Back to Journals » Cancer Management and Research » Volume 10
CYP17 polymorphisms are associated with decreased risk of breast cancer in Chinese Han women: a case–control study
Authors Yang P, Wang M, Tian T, Feng Y, Zheng Y , Yang T, Li H, Lin S , Xu P , Deng Y , Hao Q, Li N, Guan F, Dai Z
Received 7 April 2018
Accepted for publication 3 May 2018
Published 3 July 2018 Volume 2018:10 Pages 1791—1798
DOI https://doi.org/10.2147/CMAR.S167503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Pengtao Yang,1,* Meng Wang,1,* Tian Tian,1,* Yanjing Feng,2 Yi Zheng,1 Tielin Yang,3 Hongtao Li,4 Shuai Lin,1 Peng Xu,1 Yujiao Deng,1 Qian Hao,1 Na Li,1 Feng Guan,5 Zhijun Dai1
1Department of Oncology, Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710004, People’s Republic of China; 2Department of Cardiology, Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, People’s Republic of China; 3School of Life Science and Technology, Xi’an Jiaotong University, Xi’an 710049, People’s Republic of China; 4Department of Breast, Head and Neck Surgery, Affiliated Tumor Hospital of Xinjiang Medical University, Urumchi, 830000, People’s Republic of China; 5College of Life Science and Technology, Northwest University, Xi’an 710069, People’s Republic of China
*These authors contributed equally to this work
Introduction: CYP17 is the second most important enzyme in estradiol synthesis. Epidemiological studies have shown the associations between CYP17 polymorphisms and cancer risk. We conducted a case–control study to evaluate the relationship between CYP17 polymorphisms (rs743572 and rs2486758) and breast cancer (BC) risk.
Patients and methods: This case–control study included 560 BC patients and 583 age-matched healthy controls from Northwest China. Two polymorphisms (rs743572 and rs2486758) of CYP17 were genotyped by using Sequenom MassARRAY. ORs and 95% CIs were used to evaluate the relationship.
Results: Compared with the wild genotype of rs743572, we found a significantly reduced risk of BC associated with the variant genotypes (heterozygote model: OR=0.69, 95% CI=0.53–0.89; homozygote model: OR=0.68, 95% CI=0.49–0.95; dominant model: OR=0.69, 95% CI=0.54–0.87; overdominant model: OR=0.78, 95% CI=0.62–0.98; allele model: OR=0.79, 95% CI=0.66–0.93). For rs2486758 polymorphism, we did not find any difference in any of the genetic models. Further stratification analysis by clinical characteristics showed rs743572 was associated with estrogen receptor status (heterozygote model: OR=2.13, 95% CI=1.47–3.08; homozygote model: OR=3.29, 95% CI=1.94–5.58; dominant model: OR=2.39, 95% CI=1.69–3.37) and progesterone receptor status (homozygote model: OR=3.17, 95% CI=1.82–5.55), but there was no association between rs2486758 and clinical characteristics of BC. Haplotype analysis showed that Grs743572Crs2486758 haplotype was a protective factor of BC (OR=0.52, 95% CI=0.40–0.67). Survival analysis did not find that CYP17 rs743572 polymorphism was associated with triple-negative BC, either in terms of overall survival or progression-free survival.
Conclusion: Our results suggest that CYP17 polymorphisms may reduce the susceptibility to BC in Chinese women.
Keywords: CYP17, polymorphism, breast cancer, susceptibility
Introduction
Breast cancer (BC) is the most common cancer in women worldwide and the second leading cause of cancer death in the United States.1 In People’s Republic of China, BC led to 70,700 deaths in 2015 and the estimated number of new cases is 272,400.2 The development of BC is a complex interaction of genes, environment, and lifestyle.3 Based on current research, it is certain that estrogen levels are associated with the occurrence and development of endometrial cancer and BC,4 and estrogen metabolism-related genes which affect estrogen levels are considered to participate in the pathogenesis of BC. Among them, one of the most important gene is cytochrome P450, family 17 (CYP17).5,6
Various enzymes mediate the conversion of cholesterol into estrogen and CYP17 is a key enzyme in estradiol synthesis.7 It has two different catalytic reactions: the 17α-hydroxylase and 17,20-lyase reactions,5 their ratio affects the final product of steroid hormone biosynthesis. A single-nucleotide polymorphism (SNP), CYP17-34T/C polymorphism (rs743572), is located in the 5’-untranslated promoter region, it creates a recognition site for the MspAI restriction enzyme resulting in two allelic variants: T (A1 allele) and C (A2 allele).8 The A2 allele was considered to improve the transcription efficiency of the CYP17 gene, thereby increasing the activity of related enzymes and the synthesis of estradiol,9,10 therefore this SNP has received widespread attention. Another important SNP is rs2486758, which is mapped to the intergenic section near the 5’ of the CYP17 gene.11 The previous study showed that the rs2486758 minor allele increased the expression of CYP17 gene by affecting gene splicing and transcription factor binding or the sequence of noncoding RNA.12
Previous studies have confirmed the association between CYP17 gene polymorphism and risk of various cancers.8–10,13–17 Although there have been several studies about the relationship between CYP17 gene polymorphism (rs743572) and BC susceptibility of Han Chinese,18–24 the conclusions of these studies were not entirely consistent. Specifically, there were no studies regarding the association between rs2486758 and BC susceptibility of the Chinese. Therefore, we conducted this case–control study to investigate the relationship between the CYP17 polymorphisms (rs2486758 and rs743572) and BC risk in a Northwest Chinese population.
