Back to Journals » Clinical Interventions in Aging » Volume 9
Daily consumption of the collagen supplement Pure Gold Collagen® reduces visible signs of aging
Authors Borumand M, Sibilla S
Received 10 April 2014
Accepted for publication 18 July 2014
Published 13 October 2014 Volume 2014:9 Pages 1747—1758
DOI https://doi.org/10.2147/CIA.S65939
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Maryam Borumand, Sara Sibilla
Minerva Research Labs Ltd., London, UK
Abstract: With age, changes in the metabolic processes of structural components of the skin lead to visible signs of aging, such as increased dryness and wrinkle formation. The nutritional supplement, Pure Gold Collagen®, which consists of hydrolyzed collagen, hyaluronic acid, vitamins, and minerals, was developed to counteract these signs. An open-label study was conducted to investigate the effects of this nutritional supplement on skin properties. Supplementation with 50 mL of Pure Gold Collagen on a daily basis for 60 days led to a noticeable reduction in skin dryness, wrinkles, and nasolabial fold depth. In addition, a significant increase in collagen density and skin firmness was observed after 12 weeks. The data from this study suggest that Pure Gold Collagen can counteract signs of natural aging.
Keywords: hydrolyzed collagen, antiaging, wrinkles, firmness, skin
Corrigendum for this paper has been published
Introduction
Skin is the largest organ in the human body, and serves several important functions. As well as having a sensory role, it provides a physical barrier against environmental factors. The two main layers that make up the skin are the epidermis and the dermis. The former consists of cells, which are proliferating (basal), differentiated (keratinocytes), or squamous. The dermis, on the other hand, contains fibroblasts, which produce elastin and collagen, including type I and type III, among other extracellular matrix proteins.1 Embedded within the collagen fiber network are glycosaminoglycans, comprising mainly hyaluronic acid and dermatan sulfate. The molecular size of hyaluronic acid is quite important, as it affects the physicochemical properties of the skin, such as its ability to retain water, and its elasticity and viscosity. Hyaluronic acid consists of a helical chain that comprises alternating units of N-acetylglucosamine and glucuronic acid, and its average molecular weight has been shown to be in the range of 10–104 kDa.2–4
The extracellular matrix accounts for almost 80% of the skin’s dry weight.5 For the skin to function normally and appear youthful, the structure of the dermal layer must be maintained because the dermis provides structural support to the epidermis, which carries the blood vessels and supplies the skin with important nutrients for its functioning. However, natural aging can affect the structural integrity of the dermis. In addition, areas of the body exposed to the sun are prone to photoaging. Both these types of aging can be exacerbated by diet. High sugar levels lead to development of advanced glycation end products due to a chemical reaction between glucose and the free amino groups in proteins. Advanced glycation end products remain in the tissue where they form because they cannot be degraded normally by enzymes. All these factors affect fibroblasts in the dermis by causing changes in their shape and in the amount and quality of elastin and type I collagen fiber production. Moreover, aged fibroblasts synthesize less collagen, both in vitro and in vivo, when compared with young adult fibroblasts.6 This results in visible signs of aging, which are usually most prominent on the face.
The photoaged dermis contains collagen fibers and elastin that are disorganized and abnormal in nature.7,8 Recently, it has been shown that smoking alters the components of the dermis and leads to premature aging of components in the dermis.9,10 Stress also seems to affect the integrity of collagen in the skin. An important indicator of the harmful effects of chronic stress is dysregulation of the circadian cortisol/corticosterone rhythm11,12 which is known to alter both the synthesis and degradation of collagen.13
In order to be active in the deeper layers of the skin, hydrolyzed collagen has to cross the intestinal barrier and reach the bloodstream. It is worth noting that the rate of transport across the intestinal barrier could be a controlling step that affects the efficacy of these compounds in the skin. Therefore, it is imperative to demonstrate whether collagen peptides can be absorbed, and in what form and quantity, before speculating on their mechanism of action.
According to Richelle et al14 bioavailability is the amount of a nutritional bioactive compound that crosses the intestinal barrier to reach the bloodstream and is made available for either storage in the body (in this context, in the skin) or a metabolic process.
The first step in digestion consists of degradation of hydrolyzed collagen to form dipeptides and tripeptides or free amino acids. Several proteases (eg, pancreatic proteases, small intestinal brush-border proteases, peptidase) are involved in the degradation process.
