skeletal muscle of mice. Eur J Pharmacol. 2017;794:37–44. doi:10.1016/j.ejphar.2016.11.028
31. Sa-Nguanmoo P, Tanajak P, Kerdphoo S, et al. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol. 2017;333:43–50. doi:10.1016/j.taap.2017.08.005
32. Dampney RAL, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol. 2003;71:359–384. doi:10.1016/j.pneurobio.2003.11.001
33. Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi:10.1006/frne.1993.1006
34. Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi:10.1016/0165-0270(89)90150-7
35. Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi:10.1111/j.1365-2826.2008.01734.x
36. Galosy RA, Clarke LK, Vasko MR, Crawford IL. Neurophysiology and neuropharmacology of cardiovascular regulation and stress. Neurosci Biobehav Rev. 1981;5:137–175. doi:10.1016/0149-7634(81)90040-3
37. Zhou L, Podolsky N, Sang Z, et al. The medial amygdalar nucleus: a novel glucose-sensing region that modulates the counterregulatory response to hypoglycemia. Diabetes. 2010;59(10):2646–2652. doi:10.2337/db09-0995
38. Meng W, Ellsworth BA, Nirschl AA, et al. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem. 2008;51(5):1145–1149. doi:10.1021/jm701272q
39. Ems R, Garg A, Ostergard TA, Miller JP. Potential deep brain stimulation targets for the management of refractory hypertension. Front Neurosci. 2019;13:93. doi:10.3389/fnins.2019.00093
40. Kasher N, Wittbrodt MT, Alam ZS, et al. Sex differences in brain activation patterns with mental stress in patients with coronary artery disease. Biol Sex Differ. 2019;10:35. doi:10.1186/s13293-019-0248-4
41. Valenza G, Sclocco R, Duggento A, et al. The central autonomic network at rest: uncovering functional MRI correlates of time-varying autonomic outflow. NeuroImage. 2019;197:383–390. doi:10.1016/j.neuroimage.2019.04.075
42. Bremner JD, Campanella C, Khan Z, et al. Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med. 2018;80:515–525. doi:10.1097/PSY.0000000000000597
43. Shoemaker JK, Norton KN, Baker J, Luchyshyn T. Forebrain organization for autonomic cardiovascular control. Auton Neurosci. 2015;188:5–9. doi:10.1016/j.autneu.2014.10.022
44. Templin C, Hänggi J, Klein C, et al. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur Heart J. 2019;40(15):1183–1187. doi:10.1093/eurheartj/ehz068
45. Wen S, Wang C, Gong M, Zhou L. An overview of energy and metabolic regulation. Sci China Life Sci. 2019;62:771–790. doi:10.1007/s11427-018-9371-4
46. Morrison SF, Nakamura K. Central mechanisms for thermoregulation. Annu Rev Physiol. 2019;81:285–308. doi:10.1146/annurev-physiol-020518-114546
47. Walker WH, Walton JC, DeVries AC, Nelson RJ. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020;10:28. doi:10.1038/s41398-020-0694-0
48. Abreu AP, Toro CA, Song YB, et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest. 2020. doi:10.1172/JCI136564
49. Carmichael CY, Wainford RD. Hypothalamic signaling mechanisms in hypertension. Curr Hypertens Rep. 2015;17:39. doi:10.1007/s11906-015-0550-4
50. Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol. 1998;25:461–463. doi:10.1111/j.1440-1681.1998.tb02235.x
51. Alon T, Zhou L, Pérez CA, et al. Transgenic mice expressing green fluorescent protein under the control of the corticotropin-releasing hormone promoter. Endocrinology. 2009;150(12):5626–5632. doi:10.1210/en.2009-0881
52. McKinley MJ, Yao ST, Uschakov A, et al. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiologica. 2015;214(8–32):8–32. doi:10.1111/apha.12487
53. Zhou L, Sutton GM, Rochford JJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6(5):398–405. doi:10.1016/j.cmet.2007.10.008
54. Rossi F, Maione S, Berrino L. Periaqueductal gray area and cardiovascular function. Pharmacol Res. 1994;29:27–36. doi:10.1016/1043-6618(94)80095-2
55. Potts JT, Spyer KM, Paton JF. Somatosympathetic reflex in a working heart-brainstem preparation of the rat. Brain Res Bull. 2000;53:59–67. doi:10.1016/s0361-9230(00)00309-9
56. Owens NC, Verberne AJ. Medial prefrontal depressor response: involvement of the rostral and caudal ventrolateral medulla in the rat. J Auton Nerv Syst. 2000;78:86–93. doi:10.1016/s0165-1838(99)00062-4
57. Howe PR. Blood pressure control by neurotransmitters in the medulla oblongata and spinal cord. J Auton Nerv Syst. 1985;12(2–3):95–115. doi:10.1016/0165-1838(85)90054-2
58. Ito S, Sved AF. Influence of GABA in the nucleus of the solitary tract on blood pressure in baroreceptor-denervated rats. Am J Physiol. 1997;273:R1657–R1662. doi:10.1152/ajpregu.1997.273.5.R1657
59. Park JH, Gorky J, Ogunnaike B, Vadigepalli R, Schwaber JS. Investigating the effects of brainstem neuronal adaptation on cardiovascular homeostasis. Front Neurosci. 2020;14:470. doi:10.3389/fnins.2020.00470
60. Grassi G, Dell’Oro R, Quarti-Trevano F, et al. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48:1359–1365. doi:10.1007/s00125-005-1798-z
61. Elliott RH, Matthews VB, Rudnicka C, Schlaich MP. Is it time to think about the sodium glucose co-transporter 2 sympathetically? Nephrology. 2016;21:286–294. doi:10.1111/nep.12620
62. Joca HC, Santos‐Miranda A, Joviano-Santos JV, et al. Chronic sympathetic hyperactivity triggers electrophysiological remodeling and disrupts excitation-contraction coupling in heart. Sci Rep. 2020;10(1):8001. doi:10.1038/s41598-020-64949-7
63. Gronda E, Vanoli E, Sacchi S, et al. Risk of heart failure progression in patients with reduced ejection fraction: mechanisms and therapeutic options. Heart Fail Rev. 2020;25(2):295–303. doi:10.1007/s10741-019-09823-z
64. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487. doi:10.1111/dom.13126
65. Scheen AJ. Reappraisal of the diuretic effect of empagliflozin in the EMPA-REG OUTCOME trial: comparison with classic diuretics. Diabetes Metab. 2016;42:224–233. doi:10.1016/j.diabet.2016.05.006
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.