Back to Journals » Drug Design, Development and Therapy » Volume 14
Danoprevir for the Treatment of Hepatitis C Virus Infection: Design, Development, and Place in Therapy
Authors Miao M, Jing X, De Clercq E , Li G
Received 25 March 2020
Accepted for publication 12 June 2020
Published 14 July 2020 Volume 2020:14 Pages 2759—2774
DOI https://doi.org/10.2147/DDDT.S254754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Supplementary video 1 of "Danoprevir for HCV treatment" [ID 254754].
Views: 213
Miao Miao,1 Xixi Jing,1 Erik De Clercq,2 Guangdi Li1
1Hunan Provincial Key Laboratory of Clinical Epidemiology, Xiangya School of Public Health, Central South University, Changsha 410078, People’s Republic of China; 2Department of Microbiology, Immunology and Transplantation, Rega Institute for Medical Research, KU Leuven, Leuven 3000, Belgium
Correspondence: Guangdi Li Email [email protected]
Abstract: On June 8, 2018, an NS3/4A protease inhibitor called danoprevir was approved in China to treat the infections of HCV genotype (GT) 1b – the most common HCV genotype worldwide. Based on phase 2 and 3 clinical trials, the 12-week regimen of ritonavir-boosted danoprevir (danoprevir/r) plus peginterferon alpha-2a and ribavirin offered 97.1% (200/206) of sustained virologic response at post-treatment week 12 (SVR12) in treatment-naïve non-cirrhotic patients infected with HCV genotype 1b. Adverse events such as anemia, fatigue, fever, and headache were associated with the inclusion of peginterferon alpha-2a and ribavirin in the danoprevir-based regimen. Moreover, drug resistance to danoprevir could be traced to amino acid substitutions (Q80K/R, R155K, D168A/E/H/N/T/V) near the drug-binding pocket of HCV NS3 protease. Despite its approval, the clinical use of danoprevir is currently limited to its combination with peginterferon alpha-2a and ribavirin, thereby driving its development towards interferon-free, ribavirin-free regimens with improved tolerability and adherence. In the foreseeable future, pan-genotypic direct-acting antivirals with better clinical efficacy and less adverse events will be available to treat HCV infections worldwide.
Keywords: danoprevir, ITMN-191, R7227, HCV NS3/4A inhibitor, HCV genotype
Introduction
According to the WHO report in 2019, 71 million people are infected with the hepatitis C virus (HCV) worldwide. The population of HCV-infected patients in China was approximately 9.8 (6.6 to 10.8) million in 2015.1 Chronic HCV infections are known to damage and inflame the liver, leading to the development of cirrhosis and liver cancer.2 For this reason, effective anti-HCV therapeutic strategies are critical for the management of HCV.3–10
As a hepacivirus from the Flaviviridae family, HCV is a positive-sense, single-stranded RNA virus that contains a linear genome (approximately 9600 nucleotide bases) encoding 10 viral proteins.3 Due to the high genetic heterogeneity of the HCV genome, HCV strains can be classified into 8 genotypes (GT-1 to GT-8) and more than 80 subtypes.11 Globally, the most common genotype is GT-1, followed by GT-3 and GT-2, but genotype distribution varies in different countries.1 Based on a large-scale cohort of 32,030 HCV-infected patients, dominant HCV subtypes in Mainland China are characterized by GT-1b (prevalence = 52.2%), GT-2a (28.7%), GT-3b (7.1%), GT-6a (6.4%), and others.12 Pan-genotypic regimens against different HCV genotypes are highly recommended,3,5,8-10 because of the significant regional divergence of HCV genotypes.1,13
Many antivirals have been developed to treat HCV infections during the past decade.3–7 Of interest, interferon-free, all-oral regimens harboring direct-acting antivirals (DAAs) offer high clinical efficacies (>90%) to “cure” HCV infections.3,14,15 As of January 2020, more than 10 DAAs have been marketed in the USA, while eight DAA-based therapies have been approved by the National Medical Products Administration in China, including (i) asunaprevir soft capsules (Sunvepra®) approved in April 2017; (ii) daclatasvir hydrochloride tablets (Daklinza®) approved in June 2017; (iii) simeprevir capsules (Olysio®) approved in August 2017; (iv) sofosbuvir tablets (Sovaldi®) approved in September 2017; (v) ombitasvir, paritaprevir plus ritonavir tablets (Viekirax®) approved in September 2017; (vi) elbasvir and grazoprevir tablets (Zepatier®) approved in April 2018; (vii) sofosbuvir and velpatasvir tablets (Epclusa®) approved in May 2018; and (viii) danoprevir sodium tablets (Ganovo®) approved on June 8, 2018.
A decade ago, interferon treatments were standards of care for HCV-infected patients.16 However, there is a switch from interferons to DAAs which offer better clinical efficacy and less adverse events. Danoprevir (ITMN-191, R7227) is a macrocyclic small-molecule inhibitor (Movie 1) that blocks the catalytic activity of HCV NS3 protease.17 To treat genotype 1b HCV in treatment-naïve non-cirrhotic adults, danoprevir was approved in China for its use with ritonavir, peginterferon alpha-2a, and ribavirin for 12 weeks (Figure 1A). The clinical efficacy of this danoprevir-based regimen reached 97.1% (Figure 1B).
 |
Figure 1 Clinical use of danoprevir. (A) The approved use of danoprevir (100 mg twice daily) plus ritonavir (100 mg twice daily), peginterferon alpha-2a (180 μg once weekly subcutaneous injection), and ribavirin (1000 mg/day for bodyweight <75 kg, 1200 mg/day for ≥75 kg, twice daily divided dose) for 12 weeks. Sustained virologic response at 12-week posttreatment (SVR12) is defined by the undetectable level of HCV RNA (<15 IU/mL) after a treatment-free period for 12 weeks. (B) Clinical efficacy of the approved danoprevir-based regimen in the treatment of non-cirrhotic treatment-naïve patients infected with HCV genotype 1a or 1b. Clinical data of SVR12 and SVR24 are retrieved from Table 1. |
Here, we provide a comprehensive overview of danoprevir regarding its discovery, mechanisms of action, clinical efficacy, drug resistance, pharmacokinetics, and pharmacodynamics. Since danoprevir is now marketed in China, the clinical use of danoprevir will be highlighted in HCV-infected Chinese patients.