Ingesting hydrolyzed collagen could be a useful strategy to counteract the changes associated with skin aging. An experiment by Iwai et al15 showed that a significant amount of hydrolyzed collagen derived from hydroxyproline appeared in the blood of healthy human volunteers who consumed hydrolyzed collagen from cartilage, chicken feet, and porcine skin after 12 hours of fasting. After intake of collagen, the amount of hydroxyproline-containing peptides in the blood increased, reaching a peak after 2 hours followed by a decrease to half the maximum level at 4 hours after ingestion. A small peptide, proline-hydroxyproline (Pro-Hyp), was found in the blood after ingestion of hydrolyzed collagen. It was found that the amount of Pro-Hyp present in human plasma was 25–60 nmol/mL after ingesting 9.4–23 g of hydrolyzed collagen.15 The higher levels of Pro-Hyp found in blood could be partly explained by the higher quantity of the Pro-Hyp sequence in collagen. Studies by Iwai et al15 suggest that Pro-Hyp can be considered an indigestible peptide as more than 75% of Pro-Hyp was shown to persist in the blood for 24 hours after in vitro reaction with human serum.
Peptides generated by hydrolysis of a large collagen molecule can have great benefits on health and can improve skin properties. Using “gut sac” experiments, Oesser et al investigated the molecular weight of hydrolyzed collagen absorbed in the intestinal tract.16 Techniques such as high performance liquid chromatography and sodium dodecyl sulfate polyacrylamide gel electrophoresis showed that peptides in the molecular weight range of 1–10 kDa may be absorbed.
Chen et al studied the effect of different concentrations of hydrolyzed collagen derived from fish on fibroblasts and keratinocytes. In particular, they looked at proliferation and collagen production.17 They found that a collagen concentration of 48–97 μg/mL resulted in optimal proliferation (191%).12 Ohara et al18 conducted a single-blind, crossover study comparing the structure and quantity of food-derived gelatin hydrolysates in human blood from three sources of type I collagen. Five healthy male volunteers ingested type I gelatin hydrolysates from fish scales, fish skin, or porcine skin after 12 hours of fasting. It was found that about 30% of hydroxyproline-containing peptides were detected in blood even after a duration of 24 hours.
However, there is substantial evidence that peptides can be hydrolyzed in the gastrointestinal tract before they are absorbed, so that predominantly free amino acids can enter the circulation. Hydroxyproline is absorbed in two forms, ie, an amino acid form and a peptide form.19,20
The mechanism of absorption across the intestine membrane has been extensively studied. Epithelial cells are important sites of absorption of numerous nutrients. There are three ways in which intestinal transport of oligopeptides can take place: PEPT1-mediated transport of dipeptides and tripeptides mediated by PEPT1;21 transport of macromolecules such as proteins via the transcytotic route;22 and transport for peptide absorption by the passive intracellular route.23 The complete role of these pathways in intestinal oligopeptide absorption is not yet fully understood.
Transcellular transport of these peptides across intestinal epithelial cells is a two-step mechanism, which involves transport across two separate membranes, ie, uptake of peptides by epithelial cells across the brush-border membrane and absorption into the bloodstream across the basolateral membrane.24 The first step is initiated by hydrogen ion-coupled peptide transporters, namely PEPT1 and PEPT2. PEPT1 functions as an enantioselective transporter of monovalent, polyvalent, and neutral charged peptides.25 It has been shown that collagen-derived peptides (Pro-Hyp and glycine-Pro-Hyp)25 are absorbed via the PEPT1 transporter.
Distribution is usually defined as the process by which a compound reaches the target tissue through the blood circulation. Factors that can affect the rate of distribution are blood flow and the chemical features of a given compound, such as molecular size and polarity.
After ingestion, collagen peptides are digested and distributed throughout the body. Watanabe-Kamiyama et al26 studied the distribution of collagen peptides to the skin and other tissues by means of an in vivo experiment in which 14C-labeled proline or collagen peptides were administered to Wistar rats. Radioactivity was measured in different tissues 0–6 hours after ingestion of the collagen peptides and for 14 days thereafter. The results were very promising in terms of residence time in the skin and showed that radioactivity remained in skin tissue at a high level for up to 14 days. This indicates the ability of collagen peptides to reach the dermis in the skin where their main benefit is observed.
Pure Gold Collagen® (Minerva Research Labs Ltd., London, UK) is a liquid supplement containing soluble hydrolyzed collagen type I obtained from the cartilage of fish, which contains low-molecular-weight hyaluronic acid, as well as several vitamins and minerals. An open-label study was carried out in 294 subjects to analyze and examine the effects of Pure Gold Collagen on skin aging. The aim of the study was to determine whether daily Pure Gold Collagen supplementation enhances new collagen formation in the dermis and reduces the visible signs of aging.
