Back to Journals » Journal of Pain Research » Volume 9
Cytidine 5’-diphosphocholine administration prevents peripheral neuropathic pain after sciatic nerve crush injury in rats
Authors Emril D, Wibiwo S, Meliala L, Susilowati R
Received 2 July 2014
Accepted for publication 11 August 2014
Published 23 May 2016 Volume 2016:9 Pages 287—291
DOI https://doi.org/10.2147/JPR.S70481
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Dessy R Emril,1 Samekto Wibowo,2 Lucas Meliala,2 Rina Susilowati3
1Department of Neurology, Faculty of Medicine, Syiah Kuala University, Banda Aceh, 2Department of Neurology, 3Department of Histology and Cell Biology, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia
Background: Cytidine 5’-diphosphocholine (citicoline) has been shown to have beneficial effects in central nervous system injury as well as in motoric functional recovery after peripheral nerve injury. This study aimed to examine the effect of citicoline on prevention of neuropathic pain in a rat model of sciatic nerve crush injury.
Methods: Forty experimental rats were divided into four groups. In three groups, the right sciatic nerves were crushed in the mid-thigh region, and a gelatin sponge moistened with 0.4 or 0.8 mL of 100 µmol/L citicoline, or saline 0.4 mL in the control group, was applied. The fourth group of rats was sham-operated, ie the sciatic nerve was exposed with no crush. Functional assessments were performed 4 weeks after crush injury. von Frey filaments (100 g threshold) were used to assess neuropathic pain. In addition, the sciatic functional index and extensor postural thrust (EPT) tests were used to assess motoric function.
Results: The crush/citicoline 0.4 mL group had a lower percentage of pain (23.53%, n=17) compared with the crush/saline group (53.33%, n=15, P<0.005). The crush/citicoline 0.4 mL group also showed better motoric recovery, as seen in stronger EPT results (P<0.001). However, the sciatic functional index analysis did not show significant differences between groups (P=0.35). The crush/citicoline 0.8 mL group showed a higher percentage of pain (66.67%, n=18) and less EPT recovery. These results may be explained by more severe nerve injury due to compression with a larger administered volume.
Conclusion: In situ administration of 0.4 mL of 100 μmol/L citicoline prevents the occurrence of neuropathic pain and induces motoric recovery, evaluated by EPT test, 4 weeks after sciatic nerve injury.
Keywords: nerve injury, nerve regeneration, neuropathic pain
Introduction
Neuropathic pain is an issue that has not been thoroughly studied or resolved. It is defined as pain that is caused by a primary lesion in or damage to the central or peripheral nervous system. Damage may occur as a result of compression, cutting, ischemic or metabolic disorders, cellular infiltration, or a combination of the above.1 If there is damage to the nerves, then there will be a cascade reaction, starting with damage in the nerve cell membranes and progressing to cell edema, Wallerian degeneration of the distal segments, and the cell regeneration process in the form of axonal sprouting. Unsuccessful regeneration may result in neuroma, and neuroma formation after injury is one of the main mechanisms responsible for the onset of neuropathic pain.2
Neuropathic pain affects 2%–3% of the population and is very problematic because of its severity, chronicity, and resistance to treatment, as well as its effects on reducing patient quality of life. The costs of neuropathic pain treatment are high, and the success rate with the use of drugs that already exist is less than 50%.3,4 The unclear pathophysiology of neuropathic pain is one reason for the difficulty in determining the right medication. Nowadays, doctors are more likely to use drugs that inhibit the excessive transmission of sodium and calcium ions to repress ectopic discharge that arises as a result of nerve damage that becomes the source of pain.
Citicoline has been proven to induce nerve regeneration after injury in several in vitro studies.5–7 It has been widely used in clinical cases of central nervous system disorders such as ischemic stroke, cognitive impairments,8 and glaucoma.9 In situ administration of citicoline has been reported to play a role in improving motoric function and inducing the regeneration process of the damaged axons in a rat sciatic nerve injury model.5 Administration of CDP-choline and choline + cytidine several days after injury shows improved motoric function recovery and better structural nerve regeneration,6 indicating its potential in the treatment of peripheral nerve injury. However, there are no reports about whether administration of citicoline may prevent neuropathic pain after peripheral nerve injury.
Based on the above, this study aimed to investigate the effect of citicoline administration on neuropathic pain in a rat model of injured sciatic nerve. This study will assess regenerative improvement through pain behavior assessment as well as motoric functional analysis.
