Back to Journals » Research and Reports in Urology » Volume 15
Cytoplasmic Androgen Receptor, CD24 Expression and Smoking Intensity to Urothelial Carcinoma of the Bladder Invasiveness: A Cross-Sectional Study
Authors Pramod SV, Safriadi F, Hernowo BS, Dwiyana RF , Trianasari N, Egawa S
Received 3 September 2023
Accepted for publication 19 October 2023
Published 1 November 2023 Volume 2023:15 Pages 485—494
DOI https://doi.org/10.2147/RRU.S433705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Guglielmo Mantica
Sawkar Vijay Pramod,1 Ferry Safriadi,1 Bethy S Hernowo,2 Reiva Farah Dwiyana,3 Nurvita Trianasari,4 Shin Egawa5
1Department of Urology, Faculty of Medicine Universitas Padjadjaran, Hasan Sadikin Academic Medical Center, Bandung, Indonesia; 2Department of Pathology, Faculty of Medicine Universitas Padjadjaran, Hasan Sadikin Academic Medical Center, Bandung, Indonesia; 3Faculty of Medicine Universitas Padjadjaran, Bandung, Indonesia; 4Economics and Business School, Telkom University, Bandung, West Java, Indonesia; 5Department of Urology, Jikei University School of Medicine, Nishi-Shimbashi, Minato-ku, Tokyo, Japan
Correspondence: Sawkar Vijay Pramod, Department of Urology, Faculty of Medicine Universitas Padjadjaran, Hasan Sadikin Academic Medical Center, Bandung, Indonesia, Email [email protected]
Purpose: To the best of our knowledge, Androgen receptor (AR) and cluster of differentiation 24 (CD24) expression in bladder urothelial carcinoma (UC) has not yet been reported in our population. The aim of this study was to evaluate the expression of both markers in UCB using immunohistochemistry.
Materials and Methods: Data from 60 patients with UCB were obtained between 2009 and 2018. The samples were divided into four groups based on their smoking history. Group 1 included non-smokers, group 2 smoked < 20 cigarettes/day for 30 years, group 3 smoked for 31– 40 years, and group 4 smoked for > 40 years. Each group then divided into Non muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC) subgroups. The smear was stained with hematoxylin and eosin (HE) - immunohistochemistry of CD24 and RA, followed by histoscore assessment.
Results: The male to female smoking rates was 1.8. Based on gender, in the NMIBC group there were 85.7% men and 14.3% were women while in MIBC 74.4% men and 25.6% women. The mean age of the NMIBC and MIBC groups was 56.3 years and 54.5 years, respectively. There was no significant relationship between smoking status in group 2 (OR 0.31, CI 95% CI, p=0,39), group 3 (OR 013, CI 95% CI, p=0,05), and group 4 (OR 0.23, CI 95% CI, p=0215) to the UCB invasiveness. A significant relationship was observed between cytoplasmic AR expression and UCB invasiveness (OR 0.14[0,04; 0.47], CI 95%, p=0.001). There was no significant relationship between RA in the nucleus and UCB invasion (OR 1.09[0,18; 6.48] CI 95%, p=1000). No significant relationship was observed between CD24 expression and UCB invasiveness (OR 0.81[0,27– 2,45] CI 95%, p=0712).
Conclusion: Cytoplasmic AR expression is associated with UCB invasiveness. Smoking history and CD24 expression were not associated with UCB invasion.