Materials and methods
Study design
An open-label multicenter study was conducted to investigate the effect of Pure Gold Collagen ingestion on aged skin. There were three arms to the study (Figure 1). In arm 1, 217 subjects were recruited by 40 dermatologists across five different countries (USA, United Arab Emirates, Greece, the Czech Republic, and Spain). In arm 2, 13 subjects were recruited at a single site. In arm 3, 70 volunteers were recruited from a single site. In total, 294 subjects completed the study.
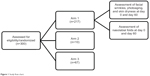  | Figure 1 Study flow chart. |
Arm 1: observational assessment of skin condition
Only subjects due to undergo a cosmetic procedure were recruited. The cosmetic procedures included any form of chemical peel or dermabrasion, non-ablative laser or fractional laser resurfacing, facial plastic surgery, and high-energy or thermage treatments. The effect of the oral supplement was evaluated by comparing the results after 60 days with baseline (day 0) values and noting the level of hydration, distribution of fine lines/wrinkles, and extent of photodamage as low, medium, or high.
Arm 2: quantitative assessment of collagen density
Ten volunteer subjects consumed the liquid food supplement on a daily basis over a 12-week period without any breaks. Their collagen density was objectively measured using high resolution ultrasound skin imaging by DermaLab® Series SkinLab USB (Cortex Technology, Hadsund, Denmark).
Arm 3: quantitative assessment of firmness
Sixty-seven subjects consumed the liquid food supplement on a daily basis for 130 days (18.5 weeks) without any breaks. The skin firmness of these subjects was objectively measured using the SkinLab USB Elasticity Module (DermaLab Series SkinLab USB) at 50, 80, and 130 days. The mean values of the measurements were recorded and considered significant when P was ≤0.05 (by analysis of variance).
Inclusion and exclusion criteria
Three hundred subjects aged 18–74 years were recruited. All subjects were provided with a written informed consent form, which they signed before participating in the study. The medical history of the subjects was recorded. The subjects were of various ethnic origins (Table 1). They were asked to continue with their regular or typical application of topical creams, and were asked not to start taking any other nutritional supplements or new antiaging topical creams during the course of the study. Subjects who were allergic to any of the ingredients present in Pure Gold Collagen or who were planning a pregnancy, pregnant, or breast feeding, were excluded from the study.
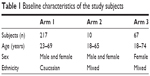  | Table 1 Baseline characteristics of the study subjects |
Product use
One 50 mL bottle of Pure Gold Collagen contains water, 5,000 mg hydrolyzed collagen, citric acid, pyridoxine hydrochloride (vitamin B6), extract of black pepper (Piper nigrum), copper, borage seed (Borago officinalis) oil, glycerol, soy lecithin, biotin soybean polysaccharide, malic acid, ascorbic acid (vitamin C), hyaluronic acid, D-α-tocopherol (vitamin E), sucralose, N-acetylglucosamine, Stevia, zinc, and biotin. The hydrolyzed collagen in Pure Gold Collagen is type I, and is extracted from farmed Tilapia and Pangasius fish by Rousselot of France. All fish used by Rousselot come from establishments that are registered in the European Union for the importation of edible fish that is fit for human consumption and also guaranteed by health certificates signed by official veterinarians.
The study volunteers were instructed to drink their food supplement once a day in the morning before breakfast and to store the product in a cool and dry place away from direct sunlight and heat. Subjects were informed that once a bottle was opened, it had to be consumed within 24 hours.
Compliance was evaluated by the investigator on a case-by-case basis. During the study, any adverse reaction to the product was reported and investigated further. The investigator was required to withdraw from the study any subject experiencing an adverse reaction. No adverse events occurred during the study period.
Baseline visit
The investigator conducted an assessment of the subject’s skin, and if possible, a photograph was taken of the full face with the eyes closed. The subject was given the product and instructions on consumption, dose, and time as well as the reporting procedure for any adverse reactions. Subjects in arm 1 of the study were asked to return in 20 days for cosmetic treatment and 40 days thereafter. Those in arm 2 of the study had their collagen levels measured at baseline (week 0) and at weeks 4, 8, and 12. Those in arm 3 had their skin firmness measured and were asked to return in 50, 80, and 130 days.