Materials and methods
Animals
Forty adult male Wistar rats weighing 200–300 g were used in this study. Acclimatization was done at least 1 week before surgery. Groups of four rats were put in plastic cages with padded floors and kept in a laboratory animal care facility with a light/dark cycle of 12/12 hours (light from 8 am to 8 pm and dark from 8 pm to 8 am). Rats were given free access to food and water. This experiment was approved by the Ethical Committee of the Faculty of Medicine of the Universitas Gadjah Mada, Yogyakarta, Indonesia (no KE/FK/478/EC). All procedures that were performed to the mice following the Law of the Republic of Indonesia Number 18/2009 on Husbandry and Animal Health and National Ethical Guidelines for Health Research 2011.
Surgical procedures
Rats were anesthetized by intramuscular injection of 30 mg/kg thiopental sodium. The fur of the gluteal area was shaved and topical povidone/iodine was applied. Incision was made through the skin and gluteal muscle until the right sciatic nerve was identified. The location to be crushed was marked with 8.0 nylon thread in the epineurium. The nerves were crushed using an arterial clamp on the strength of one tooth for 60 seconds.10 The forty rats were grouped randomly into a crush/saline control group (n=10), crush/0.4 mL citicoline treatment group (n=10), or crush/0.8 mL citicoline treatment group (n=10). The injured nerves of the groups were then wrapped with a gelatin sponge soaked with 0.4 mL of 0.9% saline solution, 0.4 mL of 100 μmol/L citicoline, or 0.8 mL of 100 μmol/L citicoline, respectively. A further group of rats was sham-operated, ie the right sciatic nerve was exposed without any injury or treatment given (n=10). Antibiotics were given in situ and the wounds were closed layer by layer. The whole surgical procedure was done by one operator.
Evaluation of neuropathic pain behavior
von Frey filament tests with a threshold of 100 mg were performed in the fourth week after surgery. Pressure was applied to the third and fourth toes until the legs bent (withdrawal response). The result was considered positive if there was a withdrawal response of seven or more out of ten repetitions.11
Sciatic functional index
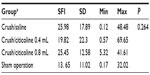
Sciatic functional index (SFI) was measured using walking tracks made from plywood (10 cm × 100 cm dimension), which were covered with white paper and led to a dark compartment. Both hind limbs of each rat were dipped in methylene blue stain. Four different measurements of the rat footprints were obtained: distance footprints, spread of fingers, distance between the footsteps, and distance between the legs. SFI was calculated based on the values obtained using the following equation:12
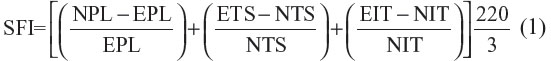
SFI=Sciatic Funtional Index, N: normal, or non-operated; E: experimental, or operated; PL: print length; TS: total toe spread, or distance between first to fifth toe; IT: intermediate toes spread, or the distance between the second and fourth toes.
Extensor postural thrust (EPT) test
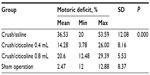
Using the examiner’s hand, each rat’s body weight was supported at the thoracic area and the right leg was made to touch to the weighing surface of the scale. When there was contact between the rat’s toes and the scale, the rat would straighten its leg and kick the scale. The numbers that appeared on the monitor of the scale were recorded in grams as the strength of the right leg muscles and regarded as experimental EPT (EEPT). The same procedure was applied for the left hind leg (normal EPT [NEPT]). Motor deficit was calculated using the following equation:13
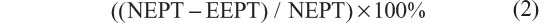
Statistical analysis
The results regarding neuropathic pain behavior were reported as frequency of postive pain behavior for each group and analyzed using the chi-square test. The mean differences between groups for the SFI and EPT were assessed using Kruskal–Wallis statistical analysis. P-values <0.05 were considered statistically significant.
Results
Neuropathic pain behavior test
The results of the von Frey filament tests, performed in the fourth postoperative week and using 100 g as the cut-off point, are shown in Table 1. Most of the rats with an injured sciatic nerve that had saline treatment showed pain behavior (8/10), whereas pain behavior was only detected in 2/10 of the injured rats that received 0.4 mL citicoline (P<0.05). More rats that were treated with 0.8 cc citicoline showed pain behavior (4/10), but there was no statistically significant difference in comparison with the saline group (P>0.05).
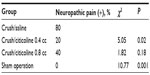  | Table 1 Pain behavior assessed with von Frey filaments (100 g threshold) 4 weeks after crush injury |
SFI
Based on the walking track analysis, SFI values, as indicators of functional recovery, were calculated, and the results are shown in Table 2. The results show that the difference between groups was not statistically significant (P=0.26) (Table 2).
EPT
EPT tests were conducted in the fourth week after surgery, and the results are shown in Table 3. As predicted, the sham-operated rats showed a better result (mean 2.47%) compared to the injured groups. Injured rats that had the 0.4 mL citicoline treatment showed the best result among the injured groups (14.28%), with the injured rats that received saline having the worst result (36.54%). The 0.8 mL citicoline administration led to a less satisfactory EPT result (20.60%) compared to the 0.4 mL citicoline group. The results were statistically significant (P=0.00).