Keywords: androgen receptor, CD24, immunohistochemistry, smoking, urothelial carcinoma of the bladder
Introduction
Data from the Global Cancer Observatory (GLOBOCAN) showed that in 2020, there were 573,278 new cases and 212,536 deaths due to urothelial bladder carcinoma worldwide. In Asia, the incidence is approximately 36.3%; in Indonesia, it is 4.7–9.9 cases per 100.000 populations per year. Approximately 75% of bladder malignancies are non-muscle invasive bladder cancers (NMIBC) that are confined to the mucosa (stage Ta, carcinoma in situ) or submucosa (stage T1).1 Our institution’s data within 12 years showed that, from 773 cases of bladder cancer, 556 cases (72%) were UCB. The most common stages were MIBC (93.41%) and NMIBC (6.59%).2
The number of people who smoke in Indonesia ranks third in the world after China and India. The prevalence of smokers in Indonesia is approximately 33% of the total population (257 million people).3 A study stated that in the United States, 23.1% of men and 18.3% of women are smokers. In 2013, the Data and Information Center of the Indonesian Ministry of Health showed that the prevalence of smoking in men in Indonesia increased from 65.8% in 2010 to 66% and in women from 4.1% in 2010 to 6.7%.4 A meta-analysis study by Van Osch et al found a three-fold increase in the incidence of urothelial bladder carcinoma in current smokers and a nearly two-fold increase in former smokers.5,6
Even with the increasing number of women who smoke in Indonesia, epidemiological studies have shown that men are 3–4 times more likely to be affected by UCB than women; however, women have a more advanced stage, higher recurrence, and lower survival rate. This may explain some of the differences in bladder tumors between men and women.7,8
Currently, Cluster of Differentiation 24 (CD24) is a renowned molecular marker. Studies have shown that CD24 is expressed in several cancers including UCB.9 However, the relationship between CD24 expression and UCB invasion has not been concluded.10
To our knowledge, the expression of Androgen receptor (AR) and cluster of differentiation 24 (CD24) expression in bladder urothelial carcinoma (UC) has not been reported yet in our population. The aim of this study was to evaluate the expression of both markers in UCB using immunohistochemistry.
Materials and Methods
Patients
This study had been reviewed by research ethics committee of Universitas Padjadjaran with the Institutional Review Board number 0520090923 and ethical approval letter number 1075/UN6.KEP/EC/2020. We found 60 patients with bladder urothelial carcinoma that were included in this study and in our institution between 2009–2018. Further inform consent is not necessary as every patient come to our hospital already sign approval for medical record disclosure for research purposes. The inclusion criteria were the histopathological diagnosis of urothelial tumors, availability of clinical data, and availability of paraffin-embedded tissue specimens. All patients underwent surgery but did not receive chemotherapy or radiotherapy prior to surgical resection. All samples were histologically evaluated for urothelial bladder carcinoma.
Smoking Assessment
This study used a cross-sectional, analytical, and retrospective design. Smoking status was obtained from the bladder cancer registry in our department and divided into four groups: non-smokers (group 1), smoke less than 20 cigarettes a day for no longer than 30 years (group 2), smoke for 31–40 years or smoke more than 20 cigarettes a day for less than 30 years (group 3), and smoke for more than 40 years (group 4).
Immunohistochemistry
For immunohistochemistry, the paraffin blocks were deparaffinized in xylene and dehydrated in a series of ethanol solutions. The slides were washed three times in phosphate-buffered saline (PBS), immersed in 0.3% hydrogen peroxide solution in methanol for 15 min, and washed three times with PBS. After extensive washing with PBS, a color reaction was performed for 3 min with 2% 3.30-diaminobenzidine in 50 mM Tris (pH 7.6) containing 0.3% hydrogen peroxidase. Finally, the slides were counterstained with hematoxylin. CD24 immunohistochemistry was performed using labeled streptavidin-biotin immunoperoxidase complex and we used antibody rabbit polyclonal CD24 (No. GTX37755, GeneTex) at a dilution of 1:50. CD24 expression in the cytoplasm is shown in brown color. Androgen receptor (AR) immunohistochemistry was performed using the mouse monoclonal antibody AR (No. GTX83309 - GeneTex) at a dilution 1:100. The expression of AR in the nucleus and cytoplasm was also brown. In cystectomy specimens, staining in the areas of the UCs and normal urothelium was evaluated separately. The histoscore was assessed by an independent pathologist, with a range of 0–12 which is the result of the intensity of tumor cells with a score of 0–3 (0: none, 1: weak, 2: moderate, 3: strong) multiplied by the distribution of tumor cells with a score of 0–4 (0: none, 1: <10%, 2:10–50%, 3:50–80%, 4: >80%).