Efficacy variables
Arm 1
On days 0 and 60, during observational assessment of the subject’s face, the skin was evaluated for hydration, fine lines/wrinkles, and photodamage. On day 0, the investigator noted the level of hydration, distribution of fine lines/wrinkles, and the extent of photodamage as low, medium, or high. The subject was asked to describe the general nature of their skin as normal, oily, or dry, pigmented or non-pigmented, tight, or wrinkled.
On day 60, the skin assessment was repeated, but this time an ordinal scale was used for comparing the above parameters with baseline as follows: 0, no difference; 1, slight difference; 2, significant difference. The nasolabial folds of the subjects were also evaluated at days 0 and 60 using a modified version of the Fitzpatrick Wrinkle Scale,27 where I indicates no wrinkling and III indicates very severe wrinkling in the nasolabial fold area.
Arm 2
Collagen measurements were performed using high resolution ultrasound skin imaging equipment (DermaLab SkinLab USB). This has been described previously in the literature.2 The device consists of a laptop/computer that is connected to a handheld probe attached to a unit. After switching on the unit, the probe is held perpendicular to the skin surface, and an acoustic pulse is then sent to the skin. Part of this pulse is reflected and the other part is transmitted deeper into the skin. The signal that reflects back is picked up by the ultrasound transmitter. A cross-sectional image is produced on the software screen of the laptop/computer, which denotes the intensity, in terms of amplitude, of the reflected/received signals captured by the ultrasound transmitter. The amount of the received signal refers to a color gradient, where bright colors denote areas with strong reflections (mainly due to significant changes in density between structures like elastin, connective tissue, and collagen) and dark colors refer to areas with low reflection (ie, little or no change in density of structures present in the skin, such as fluid, fat, and blood). A high-intensity (white/yellowish) signal from the epidermis and dermis is represented on the screen with a mix of colors, whereas a low-intensity signal (black and dark green) is produced in areas that represent muscle and subcutaneous fat.
The border between the epidermis and dermis is calculated automatically every time an ultrasound image is received by the transmitter. The unit also has the capacity to detect and calculate the average intensity as well as average thickness of the skin by ultrasound echo over the entire dermis. The intensity of the ultrasound echo (intensity score) is directly related to the density of proteins in the skin. In other words, the higher the intensity scores, the higher the density of collagen and elastin fibers in the skin.
The left ventral forearm and the zygomatic (crow’s feet) area around the left eye were the areas used for taking measurements. In each area, three consecutive measurements were performed and corresponding values were then used to calculate average intensities of the ultrasound echo (intensity score).
The volunteers consumed the oral liquid food supplement on a daily basis over a 12-week period without any breaks. Measurements were taken at baseline (week 0) and at weeks 4, 8, and 12. The study was conducted in the last 3 months of the year, when the skin is exposed to the harshest weather conditions.
Arm 3
Measurements were performed using the SkinLab USB elasticity module (DermaLab SkinLab USB Series). The elasticity module is made up of a rubber seal with a chamber in the middle that is placed at the test site on the subject. The chamber consists of a circular hole that aids in creating a vacuum to enable suction to take place on the skin surface. The circular hole is surrounded by an adhesive tape that prevents any creeping and folding of the skin under the vacuum chamber. The suction method comprises an elevation phase as well as a retraction phase. The parameters that help in describing the skin elasticity are: Young’s elasticity modulus (E), which represents firmness of the skin, and is based on the amount of force required to raise the skin surface 1.5 mm between two infrared detection levels inside the vacuum chamber; skin retraction time (time taken to retract from a 1.5 mm lift position, calculated in seconds); and viscoelasticity (VE), which combines both the raised and retraction phase.
Three measurements are taken at the same time, comprising three cycles (one cycle presents suction/release). Three successive curves that specify the heights (in mm) reached by the skin during suction and the levels of “skin return” during release times are displayed on the computer monitor. Skin elasticity values are measured using key points of evaluation, as follows:
- R0, R3, or R6, denoting the maximum stretch point of skin (ie, skin capacity), dependent on the curve (first, second, or third)
- R1, R4, or R7, denoting the minimum value (skin return), dependent on the curve (first, second, or third)
- R2, R5, or R8, denoting skin elasticity, depending on the curve that corresponds to the ratio between: R2 = R1 – R0; R5 = R4 – R3; R8 = R7 – R6.
The volunteers consumed the liquid food supplement orally on a daily basis over a 130-day period without any breaks. Measurements were taken on the left ventral forearm after 50, 80, and 130 days of treatment with Pure Gold Collagen.
Statistical methods
Mean scores and the efficacy of the instrumental data were calculated and analyzed at each time point. The paired-samples t-test and analysis of variance were used to compare baseline values and to test for change as a function of treatment and time. Mean percentage change from baseline was evaluated. Values were considered to be statistically significant when P was ≤0.05.