Discussion
To our knowledge, this is the first report showing the effect of citicoline in preventing pain behavior after peripheral nerve injury. Pain behavior testing showed that the group treated with 0.4 mL citicoline had fewer rats that had neuropathic pain compared to the other injured groups. This suggests that citicoline has the potential to prevent the onset of neuropathic pain based on its effect in inducing optimal axonal regeneration. However, more of the rats which were treated with the higher volume of citicoline (0.8 mL) showed pain behavior and this may have been due to excessive compression of the injured nerve with a gelatin sponge that had absorbed more volume. Another possibility is that the sponge’s absorbing capacity was exceeded, causing a spill of citicoline to the surrounding tissue that may have contributed to inducing more inflammation and resulted in inhibition of axonal regeneration.
The study of functional status using walking track analysis showed a nonsignificant result. This was presumably due to the difficulty in measuring footprint lengths, as the footprints were sometimes not clearly visible. Some animal is sometimes less cooperative when walking the tracks. A previous study mentioned the same constraints in SFI calculation.12 Further studies may determine methods that are easier to implement and that have more accuracy.
EPT is another motoric functional test that was conducted in this study. The EPT result was in concordance with the result of the pain behavioral test, ie 0.4 mL citicoline administration resulted in the best functional recovery, while the 0.8 mL citicoline group had a less satisfactory result. Our results also support the motoric functional recovery after citicoline administration in sciatic nerve injury reported by Ozay et al.5
The mechanism of citicoline in improving axonal regeneration post-injury needs to be clarified. CDP-choline and its metabolites, choline and cytidine, had a beneficial effect in nerve repair after peripheral nerve injury in our rat model Choline and cytidine whether alone or in combination, are important substrates for the synthesis of phosphatidylcholine and promote cell membrane synthesis and repair,14–17 as well as neurogenesis in vitro or in vivo.18,19–21 Administered CDP-choline exogenously produces some pharmacologic effects that increase cholinergic neurotransmission.22–25 Some studies have proved the effects of choline in decreasing the severity of inflammatory response26 and preventing endotoxin-induced multiorgan injury.26,27 All these effects are essential for the recovery process after nerve injury.28–30 Since oral and intravenous citicoline administration has shown potential in entering injured nervous tissue in clinical settings, we plan to test in the future whether systemic administration of citicoline may give similar results in preventing neuropathic pain in injured sciatic nerves.
Conclusion
We have shown that the administration of 0.4 mL citicoline in situ can prevent neuropathic pain after sciatic nerve crush injury in rats.
Disclosure
The authors report no conflicts of interest in this work.
References
Woolf CJ. Neuropathic pain. In: Pain. Philadelphia, Lippincott Williams Wilkins, 2004:765–795(1). | |
Wall PD, Gutnick M. Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol. 1974;43:580–593. | |
Bridges D, Thompson SW, Rice AS. Mechanisms of neuropathic pain. Br J Anaesth. 2001;87:12–26. | |
Suharjanti I. Paradigma baru penanganan nyeri sentral paska stroke fokus pada pengobatan triad nyeri. [New Paradigm of central pain treatment post stroke, concern on pain triad treatment] In: Machfoed MH, editor. Proceeding of Workshop the 12th Continuing Neurological Education. Surabaya: Neurology Department of Medical Faculty of Airlangga University/dr. Soetomo General Hospital and Indonesian Neurological Association (INA); 2010:39–44. | |
Ozay R, Bekar A, Kocaeli H, Karli N, Filiz G, Ulus IH. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surg Neurol. 2007;68:615–622. | |
Aslan E, Kocaeli H, Bekar A, Tolunay S, Ulus IH. CDP-choline and its endogenous metabolites, cytidine and choline, promote the nerve regeneration and improve the functional recovery of injured rat sciatic nerves. Neurol Res. 2011;33:766–773. | |
Kaplan T, Kafa IM, Cansev M, et al. Investigation of the dose-dependency of citicoline effects on nerve regeneration and functional recovery in a rat model of sciatic nerve injury. Turk Neurosurg. 2014;24;54–62. | |