Statistical Analysis
Data analysis was carried out with bivariate analysis between dependent and independent variables using unpaired t-test, chi-square, Fisher exact, and Mann–Whitney, followed by logistic regression multivariate analysis using SPSS version 24.0, for Windows, with a p value < 0.05, indicating statistical significance.
Results
Clinicopathological Criteria
This study was conducted using urothelial bladder carcinoma tissue samples from cystoscopy biopsy/TUR-bladder tumor procedures or cystectomy surgery at Hasan Sadikin AMC between 2009 and 2018.
The mean age of the sample recruited for this study was 55.17±12.03 years with 47 males (78.3%) and 13 females (21.7%). Table 1 shows the sex of the study participants. Urothelial bladder carcinoma occurred in 78.3% of the men and 21.7% of the women. In the NMIBC group, it occurred in 85.7% of the men and 14.3% of the women. In the MIBC group, it occurred in 74.4% of the men and 25.6% of the women. This is in accordance with the incidence obtained by Siegel (2012), who concluded that men experience urothelial bladder carcinoma to 3–4 times more than women.11
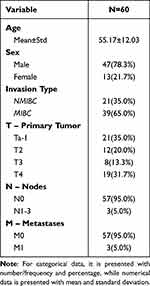 |
Table 1 Demographics Information of the Study Subjects |
In the NMIBC group, the average age was 56.33±11.150 years, consisting of 18 males (85.7%) and 3 women (14.3%). In the MIBC group, the average age was 54.54±12,574 years, consisting of 29 males (74.4%) and 10 women (25.6%). The results of statistical tests in both groups were p values > 0.05, which means that both age and sex were not significant risk factors for UCB invasiveness.
Table 2 shows that the statistical test result of age and sex were greater than 0.05 (p value > 0.05), which means that there was no association between age and sex to UCB invasiveness.
 |
Table 2 Comparison of Age and Sex in Groups of Patients Diagnosed with NMIBC and MIBC |
Association of Smoking Status with UCB Invasiveness
Table 3 shows that the statistical test results of the Smoking Group variables were greater than 0.05 (p value > 0.05), which means that there was no association between smoking status and UCB invasiveness.
 |
Table 3 Association of Smoking Status with UCB Invasiveness |
Figure 1 shows the results of the examination of Nuclear androgen receptor (N-AR) and the expression of RA in patients with urothelial bladder carcinoma. This strong expression indicates a strong relationship between cytoplasm androgen receptor (C-AR) and UCB invasiveness in urothelial bladder carcinomas.
 |
Figure 1 Nuclear androgen receptor (N-AR) and the expression of RA in patients with urothelial bladder carcinoma (A) AR + nuclear magnification 100x; (B). AR + nuclear magnification 200x. |
Figure 2 shows the distribution of CD24 in urothelial cells of urothelial bladder carcinomas. The even distribution and CD24 expression were found to be strong in patients with urothelial bladder carcinoma.
Figure 3 shows the study framework of this study, showing an illustration of smoking and its relation to AR and CD24 expression.
Association of Cytoplasm AR, Nuclear AR, CD 24 with UCB Invasiveness
In the NMIBC group, for CD24 weak category was 13 (61.9%) and the strong category and 8 (38.1%) in the strong category. In the MIBC group, for CD24 the weak category is 29 (74.4%) were in the weak category and 10 (25.6%) were in the strong category. The result of the statistical test in the research group above using chi square is a p value > 0.05, which means it is not statistically significant; thus, there is no statistically significant association between the CD24 Category variables in the NMIBC and MIBC groups.