Results
Arm 1
Improvement in skin properties
In total, 217 subjects in arm 1 completed the study. The volunteers were aged 23–69 years and all were Caucasians. All subjects had some photodamage and fine lines/wrinkles. One hundred and fifty-seven subjects had a medium or high distribution of fine lines and/or wrinkles (69 of these subjects agreed that their skin was wrinkled). At the end of the study, the dermatologists noted that 109 of these 157 subjects (69%) had either visible or significant improvement in their facial lines (Figure 2A). Interestingly, 24 of the subjects who had an improvement did not undergo any antiaging treatment such as Botox or fillers (Figure 2A). One subject had a nevus and a papilloma removed and seven subjects had cheek augmentation. The others did not have any cosmetic procedure. This suggests that Pure Gold Collagen improves wrinkles and facial lines as effectively as certain cosmetic procedures.
The Glogau photoaging classification was used to assess generalized facial photoaging.28 Eighty-six subjects had moderate to severe photoaging according to the investigator (52 of the subjects agreed that their skin was pigmented). At the end of the study, 37 of these 86 subjects (43%) had either visible or significant improvement (19 had undergone a facial treatment, and 18 either had no treatment or had localized injection of Botox or fillers, Figure 2B).
Similar results were observed for dryness, which is another change in skin properties associated with aging. Fifty-four subjects appeared to have dry skin (22 agreed they suffered from dry skin). At the end of the study, 44 of these 54 subjects (82%) had either visible or significant improvement in skin hydration. Twenty-three had undergone a facial treatment. However, an increase in hydration was noted in 21 subjects who either had no treatment or had localized injection of Botox or fillers (Figure 2C).
Reduction in nasolabial fold depth
The nasolabial folds extend from the side of the nose to the corners of the mouth. These folds may deepen with age, and as they are more prominent than other facial lines, their depth is a useful parameter for measuring the effect of antiaging products.
Measurement of nasolabial fold depth using a visual score showed that supplementation with Pure Gold Collagen led to significant improvement, with a 24% reduction in the average score from baseline (P≤0.001; Figure 3A). In 37% of subjects, a significant improvement in nasolabial fold depth was observed, with a reduction of 44% in average score (P≤0.001; Figure 3B). Interestingly, a comparable significant decrease (P≤0.05) in nasolabial fold depth was reported regardless of whether subjects underwent treatment for nasolabial folds (30%, n=23) or not (32%, n=30; Figure 3C).
After 60 days, a decrease in nasolabial fold depth was observed in subjects who underwent other cosmetic treatments. Dermatologists reported a decrease of 15% for laser treatments, 50% for Botox, 28% for fillers, 41% for treatments in the nasolabial fold area, and an 18% and 10% decrease for mesotherapy and dermabrasion, respectively (Figure 4).
  | Figure 4 Percentage of reduction in nasolabial fold depth in people who underwent other cosmetic treatments. |
In the group of subjects who had dermabrasion, facial laser treatment, or Botox in the upper area of their face for glabellar lines or crow’s feet, there was a respective 4%, 12%, and 18% reduction in the number of class 1.5 nasolabial folds (Table 2). Not surprisingly, the largest reduction in the number of class 2 wrinkles was observed in subjects who had fillers in their nasolabial folds (29%; Table 2). There was also a reduction in class 2 wrinkles among those who had mesotherapy or platelet-rich plasma therapy (25%; Table 2). In the group of subjects who had cheek or lip augmentation by fillers, there was a 25% decrease in class 3 wrinkles (Table 2).
Arm 2: enhancement of collagen content
High resolution ultrasound skin imaging (DermaLab SkinLab USB) was used to assess the effect of the liquid food supplement, Pure Gold Collagen, on dermal collagen density in healthy volunteers. The study was conducted in ten subjects. Each subject consumed either a one 50 mL daily dose of placebo or Pure Gold Collagen for 12 weeks. The subjects were measured for collagen density in the left ventral forearm and the zygomatic area around the left eye at weeks 0, 4, 8, and 12. The variation in mean ultrasound intensity scores between week 0 and week 12 as registered by DermaLab ultrasound equipment is summarized in Table 3.
A significant (P≤0.05) improvement was seen in collagen density at week 12, both in the crow’s feet area (19% increase) and on the left ventral forearm (12% increase; Table 3) when compared with the placebo group (data not shown). The magnitude of percentage increase in collagen density was greater on the face than on the forearm. The results of this study show that daily oral consumption of Pure Gold Collagen supplement does lead to a detectable improvement in skin collagen density over a 12-week period (Figure 5).