Discovery of Danoprevir
In 1996, the first crystal structure of the HCV NS3/4A protease was reported.18,19 This supported the structure-based design and optimization of HCV protease inhibitors such as danoprevir, simeprevir, telaprevir, and grazoprevir.3,20 Computational algorithms could screen large compound libraries to predict lead compounds that interact with the target of HCV NS3/4A protease. Similar to the discovery of HIV protease inhibitors,5 in silico screening of lead compounds targeting the catalytic site of HCV NS3 protease led to the discovery of danoprevir.21 (Movie 1)
The discovery of danoprevir is briefly summarized herein (Figure 2).21 Based on the principle that a peptide inhibitor could structurally mimic NS3-cleaved peptides to inhibit catalytic activities of HCV NS3 protease, a series of HCV NS3 inhibitors were screened from a virtual library harboring tetrapeptides with a carboxylic acid headgroup and diverse sidechains. Subsequent optimizations were achieved by modifications in the P1′, P2, and P3 groups of lead compounds. First, a series of initial tetrapeptide inhibitors with the carbamate attached to the P2 hydroxyproline were identified as potent hits from the structural-based screening. A lead compound with the tetrahydroisoquinoline carbamate was subsequently identified by enzymatic assays. Second, reconstructing a macrocyclic structure in the initial tetrapeptide inhibitors reduced the solvent-exposed polar surface area. The macrocyclization led to a macrocyclic tripeptide mimetic compound with better cell permeability, metabolic stability, and antiviral potency. Third, the carboxylic acid headgroup was subsequently replaced by an acid bioisostere acylsulfonamide that enhanced enzymatic and cellular potency (>2 fold changes). Fourth, potency and pharmacokinetic properties were further optimized by the modification of P2 isoindoline near the HCV NS3 active site, leading to the discovery of danoprevir (Figure 2).
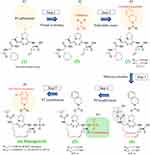 |
Figure 2 Structure-based design and discovery of danoprevir. This Figure is adapted from Jiang Y, Andrews SW, Condroski KR et al Discovery of danoprevir (ITMN-191/R7227), a highly selective and potent inhibitor of hepatitis C virus (HCV) NS3/4A protease. J Med Chem. 2014;57(5):1753–1769. Copyright (2014) American Chemical Society.21 |
Danoprevir was originally designed by InterMune, and its US patent (ID: 7,491,794 B2) was filed on October 13, 2004. In October 2010, Roche purchased the exclusive license of danoprevir for its global development and commercialization rights.17 In April 2013, Roche made an agreement with Ascletis Pharmaceutical, a Chinese biotechnology company located in Hangzhou, for the co-development of danoprevir in China. Under the agreement terms, Ascletis is fully responsible for its commercialization in China while Roche would receive royalties in return.17 The US patent of danoprevir will expire on October 13, 2024.
Mechanism of Action
The drug target of danoprevir is HCV NS3 which consists of a zinc-dependent serine protease at the N terminus (amino acids: 1 to 180) and an ATP-dependent RNA helicase at the C terminus (amino acids: 181 to 631) (Figure 3). During the viral maturation, HCV NS3 serine protease is an indispensable enzyme that cleaves HCV precursor polyproteins at four junctions (NS3-NS4A, NS4A-NS4B, NS4B-NS5A, NS5A-NS5B). This catalytic processing thereby releases five non-structural viral proteins (NS3, NS4A, NS5B, NS5A, NS5B) for their subsequent maturation. The activation of NS3 protease indispensably requires the binding of its cofactor NS4A, which is a single-pass transmembrane protein that locates and stabilizes NS3 protease facing towards the cytosol (Figure 3A). NS3 helicase coupled with adenosine triphosphate (ATP) is responsible for the RNA translocation and unwinding.22
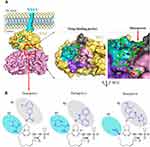 |
Figure 3 Drug binding pocket of danoprevir in the HCV NS3/4A complex. (A) Surface visualization of HCV NS3/4A with its inhibitor danoprevir (PDB: 4B76, 5EQR). The binding of NS4A to the endoplasmic reticulum membrane is presented by a schematic model. The drug-binding pocket of danoprevir is highlighted at the right. Four surface areas S1 to S4 are colored by pink, gray, cyan, and purple, respectively. (B) Chemical structures of danoprevir, paritaprevir, and simeprevir. Differences of three inhibitors are highlighted in blue, and colored cycles represent the S1 and S2 surface regions within the drug-binding pocket of HCV NS3 protease. Protein structures are visualized using the PyMOL V1.7 (https://pymol.org). |
HCV NS3 protease is the therapeutic target of many approved inhibitors such as danoprevir, boceprevir, telaprevir, paritaprevir, grazoprevir, and simeprevir.3 As illustrated in Figure 3A, these inhibitors target the same drug-binding pocket of NS3 at the catalytic site harboring three catalytic triad residues (H57, D81, S139). Similar to other macrocyclic inhibitors such as simeprevir and paritaprevir, danoprevir noncovalently binds to the catalytic site and competes with NS3 substrates. This intervention blocks the NS3-mediated proteolytic cleavages (Movie 2), thereby preventing viral maturation and replication.