The results of the statistical tests (Table 4) on the N-AR and CD24 variables had a p-value of > 0.05, which means that it was not statistically significant; thus, there was no statistically significant association between the N-AR and CD24 variables in the NMIBC and MIBC groups. Meanwhile, the p-value for the C-AR variable is less than 0.05 (p value <0.05) which means that it is statistically significant. Thus, there was a statistically significant association between C-AR variables in the NMIBC and MIBC groups. Analysis of categorical and numerical data showed that the p value for the C-AR variable was < 0.05, indicating an association between C-AR and UCB invasiveness. Based on the analysis of categorical and numerical data for N-AR and CD24 variables, a p-value >0.05. This means that there was no statistically significant relationship between N-AR and CD24 and the invasiveness of UCB. Histoscore analysis based on sex disparity showed AR and CD 24 were higher in men than in women (Table 5). Lombard 2015 concluded males have higher AR expression, and Farid et al 2019 also found that CD24 expression was higher in men than in women.10,12
 |
Table 4 Association of Cytoplasm AR (C-AR), Nuclear AR (N-AR), CD 24 with UCB Invasiveness |
 |
Table 5 Histoscore Androgen Receptor and CD24 in Gender Disparity |
Bivariate analysis was followed by multivariate analysis (Table 6) to assess the prognostic value of the combination of all independent variables in relation to UCB invasiveness. The independent variables included in the model were smoking status and the N-AR, C-AR, and CD24 levels. Four independent variables were tested to determine the dominant variable that most influential. From the multivariate analysis, no p values were smaller than 0.05 (p<0.05), indicating that the combination of smoking status and N-AR, C-AR, and CD24 did not predict UCB invasiveness.
 |
Table 6 Multivariate Statistical Analysis Between Smoking Status, N-AR, C-AR and CD24 on UCB Invasiveness |
Discussion
Smoking is a major risk factor for urothelial bladder carcinoma, accounting for > 50% of urothelial bladder carcinomas. Smokers have a 4–7 times higher risk of developing urothelial bladder carcinoma than non-smokers.13,14 In this study, male gender with urothelial bladder carcinoma were smokers. Among women who experienced urothelial bladder carcinoma, 45.5% were smokers. These data show that men with urothelial bladder carcinoma smoked significantly more than women with urothelial bladder carcinoma.15,16
Tobacco is a rich source of carcinogenic compounds such as aromatic amines.6 These compounds can cause DNA damage in both single- and double-stranded DNA. With increasing intensity and duration of smoking, the tumor increases in size and invades the bladder muscle layer (detrusor muscle).7 In this study, different results were obtained, that smoking status did not correlate with the UCB invasiveness (p>0.05). This indicates that as individuals develop a habit of smoking on a daily basis, there is no difference between those who smoke frequently and those who smoke less frequently.
A study conducted by Barbosa found a slight correlation between increased smoking intensity and a higher chance of developing a more aggressive tumor type; however, this correlation was not observed in current smokers. This contradictory finding implies that there is no direct link between smoking intensity and bladder cancer.6 This finding is also supported by research by van Roekel et al, which likewise found no dose-response relationship between tumor features and smoking intensity.17 A study by Jiang demonstrated the opposite, demonstrating that the risk of UBC decreases with the number of years since quitting, but that there is no difference between subgroups of tumor aggressiveness.18
Urothelial bladder carcinoma is not considered an endocrine-related cancer; however, androgens and their receptors (AR) play a role in carcinogenesis and progression. Androgen receptors in cells are located in the cytoplasm and nucleus. In the absence of androgens, AR are localized in the cytoplasm. According to Kimura, AR is exclusively expressed in the nucleus and can act as a transcription factor.19 The withdrawal process from androgens will cause transfer of unbound AR from the nucleus to the cytoplasm.