Arm 3: enhancement of skin firmness
The DermaLab SkinLab USB elasticity module was used to assess the effect of Pure Gold Collagen on skin firmness in 67 healthy volunteers. Measurements were carried out on the ventral left forearm after 50, 80, and 130 days of treatment with Pure Gold Collagen. Mean values were recorded and considered significant when P was ≤0.05 (by analysis of variance). In 37% of subjects taking Pure Gold Collagen, an increase in skin firmness was observed at 80 and 130 days. This improvement was statistically significant at 80 days (83%; P≤0.05) and at 130 days (94%; P≤0.01; Figure 6).
  | Figure 6 Increase in skin firmness after 80 and 130 days of treatment with Pure Gold Collagen®. |
Adverse events
No adverse events were reported by any of the subjects during the study, indicating that daily oral intake of Pure Gold Collagen 50 mL was well tolerated by the study population.
Discussion
Structural changes in the dermis are the key cause of facial skin aging, and lead to dry and loose skin with appearance of furrows or wrinkles. The results of this study suggest that daily supplementation with Pure Gold Collagen can help to counteract such signs of aging.
We found that 15% of subjects taking Pure Gold Collagen had fewer facial lines and wrinkles after 60 days, even though they did not undergo any form of cosmetic procedure. Moreover, 32% had an improvement in the level of photoaging and 39% had less skin dryness. These subjects either had no form of cosmetic treatment or had only a localized procedure, indicating that the effects were due to Pure Gold Collagen.
Given that the product is an oral supplement, the improvements observed most likely resulted from changes in protein turnover within the dermal layer of the skin. Pure Gold Collagen has a dual mechanism of action. First, the product contains peptides that increase the amount of collagen in the dermis, and the enhanced fibrillar network improves the overall integrity of the skin, leading to fewer wrinkles. Second, the dermal tissue contains fibroblasts that are stimulated by collagen peptides to produce new collagen, elastin, and hyaluronic acid.
After 60 days, we observed an 18% decrease in class 1.5 nasolabial folds in volunteers who had Botox in the upper area of the face. An even larger (25%) improvement was found in class 3 wrinkles among those who underwent cheek or lip augmentation. The fact that these subjects had an improvement in nasolabial fold depth even though they did not have any fillers in that area suggests the effect was likely to have been due to Pure Gold Collagen. Even more interesting is the fact that the percentage improvement in both cases was similar to that in patients who had injection of fillers into their nasolabial folds. A placebo-controlled clinical trial is now required to confirm the true effect of Pure Gold Collagen on nasolabial fold depth when compared with other cosmetic procedures. An increased level of collagen in the dermis was detected in individual subjects after 12 weeks.
Other treatments are widely used for wrinkled and photoaged skin, with products containing retinoid recognized as the current benchmark treatment. Retinoids are a family of compounds that are made up of various mixtures of vitamin A and its derivatives. They are synthetic molecules that act through the same pathway. Retinoids are used either topically or orally for a number of skin conditions (primarily acne). Retinoid treatments gained popularity in the 1980s in large part because of the work reported by Kligman et al.29 Studies have shown that the collagen content in the upper papillary dermis can be increased by retinoids. This process occurs by inhibiting collagen degradation thereby causing an increase in collagen levels. Previous studies have also reported that biosynthesis of type I procollagen can be enhanced by retinoids.30,31 Histology staining techniques such as immunohistochemistry have shown reorganization of dermal collagen to form new woven bundles as well as enhanced levels of collagen types I, III, and VII (dermal-epidermal anchoring fibrils).32 Retinoid creams have also been studied in patients prior to chemical peel33 and dermabrasion.34 It was found that patients pretreated with retinoid creams had less post inflammatory hyperpigmentation and a substantial increase in the area of re-epithelialized skin. The results achieved by pretreating patients before microdermabrasion and chemical peels led to animal studies that investigated the effect of a retinoid before procedures such as carbon dioxide laser resurfacing. Some studies have documented mild and transient adverse effects