Assuming a two-step mechanism, the surface plasmon resonance biosensor-based analysis showed the equilibrium dissociation constants (KD) of danoprevir to the NS3 protease (without the NS4A cofactor) in GT-1a (KD = 2.5 nM), GT-1b (1.6 nM), and GT-3a (21 nM).23 Moreover, the KD values of danoprevir to the NS3/4A protease were 4.0 nM and 6.5 nM in GT-1a and GT-1b, respectively.23 The binding of danoprevir to NS3 protease is characterized by the slow and tight association and an extremely slow dissociation, thereby allowing the persistent inhibition of NS3 protease activity.23,24
Clinical Efficacy
The clinical efficacy of danoprevir has been evaluated by nine clinical studies (Table 1). In addition to the RUSHMORE study (phase 2a, NCT01483742) which recruited cirrhotic patients,25 the other eight studies reported the clinical efficacy of danoprevir in non-cirrhotic patients, including the MANASA study (phase 3, NCT03020082),26 a phase 2/3 study (NCT03362814),27 the DAUPHINE study (phase 2b, NCT01220947),28 the MAKALU study (phase 2, NCT03020004),29 the EVEREST study (phase 2, NCT03020095),30 the INFORM-SVR study (phase 2, NCT01278134),31 a phase 2 study (NCT01331850),32 and a phase 1 study (NCT01185860).33 Among these studies, the approved danoprevir-based regimen (danoprevir 100 mg twice daily (BID) plus ritonavir 100 mg twice daily; peginterferon alpha-2a 180 μg once weekly; and ribavirin 1000 mg/day for bodyweight <75 kg, 1200 mg/day for ≥75 kg, twice daily for 12 weeks) in treatment-naïve non-cirrhotic patients was evaluated by the MANASA study,26 the MAKALU study,29 the DAUPHINE study,28 and a phase 1 study (NCT01185860).33
 |
Table 1 Clinical Efficacy of Danoprevir 100 mg in Major Clinical Trials of GT-1 |
In the MANASA study (phase 3), 141 treatment-naïve non-cirrhotic adults infected with either HCV GT-1a (n = 3) or GT-1b (N = 138) were recruited from 23 medical centers in China and 140 (99.3%) patients completed the full course of the approved danoprevir regimen.26 Detection of sustained virologic response (HCV RNA <15 IU/mL) at post-treatment week 12 (SVR12) was observed in 136 (97.1%) patients. The drug-related serious adverse events included alanine aminotransferase elevation and liver dysfunction.26
In the MAKALU study (phase 2), 70 treatment-naïve non-cirrhotic patients infected with HCV GT-1b were recruited in China and 69 (98.6%) patients completed the 12-week treatment of the approved danoprevir regimen.29 One patient discontinued the therapy due to adverse events. SVR12 was observed in 66 (96.0%) of 69 patients, and a virologic breakthrough was not observed. The common adverse events were nausea and diarrhea (approximately 10%).29
In the DAUPHINE study (phase 2b), treatment-naïve non-cirrhotic adults infected with either HCV GT-1a (n = 244), GT-1b (n = 135), GT-3 (n = 1), or GT-4 (n = 33) randomly received different doses of danoprevir (50 mg, 100 mg, 200 mg) plus ritonavir, peginterferon alpha-2a, and ribavirin.28 After the 12-week treatment of danoprevir/r (100 mg/100 mg) plus peginterferon alpha-2a and ribavirin, SVR24 rates were 63.0% (17/27), 73.9% (17/23), and 100.0% (6/6) against GT-1a, GT-1b, and GT-4 (Table 2), respectively.28 Moreover, headache, fatigue, and pyrexia were the most common adverse events (>30%).28 Incidences of adverse events such as fatigue, myalgia, and chills were similar between the danoprevir/r plus peginterferon alpha-2a/ribavirin arm and the peginterferon alpha-2a/ribavirin arm.28
 |
Table 2 Clinical Efficacy of Danoprevir 100 mg in Patients Infected with HCV GT-4 |
The accumulated data from the MANASA study (phase 3),26 the DAUPHINE study (phase 2b),28 and a phase 1 study (NCT01185860)33 suggested that the most common adverse events were anemia (48.5%), fatigue (36.3%), fever (35.5%), and headache (32.4%). Table 3 summarizes the important adverse events of the danoprevir-based regimen in the aforementioned three clinical trials. Many adverse events have already been reported in HCV-infected patients who received the regimen of peginterferon alpha-2a plus ribavirin (Table 3). Therefore, the clinical use of danoprevir/r + peginterferon alpha-2a + ribavirin should be closely monitored and dose reduction or treatment discontinuation may be considered. Moreover, adverse events of danoprevir are yet to be evaluated by single-drug arms in clinical trials.
 |
Table 3 Adverse Events of Danoprevir/r (100 mg/100 mg) Twice Daily Plus Peginterferon Alpha-2a and Ribavirin in Major Clinical Trials |
In addition to the approved regimen, danoprevir in combinational therapeutics were also examined by several clinical trials. For instance, (i) danoprevir/r plus ravidasvir (NS5A inhibitor) and ribavirin in the EVEREST study offered 100% of SVR12 and SVR24 in treatment-naïve non-cirrhotic HCV GT-1-infected patients who received danoprevir/r (100 mg/100 mg twice daily) plus ravidasvir (200 mg once daily) and ribavirin for 12 weeks.30 This regimen also exhibited a high rate of SVR12 (99%, 306/309) in another phase 2/3 trial (NCT03362814).27 (ii) Danoprevir/r plus mericitabine (NS5B inhibitor) and peginterferon alpha-2a/ribavirin for 24 weeks in the MATTERHORN study offered a high rate of SVR24 (98.2%, 55/56) in non-cirrhotic HCV GT-1b-infected patients who previously experienced partial or null responses.32 Another phase 2b study (RUSHMORE) reported SVR24 (61.1%, 11/18) of the above regimen in cirrhotic HCV GT-1-infected patients who previously exhibited null responses.25 (iii) Danoprevir/r plus mericitabine and ribavirin for 24 weeks offered SVR24 <65% in non-cirrhotic HCV-infected patients based on two phase 2 studies: INFORM-SVR31 and MATTERHORN (Table 1).32 The above findings revealed the potential of danoprevir/r in interferon-free regimens, but future studies are yet to evaluate the interferon-free ribavirin-free regimens of danoprevir plus other DAAs such as sofosbuvir.