In this study, based on histoscores, we found strong (histoscore ≥ 6) and weak (histoscore <6) AR expression in both the cytoplasm and nucleus. Subsequent analysis revealed that only cytoplasmic expression was associated with UCB invasion (p = 0.001). In this study, we observed an association between cytoplasmic AR expression and UCB invasiveness. An interesting finding of this study is that cytoplasmic AR expression is associated with the level of invasion, which has not been previously explained. Androgen receptors that are translocated into the nucleus bind to the androgen response element (ARE) and reduce the expression of AR. Several studies have shown that protein degradation is necessary for maintaining cellular function. A literature review by Lee and Chang showed that AR is also degraded in the nucleus, which could be the cause of the reduced AR expression in the nuclear.20 Another thing that causes reduced expression of nuclear AR is the withdrawal process from androgens, causing AR to move from the nuclear into the cytoplasm. Androgen-sensitive AR can become androgen-insensitive AR, which occurs in castration-resistant prostate cancer. Several mechanisms underlying these changes include mutations, changes in the AR gene, and an increase in variants of AR; thus, the expression of AR in the nucleus is reduced with CPI examination.21
Several studies have evaluated the expression of AR in primary tumors and their metastatic sites. Kutasovic (2019) argued that in metastatic specimens AR expression was found.22 A study conducted by Bronte (2018) showed that only 60% of AR were found concurrently in primary and metastatic tumors. This implies that clinicians who provide anti-AR therapy must have data on the status of AR both in primary tumor tissue and metastatic tissue, given that the expression of AR in primary tumors can be different from the site of metastasis. As much as 5% of urothelial bladder carcinomas have spread to regional lymph nodes or metastases to other organs; therefore, the assessment of AR in primary tumors in the bladder can show different expression compared to the site of metastasis.23 This is in accordance with a study conducted by Kraby (2018), who concluded that there was an overexpression of AR (21.4%) in regional lymph tissue and metastatic sites compared to primary tumor tissue.24 Cytoplasm AR expression may initiate a signal transduction pathway to regulate cellular proliferation and migration, known as the non-genomic pathway.25 This could explain the results that we obtained. Martinez-Rojo showed that the binding of androgens to androgen receptors in the cytoplasm can initiate a transduction process for the regulation of cell proliferation known as the non-genomic pathway (AR non-genomic pathway). In addition, non-genomic RA signaling pathways are mediated by molecules in the cytoplasm and cell membranes and do not undergo androgen receptor-mediated transcription in the cell nucleus.
CD24 is a cell surface molecule linked to the glycosyl-phosphatidylinositol protein, which functions as a cancer cell adhesion molecule during invasion and metastasis. CD24 acts as a ligand for P-selectin adhesion receptor (a protein present on the surface of endothelial cells and platelets). The interaction between cancer cells and P-selectin via CD24 is an important adhesion pathway for cancer progression and metastasis. Based on research by Lim et al on CPI examination, CD24 expression can be observed in cell membranes and cytoplasm.26
In this study, there was no association between CD24 expression and UCB invasiveness (p = 0.315). The mechanism that can explain the absence of this association is that the androgen receptor expressed in the cytoplasm first binds to other co-regulators such as -catenin, cyclin-d1 which actively play a role in UCB invasiveness. Our results are in accordance with those of a study by Liu et al, which found no association between CD24 and urothelial bladder cancer progression. Liu et al found that 36% of patients with bladder urothelial carcinoma do not express CD24. In samples expressing CD24, no association was observed with UCB invasiveness. However, in Liu’s study, the subjects were limited to patients with non-invasive bladder cancer.9
In normal bladder urothelial cells, small amounts of CD24 are found in the apical cytoplasm. In noninvasive superficial tumors, CD24 expression was higher in the apical cytoplasm. In other words, superficial tumor cells have the same polarity as normal urothelial cells, but have a higher level of CD24 expression. In some malignant tumors, there is a change in polarity (loss of polarity), where CD24 expression is evenly distributed in the cytoplasm.27 Increased CD24 expression in the cytoplasm and loss of polarity of CD24 are associated with increased UCB invasiveness, this is in accordance with Kristiansen’s study et al 2002.28 Apical polarization properties of CD24 expression are found in normal urothelial, to non-invasive superficial tumors.24 However, when the tumor has progressed to invasive or metastatic direction, the apical polarity trait is lost (non-polar expression).24 This causes urothelial carcinoma cells to spread more easily and quickly to regional lymph nodes and metastatic organs, so that the picture of CD24 expression in primary tumors (urothelial bladder carcinoma) is reduced (Choi et al, 2007).29,30 The results obtained in this study are in line with the conclusions of the study. Another possible explanation is that, according to a previous study by Sottnik, cluster expression of differentiation is inversely correlated with AR in primary tumors.31
The last finding in this study, namely, from the multivariate analysis, showed that the combination of all independent variables (smoking status, N-AR and C-AR expression, and CD24) was not associated with UCB invasiveness. Mitra et al 2013 concluded that the combination of changes in molecular markers and smoking intensity variables (multivariable) can predict the level of invasion in individuals with UCB if each of these variables has an influence on invasiveness.32
To the best of our knowledge, this is the first report on the correlation between UCB and RA expression in the cytoplasm, which was limited because the study design was retrospective, the number of samples used was minimal, and the tissue samples in paraffin blocks were obtained from various procedures (biopsy results/TUR-bladder tumor and cystectomies). We also did not follow up the patients, which can be the objective of further study the outcome or impact of the biomarker finding.