from using retinol cream, including skin irritation, dryness, burning, and erythema; however, the results of these studies are not significant due to a lack of consistency in study designs.35 Published in vivo studies confirm the efficacy of collagen peptides. The effect of ingestion of two oral doses (0.2 g/kg and 1.0 g/kg body weight) of hydrolyzed collagen for 56 days on the extracellular matrix of rabbit Achilles tendon was investigated by Minaguchi et al.36 The size of the collagen fibrils and quantity of glycosaminoglycans were measured and compared with those in rabbits fed a control protein (lactalbumin) or water alone. It was found that even though there was an increase in collagen fibril diameter, the collagen fibril appeared much thicker in the rabbits that ingested oral collagen peptides. Statistical analysis showed that fibrils with a diameter range of 20–60 nm were more likely to be found in the water group; however, in the test group, in the presence of hydrolyzed collagen, the highest diameters were in the range of 160–180 nm. These results suggest that hydrolyzed collagen has benefits for the skin, given that type I collagen is the major element present in the extracellular matrix in both skin and tendon tissue. It has also been previously reported that Pro-Hyp is present in the human blood following the oral administration of hydrolyzed collagen and that aids in producing hyaluronic acid in vitro by stimulating human dermal fibroblasts.37
The aim of a recent study conducted by Proksch et al38 was to investigate the effect of collagen hydrolysate consisting of collagen peptides on skin structures and processes related to cutaneous aging. A placebo-controlled, double-blind trial was conducted in 69 women aged 35–55 years who were randomized to receive 2.5 g or 5 g of collagen hydrolysate or placebo once daily for 8 weeks. The biophysical properties of skin, including elasticity, skin moisture, transepidermal water loss, and skin roughness, were measured before the first orally administered dose, after 4 weeks, and finally after 8 weeks. After the study concluded, the investigators reported that skin elasticity in both collagen hydrolysate dosage groups was significantly improved in comparison with placebo. Skin moisture and evaporation were also positively influenced by treatment with collagen hydrolysate, but the results were not statistically significant.
Interestingly, Ohara et al used human dermal fibroblasts in culture to study the effect of collagen peptides on extracellular matrix mechanisms and cell proliferation. Pro-Hyp was one of the major collagen peptides and was shown to enhance cell proliferation and synthesis of hyaluronic acid by 1.5-fold and 3.8-fold, respectively. It was also found that phosphorylation of signal transducer and activator of transcription 3 was enhanced within 60 minutes, suggesting that Pro-Hyp not only stimulates cell mitotic activity but also synthesis of hyaluronic acid.37
Our supplement, Pure Gold Collagen, most likely increases collagen content by changing the balance between production and degradation of collagen in human dermal fibroblasts. The hydrolyzed collagen present in Pure Gold Collagen is primarily type I, a type of hyaline cartilage derived from fish skin, and is the same as that found in the dermis. Published in vivo studies26 confirm the efficacy of collagen peptides. In this study, we have shown for the first time that daily ingestion of Pure Gold Collagen, an oral nutritional supplement, improves collagen density, suggesting increased formation of new collagen fibers. This study supports the published literature demonstrating increased collagen synthesis in the skin as a result of daily oral ingestion of collagen peptides.39
Conclusion
The study presented here provides evidence that ingestion of Pure Gold Collagen can reduce the signs of aging. A larger in vivo study is needed to confirm the antiaging effect of this supplement. Additional in vitro studies will also help us to understand the potential mechanism of action of this nutritional antiaging supplement.
Acknowledgments
We would like to thank Thane Aung for his contribution to the data collection and Dr Martin Godfrey for his time and expertise in critically reviewing the manuscript. We would also like to thank Dr Sushmita Roy-Nawathe for her contribution to reviewing and editing the manuscript as well as all the dermatologists who collected the data.
Disclosure
The study was funded by Minerva Research Labs Ltd., London, UK. Further information is available at http://www.gold-collagen.com. The authors report no other conflicts of interest in this work.