Drug Resistance
Drug resistance mutations may significantly reduce the potency of NS3 protease inhibitors.38 Table 4 summarizes drug resistance mutations in HCV GT-1-infected patients who experienced the treatment failure of the approved danoprevir regimen. It is known that substitutions at amino acid positions 36, 80, 155, 156, and 168 induce drug resistance to simeprevir and paritaprevir.39 Given the similar chemical structures of macrocyclic NS3/4A inhibitors (Figure 3B), drug resistance profiles may share common features between danoprevir, simeprevir, and paritaprevir.
 |
Table 4 Drug Resistance Mutations of Danoprevir in Clinical Studies |
In a phase 3 study, R155K (n = 1) and D168A (n = 3) were observed in four treatment-naïve HCV GT-1-infected patients who showed no SVR12 after receiving danoprevir/r plus pegylated-interferon alpha-2a and ribavirin for 12 weeks.26 In the phase 2 study called INFORM-SVR, R155K was detected in all danoprevir-treated HCV GT-1a-infected patients (n = 97) at the time of breakthrough, relapse, or discontinuation.31 D168A was also observed in HCV GT-1b-infected patients with virological breakthrough or relapse.31 R155K and D168A could interrupt the favorable cation-π stacking interactions between danoprevir and the drug-binding pocket, thereby reducing the binding affinity of danoprevir to HCV NS3 protease.40
Naturally occurring polymorphisms in different HCV genotypes, such as Q168 in GT-3,38 could induce drug resistance and prevent the pan-genotypic antiviral activity of danoprevir.41 Three active site polymorphisms (R123T, I132L, D168Q) are mainly responsible for the lost potency of protease inhibitors against HCV GT-3.41 This finding was supported by comparing the enzyme inhibition constant (Ki) of danoprevir in different HCV strains: (i) Ki = 1 ± 0.13 nM in a wildtype strain of GT-1a, (ii) Ki = 29.4 ± 3.4 nM in a GT-1a mutant with D168Q, (iii) Ki = 879 ± 39 nM in a wildtype strain of GT-3a, and (iv) Ki = 1056 ± 376 nM in a GT-1a mutant with R123T, I132L, and D168Q.41 Furthermore, GT-1a mutations on the surface area S2 showed modest to significant drug resistance to danoprevir, including R155K (EC50: 447 nM), D168A (153 nM), D168E (75 nM), and A156V (63 nM).42
Based on clinical findings in the literature, amino acid substitutions Q80K/R, R155K, and D168A/E/H/N/T/V are likely associated with drug resistance to danoprevir (Table 4). In treatment-failure patients, the most common mutation is R155K (96.9% in GT-1a, 35.5% in GT-1b), followed by D168A/E/H/N/T/V (4.8% in GT-1a, 70.8% in GT-1b), and Q80K/R (20% in GT-1a, 7.1% in GT-1b). R155K is often observed in GT-1a but not GT-1b because the amino acid substitution from R to K only needs one nucleotide substitution in GT-1a (AGA to AAA), but two nucleotide substitutions are required in GT-1b (CGA to AAA).43 In treatment-naïve GT-1b-infected patients, R155K (prevalence: 0%) is not a natural polymorphism conferring resistance to danoprevir and the prevalence of D168A/E/H/N/T/V is less than 1% (8/940).44,45 However, Q80R/K in non-1b genotypes is a major concern because its estimated prevalence is 98.7% (299/303) in GT-6a,44 95.5% (84/88) in GT-1a,45 and 0.6% (1/165) in GT-1b.44 An in vitro study reported the resistance towards danoprevir by protease substitutions Y56H and D168A,46 but Y56H could not be observed in the six clinical studies above. In another in vitro study, D168E and D168Y rapidly emerged during the phage-assisted continuous evolution of NS3/4A protease,47 while D168Y was neither reported by clinical studies. This disagreement might be due to inevitable differences between in vitro assays and clinical subjects.