Conclusion
We concluded that cytoplasmic androgen receptor expression can lead to the progression of urothelial bladder carcinoma. Smoking intensity and CD24 expression were not associated with urothelial bladder carcinoma invasiveness in current smokers. This indicates that as someone develops a habit of smoking on a daily basis, there is no difference between those who smoke frequently and those who smoke less frequently (non-linear dose-response relationship). Further research is needed to obtain uniform samples of tumor tissue, examine AR and CD24 by methods other than immunohistochemistry, and administer anti-androgens to bladder urothelial carcinoma cells.
Abbreviations
UCB, Urothelial Carcinoma of the Bladder; CAM, Cellular adhesion molecule; NMIBC, non-muscle invasive bladder cancer; MIBC, muscle invasive bladder cancer; HE, hematoxylin and eosin; AR, androgen receptor; PBS, phosphate-buffered saline;C-AR, cytoplasmic AR; N-AR, nuclear AR.
Funding
Thank you for financial support for publication from Directorate of Research Engagement of Universitas Padjadjaran.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Safriadi F, Palgunadi N. Karakteristik Non-Muscle Invasive Bladder Cancer Di RSUP Dr Hasan Sadikin 2008 – 2017. Unpad; 2019.
3. Ministry of Health Indonesia. Pusat Data Dan Informasi (Pusdatin) Perilaku Merokok Masyarakat Indonesia. Ministry of Health of Indonesia; 2013.
4. Islami F, Stoklosa M, Drope J, Jemal A. Global and regional patterns of tobacco smoking and tobacco control policies. Eur Urol Focus. 2015;1(1):3–16. doi:10.1016/j.euf.2014.10.001
5. van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP. Quantified relations between exposure to tobacco smoking and bladder cancer risk: a meta-analysis of 89 observational studies. Int J Epidemiol. 2016;45(3):857–870. doi:10.1093/ije/dyw044
6. Barbosa ALA, Vermeulen SH, Aben KK, Grotenhuis AJ, Vrieling A, Kiemeney LA. Smoking intensity and bladder cancer aggressiveness at diagnosis. PLoS One. 2018;13(3):e0194039. doi:10.1371/journal.pone.0194039
7. Pramod SV, Safriadi F, Hernowo BS, Dwiyana RF, Bonar A. Smoking duration and intensity associated with tumour aggressiveness in patients with urothelial carcinoma of the bladder: a correlation study. J Clin Urol. 2020;14(5):355–360. doi:10.1177/2051415820947239
8. Fajkovic H, Halpern JA, Cha EK, et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol. 2011;29(4):457–463. doi:10.1007/s00345-011-0709-9
9. Liu C, Zheng S, Shen H, et al. Clinical significance of CD24 as a predictor of bladder cancer recurrence. Oncol Lett. 2013;6(1):96–100. doi:10.3892/ol.2013.1357
10. Lombard AP, Mudryj M. The emerging role of the androgen receptor in bladder cancer. Endocr Relat Cancer. 2015;22(5):R265–77. doi:10.1530/ERC-15-0209
11. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi:10.3322/caac.20138
12. Farid RM, Sammour SAE, Shehab ZA, Salman MI, Omran TI. Expression of CD133 and CD24 and their different phenotypes in urinary bladder carcinoma. Cancer Manag Res. 2019;11:4677–4690. doi:10.2147/CMAR.S198348
13. Ouerhani S, Rouissi K, Kourda N, et al. Combined analysis of smoking, TP53, and FGFR3 mutations in Tunisian patients with invasive and superficial high-grade bladder tumors. Cancer Invest. 2009;27(10):998–1007. doi:10.3109/07357900902849707
14. Li P, Chen J, Miyamoto H. Androgen receptor signaling in bladder cancer. Cancers. 2017;9(2):20. doi:10.3390/cancers9020020