Milani M. Citicoline as coadiuvant treatment of cognitive impairment in chronic degenerative central nervous system diseases and in ischemic stroke: a review of available data. Online Journal of Medicine and Medical Science Research. 2013;13–18. | |
Levin LA, Peeples P. History of neuroprotection and rationale as a therapy for glaucoma. Am J Manag Care. 2008;14:S11–S14. | |
Mavrogenis AF, Pavlakis K, Stamatoukou A, et al. Intraneural 0X7-saporin for neuroma-in-continuity in a rat model. European Journal of Orthopaedic Surgery & Traumatology. 2013;23(3):263–272. | |
Luo ZD. (Volume Editor) Pain Research: Methods and Protocols. In: Walker JM, editor. Methods in Molecular Medicine series. Vol 99. The Humana Press Inc., Totowa, NJ: 2004. | |
Carlton JM, Goldberg NH. Quantitating integrated muscle function following reinnervation. Surg Forum. 1986;37:611–614. | |
Amado S, Rodrigues JM, Luis AL, et al. Effects of collagen membranes enriched with in vitro-differentiated N1E-115 cells on rat sciatic nerve regeneration after end-to-end repair. J Neuroeng Rehabil. 2010;7;7. | |
López-Coviella I, Agut J, Savci V, Ortiz JA, Wurtman RJ. Evidence that 5’- cytidinediphosphocholine can affect brain phospholipid composition by increasing choline and cytidine plasma level. J Neurochem. 1995;65:889–894. | |
Richardson UI, Watkins CJ, Pierre C, Ulus IH, Wurtman RJ. Stimulation of CDP-choline synthesis by uridine or cytidine in PC12 rat pheochromocytoma cells. Brain Res. 2003;971:161–167. | |
Ulus IH, Watkins CJ, Cansev M, Wurtman RJ. Cytidine and uridine increase striatal CDP-choline levels without decreasing acetylcholine synthesis or release. Cell Mol Neurobiol. 2006;26:563–577. | |
Watkins CJ, Cansev M, Ulus IH, Wurtman RJ. Uridine increase striatal CPD-choline levels without decreasing acetycholine synthesis and release. Cell Mol Neurobiol. 2006;60:989–992. | |
Wurtman RJ, Ulus IH, Cansev M, Watkins CJ, Wang L, Marzloff G. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006;1088:83–92. | |
Cansev M, Watkins CJ, van der Beek EM, Wurtman RJ. Oral uridine 5’-monophosphate (UMP) increases brain CDP-choline levels in gerbils. Brain Res. 2005;1058:101–108. | |
Cansev M, Wurtman RJ, Sakamoto T, Ulus IH. Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new bran synapses. Alzheimers Dement. 2008;4(Suppl 1):S153–S168. | |
Sakamoto T, Cansev M, Wurtman RJ. Oral supplementation with docosahexaenoic acid and uridine-5’-monophosphate increase dendritic spine density in adult gerbil hippocampus. Brain Res. 2007;1182:50–59. | |
Cansev M, Ilcol YO, Yilmaz MS, Hamurtekin E, Ulus IH. Peripheral administration of CDP-choline, phosphocholine or choline increases plasma adrenaline and noradrenaline concentrations. Auton Autocoid Pharmacol. 2008;28:41–58. | |
Cansev M, Yilmaz MS, Ilcol YO, Hamurtekin E, Ulus IH. Cardiovascular effects of CDP-choline and its metabolites: involvement of peripheral autonomic nervous system. Eur J Phamacol. 2007;577:129–142. | |
Ilcol YO, Cansev M, Yilmaz MS, Hamurtekin E, Ulus IH. Intraperitoneal administration of CDP-choline and its cholinergic and pyrimidinergic metabolites induce hyperglycemia in rats: involvement of the sympathoadrenal system. Arch Physiol Biochem. 2007;113:186–201. | |
Ilcol YO, Cansev M, Yilmaz MS, Hamurtekin E, Ulus IH. Peripheral administration of CDP-choline and its cholinergic metabolites increases serum insulin: muscarinic and nicotinic acetylcholine receptors are both involved in their actions. Neurosci Lett. 2008;431:71–76. | |
Ilcol YO, Yilmaz Z, Ulus IH. Endotoxin alters serum-free choline and phospholipid-bound choline concentrations, and choline administration attenuates endotoxin-induced organ injury in dogs. Shock. 2005;24:288–293. | |
Ilcol YO, Yilmaz Z, Cansev M, Ulus IH. Choline or CDP-choline alters serum lipid responses to endotoxin in dogs and rats: involvement of the peripheral nicotinic acetylcholine receptors. Shock. 2009;32:286–294. | |
Adibhatla RM, Hatcher. Cyticoline 5’-diphosphocholine (CPD-choline) in stroke and other CNS disorders. Neurochem Res. 2005;30(1):15–23. | |
Saver JL. Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair. Rev Neurol Dis. 2008;5:167–177. | |
Secades JJ, Lorenzo JL. Citicoline: pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol. 2006:28 (Suppl B):1–56. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.