The above findings support the use of danoprevir against GT-1b, but not GT-1a and GT-6a. Due to similar inhibitory potentials between macrocyclic NS3/4A inhibitors,48 it is not surprising that Q80K/R, R155K, and D168A/E/H/N/T/V (Figure 4) are listed as drug resistance mutations of macrocyclic NS3/4A protease inhibitors such as simeprevir and paritaprevir.39 Based on the resistance profiles, potent macrocyclic analogs with flexible moieties at the P2 position (Figure 2) would maintain active inhibition against HCV strains with drug resistance mutations in NS3/4A protease.49
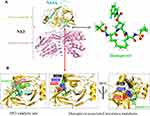 |
Figure 4 HCV NS3 protease catalytic sites and drug resistance mutations. (A) Crystal structure of HCV NS3/4A protease with its inhibitor danoprevir (PDB: 4B76, 5EQR). HCV NS3 protease, NS3 helicase, and the NS4A cofactor are colored by yellow, pink, and cyan, respectively. Crystal structure of danoprevir (PDB code: 5EQR). (B) NS3 catalytic site with three catalytic triad residues: H57, D81, and S139 (PDB code: 3SU0). Three amino acid substitutions (R155K, Q80K, D168A) associated with the drug resistance of danoprevir (PDB codes: 3SU0, 3SU1). Protein structures are visualized using the PyMOL V1.7 (https://pymol.org). |
Table 5 summarizes the EC50 values and fold changes of danoprevir-associated resistance mutants in HCV-infected patients who received danoprevir. The susceptibility to danoprevir was significantly reduced by amino acid substitutions such as R155K/Q and D168A/E/H/T/V (fold changes of EC50 >10). This implies that 155 and 168 substitutions exert an important impact on the potency of danoprevir.50
 |
Table 5 Drug Resistance Profiles of Danoprevir-Associated NS3 Mutations |
Based on the list of danoprevir-associated variants in Table 5, we used HCV NS3 sequences from our previous study38 to report the proportions of danoprevir-associated variants in six HCV genotypes. As shown in Table 6, danoprevir-associated resistance mutations are natural variants in several HCV genotypes. As a drug resistance mutation of danoprevir, D168E has a high replication capacity (34%) and fold changes of EC50 (75 folds), while it is highly prevalent in GT-5 (62.5%) and GT-6 (3.8%).
 |
Table 6 Proportions of Danoprevir-Associated Variants in Six HCV Genotypes Based on NS3 Sequences in Literature38 |
Pharmacokinetics and Pharmacodynamics
Inhibitory and Effective Concentrations
Based on a continuous fluorescent resonance energy transfer (FRET)-based assay, the half-maximal inhibitory concentrations (IC50) of danoprevir against the NS3/4A protease were measured for different clinical isolates: (i) 0.2 ± 0.01 nM in a GT-1a clinical isolate FJ024486; (ii) 0.29 ± 0.07 nM in a GT-1b reference strain FJ031985; (iii) 1.6 ± 0.1 nM in a GT-2b isolate FJ024487; (iv) 3.5 ± 0.5 nM in GT-3a isolate FJ024488; (v) 0.24 ± 0.02 nM in GT-4 isolate FJ024489; (vi) 0.35 ± 0.01 nM in GT-5 isolate FJ024490; and (vii) 0.45 ± 0.01 nM in GT-6 isolate FJ024491.52 In the evaluation of HCV viral RNA replication in Huh-7 hepatoma cells compared with a DMSO-treated control signal, the half-maximal effective concentration (EC50) of danoprevir was 1.6 nM against GT-1b and 20 nM against GT-2 and GT-3.21
An in vitro phenotypic analysis was designed to evaluate the susceptibility of different HCV genotypes to danoprevir using intra- and inter-genotypic chimeric recombinant viruses.53 Different HCV genotypes conferred varied susceptibility to danoprevir, including GT-1b (IC50 = 3 nM), GT-2a (750 nM), GT-3a (280 nM), GT-4a (2 nM), GT-5a (670 nM), and GT-6a (2 nM).53 This revealed the high potency of danoprevir to inhibit GT-1, GT-4, and GT-6.53
Bioavailability and Excretion
Danoprevir can be orally administered with or without food (Table 7). The absolute bioavailability of danoprevir 100 mg is only 1.15%, but this value increases to 3.86% when ritonavir is co-administered.54 Excretion of oral danoprevir (100 mg) or intravenous danoprevir (2 mg, 6 mg) was almost completed 6 hours after the administration in healthy nonsmoking subjects (age range: 18 to 55 years, body mass index: 18 to 30 kg/m2).54 After the intravenous administration, nearly 7% of an intravenous dose is excreted in the urine.54 Over 24 hours, 0.1% of oral danoprevir (100 mg) is excreted in urine and its urine excretion increases to 0.4% when danoprevir is orally administered with ritonavir 100 mg.54
 |
Table 7 Pharmacokinetic Parameters of Danoprevir (100 mg) Plus Ritonavir (100 mg) |
Synergistic Effects Between Danoprevir and Ritonavir/Peginterferon Alpha-2a
Ritonavir acts as a pharmacologic booster of danoprevir because ritonavir inhibits the metabolism of danoprevir and improves its pharmacokinetic profile.55 Since danoprevir is mainly metabolized by the cytochrome P450 (CYP) 3A4, its concentration is significantly increased by CYP3A inhibitors (such as ritonavir, ketoconazole) or reduced by CYP3A inducers such as rifampin, rifabutin, and carbamazepine.54 Moreover, peginterferon alpha-2a can reduce the concentration of danoprevir required for eliminating HCV replicon.52
The steady-state pharmacokinetic parameters of danoprevir/r (100 mg/100 mg twice daily) were tested in treatment-naïve patients infected with HCV GT-1.55 Its parameters were reported as follows: (i) the steady-state area under the curve over the dosing interval (AUC0-T,ss) = 87.0 ng×h/mL; (ii) observed maximum plasma concentration at steady state (Cmax,ss) = 40.1 ng/mL; (iii) median time to Cmax,ss (Tmax,ss) = 2 h; and (iv) observed trough concentration at steady state (Ctrough,ss) = 0.352 ng/mL.55
Pharmacokinetic Parameters
According to the approved label of Ganovo®, a single dose of danoprevir 100 mg could be rapidly absorbed in healthy Chinese subjects, and the maximal plasma concentration (Tmax) is reached at approximately 1.5 hours. Furthermore, the median observed maximal plasma concentration (Cmax) of danoprevir 100 mg is 8.7 ng/mL and the median area under the plasma concentration-time curve at hour 24 (AUC0-24) is 15.2 ng×h/mL. In healthy subjects, a single dose of danoprevir 100 mg is quickly cleared since its mean elimination half-life (T1/2) is approximately one hour and its apparent volume of distribution (Vd) is 8600 L. Compared to danoprevir alone, the median Cmax, median AUC0-24, and the mean T1/2 of danoprevir plus ritonavir increase to 29.7 ng/mL, 117.9 ng×h/mL, and 3.2 hours, respectively.