15. Agudo A, Bonet C, Travier N, et al. Impact of cigarette smoking on cancer risk in the European prospective investigation into cancer and nutrition study. J Clin Oncol. 2012;30(36):4550–4557. doi:10.1200/JCO.2011.41.0183
16. Tsai YW, Tsai TI, Yang CL, Kuo KN. Gender differences in smoking behaviors in an Asian population. J Womens Health. 2008;17(6):971–978. doi:10.1089/jwh.2007.0621
17. Van Roekel EH, Cheng KK, James ND, et al. Smoking is associated with lower age, higher grade, higher stage, and larger size of malignant bladder tumors at diagnosis. Int J Cancer. 2013;133(2):446–454. doi:10.1002/ijc.28017
18. Jiang X, Castelao JE, Yuan J, et al. Cigarette smoking and subtypes of bladder cancer. Int J Cancer. 2012;130(4):896–901. doi:10.1002/ijc.26068
19. Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem. 1993;41(5):671–678. doi:10.1177/41.5.8468448
20. Lee DK, Chang C. Endocrine mechanisms of disease: expression and degradation of androgen receptor: mechanism and clinical implication. J Clin Endocrinol Metab. 2003;88(9):4043–4054. doi:10.1210/jc.2003-030261
21. Henzler C, Li Y, Yang R, et al. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat Commun. 2016;7(1):13668. doi:10.1038/ncomms13668
22. Kutasovic JR, McCart Reed AE, Males R, et al. Breast cancer metastasis to gynaecological organs: a clinico-pathological and molecular profiling study. J Pathol Clin Res. 2019;5(1):25–39. doi:10.1002/cjp2.118
23. Bronte G, Bravaccini S, Ravaioli S, et al. Androgen receptor expression in breast cancer: what differences between primary tumor and metastases? Transl Oncol. 2018;11(4):950–956. doi:10.1016/j.tranon.2018.05.006
24. Kraby MR, Valla M, Opdahl S, et al. The prognostic value of androgen receptors in breast cancer subtypes. Breast Cancer Res Treat. 2018;172(2):283–296. doi:10.1007/s10549-018-4904-x
25. Martínez-Rojo E, Berumen LC, García-Alcocer G, Escobar-Cabrera J. The role of androgens and androgen receptor in human bladder cancer. Biomolecules. 2021;11(4):594. doi:10.3390/biom11040594
26. Lim SC. CD24 and human carcinoma: tumor biological aspects. Biomed. Pharmacother. 2005;59(2):S351–4. doi:10.1016/s0753-3322(05)80076-9
27. Lim SC, Oh SH. The role of CD24 in various human epithelial neoplasias. Pathol Res Pract. 2005;201(7):479–486. doi:10.1016/j.prp.2005.05.004
28. Kristiansen G, Denkert C, Schlüns K, Dahl E, Pilarsky C, Hauptmann S. CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. Am J Pathol. 2002;161(4):1215–1221. doi:10.1016/S0002-9440(10)64398-2
29. Overdevest JB, Knubel KH, Duex JE, et al. CD24 expression is important in male urothelial tumorigenesis and metastasis in mice and is androgen regulated. Proc Natl Acad Sci U S A. 2012;109(51):E3588–96. doi:10.1073/pnas.1113960109
30. Choi YL, Lee SH, Kwon GY, et al. Overexpression of CD24: association with invasiveness in urothelial carcinoma of the bladder. Arch Pathol Lab Med. 2007;131(2):275–281. doi:10.5858/2007-131-275-OOCAWI
31. Sottnik JL, Vanderlinden L, Joshi M, et al. Androgen receptor regulates CD44 expression in bladder cancer androgen receptor regulation of CD44 in bladder cancer. Cancer Res. 2021;81(11):2833–2846. doi:10.1158/0008-5472.CAN-20-3095
32. Mitra AP, Castelao JE, Hawes D, et al. Combination of molecular alterations and smoking intensity predicts bladder cancer outcome: a report from the Los Angeles cancer surveillance program. Cancer. 2013;119(4):756–765. doi:10.1002/cncr.27763
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.