References
Champion RH, Rook A, Burton JL, Ebling FJG, Wilkinson DS. Textbook of Dermatology. Oxford, UK: Blackwell Scientific; 1992. | ||
Shimada E, Matsumura G. Viscosity and molecular weight of hyaluronic acids. J Biochem. 1975;78(3):513–517. | ||
Tammi R, Saamanen AM, Maibach HI, Tammi M. Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J Invest Dermatol. 1991;97(1):126–130. | ||
Scott JE. Secondary structures in hyaluronan solutions: chemical and biological implications. Ciba Found Symp. 1989;143:6–15. | ||
Uitto J. Understanding premature skin aging. N Engl J Med. 1997; 337(20):1463–1465. | ||
Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006; 168(6):1861–1868. | ||
El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs photo-aging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):398–405. | ||
Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. | ||
Tanaka H, Ono Y, Nakata S, Shintani Y, Sakakibara N, Morita A. Tobacco smoke extract induces premature skin aging in mouse. J Dermatol Sci. 2007;46(1):69–71. | ||
Yin L, Morita A, Tsuji T. Alterations of extracellular matrix induced by tobacco smoke extract. Arch Dermatol Res. 2000;292(4):188–194. | ||
Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286–306. | ||
Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12):994–1000. | ||
Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4(10):525–533. | ||
Richelle M, Sabatier M, Steiling H, Williamson G. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96(2):227–238. | ||
Iwai K, Hasegawa T, Taguchi Y, et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem. 2005;53(16):6531–6536. | ||
Oesser S, Adam M, Babel W, Seifert J. Oral administration of (14)C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J Nutr. 1999;129(10):1891–1895. | ||
Chen JK, Shen CR, Liu CL. N-acetylglucosamine: production and applications. Mar Drugs. 2010;8(9):2493–2516. | ||
Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem. 2007;55(4):1532–1535. | ||
Boullin DJ, Crampton RF, Heading CE, Pelling D. Intestinal absorption of dipeptides containing glycine, phenylalanine, proline, beta-alanine or histidine in the rat. Clin Sci Mol Med. 1973;45(6):49–58. | ||
Matthews DM, Laster L. Absorption of protein digestion products: a review. Gut. 1965;6(5):411–426. | ||
Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. | ||
Sai Y, Kajita M, Tamai I, Wakama J, Wakamiya T, Tsuji A. Adsorptive-mediated endocytosis of a basic peptide in enterocyte-like Caco-2 cells. Am J Physiol. 1998;275(3 Pt 1):G514–G520. | ||
Adson A, Raub TJ, Burton PS, et al. Quantitative approaches to delineate paracellular diffusion in cultured epithelial cell monolayers. J Pharm Sci. 1994;83(11):1529–1536. | ||
Christensen N. Role of amino acid transport and contertransport in nutrient and metabolism. Physiologie. 1990;70:43–77. | ||
Aito-Inoue M, Lackeyram D, Fan MZ, Sato K, Mine Y. Transport of a tripeptide, Gly-Pro-Hyp, across the porcine intestinal brush-border membrane. J Pept Sci. 2007;13(7):468–474. | ||
Watanabe-Kamiyama M, Shimizu M, Kamiyama S, et al. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J Agric Food Chem. 2010;58(2):835–841. | ||
Shoshani D, Markovitz E, Monstrey SJ, Narins DJ. The modified Fitzpatrick Wrinkle Scale: a clinical validated measurement tool for nasolabial wrinkle severity assessment. Dermatol Surg. 2008;34 Suppl 1:S85–S91. | ||
Puizina-Ivic N. Skin aging. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17(2):47–54. | ||
Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. J Am Aacd Dermatol. 1986;15(4 Pt 2):836–859. | ||
Fisher GJ, Datta S, Wang Z, et al. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106(5):663–670. | ||
Kim HJ, Bogdan NJ, D’Agostaro LJ, Gold LI, Bryce GF. Effect of topical retinoic acids on the levels of collagen mRNA during the repair of UVB-induced dermal damage in the hairless mouse and the possible role of TGF-beta as a mediator. J Invest Dermatol. 1992;98(3):359–363. | ||
Woodley DT, Zelickson AS, Briggaman RA, et al. Treatment of photoaged skin with topical tretinoin increases epidermal-dermal anchoring fibrils. A preliminary report. JAMA. 1990;263(22):3057–3059. | ||
Hevia O, Nemeth AJ, Taylor JR. Tretinoin accelerates healing after trichloroacetic acid chemical peel. Arch Dermatol. 1991;127(5):678–682. | ||
Mandy SH. Tretinoin in the preoperative and postoperative management of dermabrasion. J Am Acad Dermatol. 1986;15(4 Pt 2):878–879, 888–889. | ||
Rivas S, Pandya AG. Treatment of melasma with topical agents, peels and lasers: an evidence-based review. Am J Clin Dermatol. 2013;14(5):359–376. | ||
Minaguchi J, Koyama Y, Meguri N, et al. Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in Achilles tendon. J Nutr Sci Vitaminol (Tokyo). 2005;51(3):169–174. | ||
Ohara H, Ichikawa S, Matsumoto H, et al. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J Dermatol. 2010;37(4): 330–338. | ||
Proksch E, Segger D, Degwert J, Schunck M, Zague V, Oesser S. Oral supplemention of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27(1):47–55. | ||
Schwartz SR, Park J. Ingestion of BioCell Collagen®, a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267–273. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.