In HCV-infected patients, the plasma concentration of danoprevir ranges from 0.2 to 11.1 ng/mL at steady state after the administration of danoprevir/r for 6 to 7 days. The median area under the plasma concentration-time curve at hour 12 (AUC0-12) is approximately 2.8 ng×h/mL after multiple doses of danoprevir/r.
Conclusion
We provide a comprehensive overview of danoprevir to highlight its preclinical discovery, clinical efficacy, pharmacokinetics, and pharmacodynamics. Based on clinical trials at phases 2 and 3, SVR12 of the danoprevir-based regimen (danoprevir/r plus pegylated-interferon alpha-2a and ribavirin) reached 97.1% (200/206) to treat non-cirrhotic treatment-naïve patients infected with HCV GT-1b (Figure 1). Host variations are also associated with treatment responses in HCV-infected patients.56 Based on genome-wide association studies, a key nucleotide polymorphism (rs12979860) in the host interleukin 28B (IL28B) determines clinical outcomes in HCV-infected patients.56,57 Most HCV-infected Han Chinese patients harbor the IL28B genotype CC (rs12979860), probably leading to better treatment responses in patients with the peginterferon/ribavirin regimen.57
Although danoprevir has been marketed at a relatively cheap price in China, the approved danoprevir-based regimen is only limited to the treatment of HCV GT-1b and should be closely monitored due to its adverse events. Of note, the recent development of pan-genotypic DAAs could provide interferon-free once daily regimens to effectively treat different HCV genotypes.3–10 For instance, Epclusa® is a fixed-dose single-pill tablet of sofosbuvir and velpatasvir. This 12-week regimen offers more than 95% of SVR12 against different HCV genotypes (GT-1 to GT-6) even in treatment-experienced cirrhotic patients.3
To develop interferon-free, ribavirin-free regimens administered with oral tablets, possible regimens such as danoprevir 100 mg twice daily plus sofosbuvir 400 mg once daily (NCT03887637) or danoprevir/r plus ravidasvir (NCT03020095, NCT03362814) are still under evaluation. Future studies need to investigate the clinical use of danoprevir with other DAAs in worldwide populations. Overall, the approval of danoprevir has boosted the popularity of direct-acting antivirals against HCV infections in China. In the foreseeable future, pan-genotypic DAAs with better efficacy and less adverse events will be approved in China and other countries.
Abbreviations
ATP, adenosine triphosphate; AUC, area under the plasma concentration-time curve; BID, twice daily; Cmax, maximum plasma concentration; Cmax,ss, observed maximum plasma concentration at steady state; Ctrough,ss, observed trough concentration at steady state; CYP, cytochrome P450; DAA, direct-acting antiviral; Danoprevir/r, ritonavir-boosted danoprevir; EC50, half-maximal effective concentration; GT, genotype; HCV, hepatitis C virus; IC50, half maximal inhibitory concentration; SVR, sustained virologic response; T1/2, elimination half-life; Tmax, time to maximum plasma concentration; UK, United Kingdom; USA or US, United States of America; Vd, apparent volume of distribution.
Acknowledgments
We are grateful for Prof. Lai Wei who shared with us the SVR12 data from the MANASA and MAKALU studies. This work was supported by the National Nature Science Foundation of China (grant numbers 31871324, 81730064, 31571368), Natural Science Foundation of Hunan Province (grant number 2018JJ3713), Hunan Youth Elite Project (grant number 2018RS3006), and National Science and Technology Major Project (grant number 2018ZX10715004).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Blach S, Zeuzem S, Manns M; Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176. doi:10.1016/S2468-1253(16)30181-9
2. Foster GR, Irving WL, Cheung MC, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. doi:10.1016/j.jhep.2016.01.029
3. Li G, De Clercq E. Current therapy for chronic hepatitis C: the role of direct-acting antivirals. Antiviral Res. 2017;142:83–122. doi:10.1016/j.antiviral.2017.02.014
4. De Clercq E. Development of antiviral drugs for the treatment of hepatitis C at an accelerating pace. Rev Med Virol. 2015;25(4):254–267. doi:10.1002/rmv.1842
5. De Clercq E, Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29(3):695–747. doi:10.1128/CMR.00102-15
6. De Clercq E. C-nucleosides to be revisited. J Med Chem. 2016;59(6):2301–2311. doi:10.1021/acs.jmedchem.5b01157
7. De Clercq E. Current race in the development of DAAs (direct-acting antivirals) against HCV. Biochem Pharmacol. 2014;89(4):441–452. doi:10.1016/j.bcp.2014.04.005
8. Chung RT, Ghany MG, Kim AY; AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis. 2018;67(10):1477–1492. doi:10.1093/cid/ciy585
9. Pawlotsky J-M, Negro F, Aghemo A; European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511. doi:10.1016/j.jhep.2018.03.026
10. Kanda T, Lau GKK, Wei L, et al. APASL clinical practice recommendation: how to treat HCV-infected patients with renal impairment? Hepatol Int. 2019;13(2):103–109. doi:10.1007/s12072-018-9915-5
11. Hedskog C, Parhy B, Chang S, et al. Identification of 19 novel hepatitis C virus subtypes-further expanding HCV classification. Open Forum Infect Dis. 2019;6(3):ofz076. doi:10.1093/ofid/ofz076
12. Chen Y, Yu C, Yin X, Guo X, Wu S, Hou J. Hepatitis C virus genotypes and subtypes circulating in Mainland China. Emerg Microbes Infect. 2017;6(1):e95. doi:10.1038/emi.2017.77
13. Zhang Y, Chen LM, He M. Hepatitis C virus in mainland China with an emphasis on genotype and subtype distribution. Virol J. 2017;14(1):41. doi:10.1186/s12985-017-0710-z
14. Rivero-Juarez A, Brieva T, Frias M, Rivero A. Pharmacodynamic and pharmacokinetic evaluation of the combination of daclatasvir/sofosbuvir/ribavirin in the treatment of chronic hepatitis C. Expert Opin Drug Metab Toxicol. 2018;14(9):901–910. doi:10.1080/17425255.2018.1506765
15. Cotter TG, Jensen DM. Glecaprevir/pibrentasvir for the treatment of chronic hepatitis C: design, development, and place in therapy. Drug Des Devel Ther. 2019;13:2565–2577. doi:10.2147/DDDT.S172512
16. Ellis S. Chinese approval for Ascletis’ HCV drug is first homegrown success. Nat Biotechnol. 2018;36(8):675–676. doi:10.1038/nbt0818-675a
17. Markham A, Keam SJ. Danoprevir: first global approval. Drugs. 2018;78(12):1271–1276. doi:10.1007/s40265-018-0960-0
18. Kim JL, Morgenstern KA, Lin C, et al. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell. 1996;87(2):343–355. doi:10.1016/S0092-8674(00)81351-3
19. Love RA, Parge HE, Wickersham JA, et al. The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell. 1996;87(2):331–342. doi:10.1016/S0092-8674(00)81350-1
20. Mak LY, Seto WK, Lai CL, Yuen MF. An update on the toxicological considerations for protease inhibitors used for hepatitis C infection. Expert Opin Drug Metab Toxicol. 2018;14(5):483–491. doi:10.1080/17425255.2018.1472236
21. Jiang Y, Andrews SW, Condroski KR, et al. Discovery of danoprevir (ITMN-191/R7227), a highly selective and potent inhibitor of hepatitis C virus (HCV) NS3/4A protease. J Med Chem. 2014;57(5):1753–1769. doi:10.1021/jm400164c
22. Dumont S, Cheng W, Serebrov V, et al. RNA translocation and unwinding mechanism of HCV NS3 helicase and its coordination by ATP. Nature. 2006;439(7072):105–108. doi:10.1038/nature04331
23. Svahn Gustafsson S, Ehrenberg A, Schmuck B, Anwar MI, Danielson UH. Identification of weak points of hepatitis C virus NS3 protease inhibitors using surface plasmon resonance biosensor-based interaction kinetic analysis and genetic variants. J Med Chem. 2014;57(5):1802–1811. doi:10.1021/jm401690f
24. Rajagopalan R, Misialek S, Stevens SK, et al. Inhibition and binding kinetics of the hepatitis C virus NS3 protease inhibitor ITMN-191 reveals tight binding and slow dissociative behavior. Biochemistry. 2009;48(11):2559–2568. doi:10.1021/bi900038p
25. Gane EJ, Rouzier R, Hassanein T, et al. Ritonavir-boosted danoprevir-based regimens in treatment-naive and prior null responders with HCV genotype 1 or 4 and compensated cirrhosis. Hepatol Int. 2016;10(3):478–487. doi:10.1007/s12072-015-9699-9
26. Wei L, Shang J, Ma Y, et al. Efficacy and safety of 12-week interferon-based danoprevir regimen in patients with genotype 1 chronic hepatitis C. J Clin Transl Hepatol. 2019;7(3):221–225. doi:10.14218/JCTH.2019.00018
27. Xu X, Feng B, Guan Y, et al. Efficacy and safety of all-oral, 12-week ravidasvir plus ritonavir-boosted danoprevir and ribavirin in treatment-naive noncirrhotic HCV genotype 1 patients: results from a phase 2/3 clinical trial in China. J Clin Transl Hepatol. 2019;7(3):213–220. doi:10.14218/JCTH.2019.00033
28. Everson G, Cooper C, Hezode C, et al. DAUPHINE: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha-2a/ribavirin in HCV genotypes 1 or 4. Liver Int. 2015;35(1):108–119. doi:10.1111/liv.12471
29. Zheng S, Hua R, Xie Q, et al. MAKALU: twelve-week of treatment with ritonavir-boosted danoprevir plus peginterferon and ribavirin produces 96% SVR12 in HCV genotype 1-infected non-cirrhotic chinese patients [abstract no. LB010]. Hepatol Int. 2017.
30. Kao JH, Yu ML, Chen CY, et al. Twelve-week ravidasvir plus ritonavir-boosted danoprevir and ribavirin for non-cirrhotic HCV genotype 1 patients: a phase 2 study. J Gastroenterol Hepatol. 2018;33(8):1507–1510. doi:10.1111/jgh.14096
31. Gane EJ, Pockros PJ, Zeuzem S, et al. Mericitabine and ritonavir-boosted danoprevir with or without ribavirin in treatment-naive HCV genotype 1 patients: INFORM-SVR study. Liver Int. 2015;35(1):79–89. doi:10.1111/liv.12588
32. Feld JJ, Jacobson IM, Jensen DM, et al. Randomized study of danoprevir/ritonavir-based therapy for HCV genotype 1 patients with prior partial or null responses to peginterferon/ribavirin. J Hepatol. 2015;62(2):294–302. doi:10.1016/j.jhep.2014.09.013
33. Gane EJ, Rouzier R, Wiercinska-Drapalo A, et al. Efficacy and safety of danoprevir-ritonavir plus peginterferon alfa-2a-ribavirin in hepatitis C virus genotype 1 prior null responders. Antimicrob Agents Chemother. 2014;58(2):1136–1145. doi:10.1128/AAC.01515-13
34. Di Bisceglie AM, Ghalib RH, Hamzeh FM, Rustgi VK. Early virologic response after peginterferon alpha-2a plus ribavirin or peginterferon alpha-2b plus ribavirin treatment in patients with chronic hepatitis C. J Viral Hepat. 2007;14(10):721–729. doi:10.1111/j.1365-2893.2007.00862.x
35. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. doi:10.1056/NEJMoa020047
36. McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361(6):580–593. doi:10.1056/NEJMoa0808010
37. Shiffman ML, Di Bisceglie AM, Lindsay KL, et al. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126(4):
38. Cuypers L, Li G, Libin P, Piampongsant S, Vandamme A-M, Theys K. Genetic diversity and selective pressure in hepatitis C virus genotypes 1-6: significance for direct-acting antiviral treatment and drug resistance. Viruses. 2015;7(9):5018–5039. doi:10.3390/v7092857
39. Sorbo MC, Cento V, Di Maio VC, et al. Hepatitis C virus drug resistance associated substitutions and their clinical relevance: update 2018. Drug Resist Updat. 2018;37:17–39. doi:10.1016/j.drup.2018.01.004
40. Romano KP, Ali A, Aydin C, et al. The molecular basis of drug resistance against hepatitis C virus NS3/4A protease inhibitors. PLoS Pathog. 2012;8(7):e1002832. doi:10.1371/journal.ppat.1002832
41. Soumana DI, Kurt Yilmaz N, Ali A, Prachanronarong KL, Schiffer CA. Molecular and Dynamic Mechanism Underlying Drug Resistance in Genotype 3 Hepatitis C NS3/4A Protease. J Am Chem Soc. 2016;138(36):11850–11859. doi:10.1021/jacs.6b06454
42. Romano KP, Ali A, Royer WE, Schiffer CA. Drug resistance against HCV NS3/4A inhibitors is defined by the balance of substrate recognition versus inhibitor binding. Proc Natl Acad Sci U S A. 2010;107(49):20986–20991. doi:10.1073/pnas.1006370107
43. Palanisamy N, Danielsson A, Kokkula C, et al. Implications of baseline polymorphisms for potential resistance to NS3 protease inhibitors in Hepatitis C virus genotypes 1a, 2b and 3a. Antiviral Res. 2013;99(1):12–17. doi:10.1016/j.antiviral.2013.04.018
44. Zhou K, Liang Z, Wang C, et al. Natural polymorphisms conferring resistance to HCV protease and polymerase inhibitors in treatment-naive HIV/HCV co-infected patients in China. PLoS One. 2016;11(6):e0157438. doi:10.1371/journal.pone.0157438
45. Liu G, Cai Q, Li Z, et al. Effect of drug resistance mutations on antiviral agents in HCV patients. Antivir Ther. 2016;21(5):369–375. doi:10.3851/IMP2852
46. Matthew AN, Leidner F, Newton A, et al. Molecular mechanism of resistance in a clinically significant double-mutant variant of HCV NS3/4A protease. Structure. 2018;26(10):1360–1372 e1365. doi:10.1016/j.str.2018.07.004
47. Dickinson BC, Packer MS, Badran AH, Liu DR. A system for the continuous directed evolution of proteases rapidly reveals drug-resistance mutations. Nat Commun. 2014;5(1):5352. doi:10.1038/ncomms6352
48. Koizumi Y, Ohashi H, Nakajima S, et al. Quantifying antiviral activity optimizes drug combinations against hepatitis C virus infection. Proc Natl Acad Sci U S A. 2017;114(8):1922–1927. doi:10.1073/pnas.1610197114
49. Ali A, Aydin C, Gildemeister R, et al. Evaluating the role of macrocycles in the susceptibility of hepatitis C virus NS3/4A protease inhibitors to drug resistance. ACS Chem Biol. 2013;8(7):1469–1478. doi:10.1021/cb400100g
50. Lim SR, Qin X, Susser S, et al. Virologic escape during danoprevir (ITMN-191/RG7227) monotherapy is hepatitis C virus subtype dependent and associated with R155K substitution. Antimicrob Agents Chemother. 2012;56(1):271–279. doi:10.1128/AAC.05636-11
51. Lenz O, Verbinnen T, Lin TI, et al. In vitro resistance profile of the hepatitis C virus NS3/4A protease inhibitor TMC435. Antimicrob Agents Chemother. 2010;54(5):1878–1887. doi:10.1128/AAC.01452-09
52. Seiwert SD, Andrews SW, Jiang Y, et al. Preclinical characteristics of the hepatitis C virus NS3/4A protease inhibitor ITMN-191 (R7227). Antimicrob Agents Chemother. 2008;52(12):4432–4441. doi:10.1128/AAC.00699-08
53. Imhof I, Simmonds P. Genotype differences in susceptibility and resistance development of hepatitis C virus to protease inhibitors telaprevir (VX-950) and danoprevir (ITMN-191). Hepatology. 2011;53(4):1090–1099. doi:10.1002/hep.24172
54. Brennan BJ, Poirier A, Moreira S, et al. Characterization of the transmembrane transport and absolute bioavailability of the HCV protease inhibitor danoprevir. Clin Pharmacokinet. 2015;54(5):537–549. doi:10.1007/s40262-014-0222-6
55. Gane EJ, Rouzier R, Stedman C, et al. Antiviral activity, safety, and pharmacokinetics of danoprevir/ritonavir plus PEG-IFN α-2a/RBV in hepatitis C patients. J Hepatol. 2011;55(5):972–979. doi:10.1016/j.jhep.2011.01.046
56. Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798–801. doi:10.1038/nature08463
57. Rao H, Wei L, Lopez-Talavera JC, et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2014;29(3):545–553. doi:10.1111/jgh.12398
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