Patients and methods
Ethics statement
The study was approved by the Institutional Review Board of the Xi’an Jiaotong University (Xi’an, People’s Republic of China). During the time of recruitment, all participants signed a written informed consent form for the study.
Study population
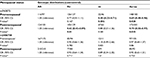
Our study consisted of 560 BC patients who were consecutively recruited between January 2013 and October 2014 at the Second Affiliated Hospital of Xi’an Jiaotong University, People’s Republic of China. There was no age limit for recruiting patients. All patients were pathologically confirmed as having BC. Patients who received chemotherapy or radiotherapy before surgery or had other types of cancer were excluded. A total of 583 cancer-free healthy controls, who were receiving health care (without any underlying illnesses) from outpatient departments, were recruited. Controls were frequency aged-matched to the cases (±5 years). The methods were carried out in accordance with the approved guidelines.34 After obtaining written informed consent, we obtained participants’ relevant information through a self-administered questionnaire, including age, ethnicity, place of residence, education level, and other potential confounding factors of interest. Clinical characteristics were collected and regularly updated through follow-up, including menopausal status, tumor size, axillary lymph node metastasis, ER status, PR status, Her-2 status, and Ki67 status. In addition, 48 cases of TNBC were followed up every month by telephone up to October 31, 2017. OS was calculated from the date of pathological diagnosis to the date of death or the last follow-up. PFS was calculated from the date of pathologically confirmed diagnosis to the progression of the disease, death without progression, or last clinical follow-up. Survival distributions were estimated by using the Kaplan–Meier method and difference in the survival was tested using the log-rank test.
Genotyping assay
The blood samples were collected from the peripheral vein and placed into EDTA-coated tubes. All samples were stored at −80°C, after centrifugation, whole blood cells were collected for further analysis.35 Standard phenol–chloroform extraction method was used to extract genomic DNA from blood leukocytes. The DNA concentration was checked by spectrometry (DU530 UV/VIS spectrophotometer, Beckman Instruments, Fullerton, CA, USA), which we described in our previous studies.25,36,37 Two tag-SNPs (rs2486758 and rs743572) were selected in our study based on minor allele frequency data from HapMap to achieve 80% power (http://www.hapmap.org).38 Sequenom MassARRAY Assay Design 3.0 Software (Agena Bioscience, San Diego, CA, USA) was used to design multiplexed SNP MassEXTEND assay. CYP17 genotyping was performed by using Sequenom MassARRAY RS1000 according to the manufacturer’s standard recommended protocol. The corresponding primers for each SNP in this study were listed in Table 7. Sequenom Typer 3.0 Software (Sequenom Inc., San Diego, CA, USA) was used for data analysis.
Statistical analyses
The differences in the distributions of demographic characteristics, selected variables, and frequencies of the two SNP genotypes between the cases and controls were compared using Student’s t-test or χ2 test. In control subjects, any departure from HWE was tested by applying goodness of fit χ2 test before analysis. The association between CYP17 SNPs and BC risk were estimated by computing ORs and 95% CIs, using univariate and multivariate logistic regression analysis with adjustment for age and BMI Online SHEsis software (http://analysis.bio-x.cn/myAnalysis.php) was used to evaluate LD. Phase 2.1 (downloaded from http://stephenslab.uchicago.edu/phase/download.html) software was used for haplotype analysis and for each haplotype, an OR and 95% CI was estimated by using χ2 test. All the statistical analyses were performed using the software SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA), and a two-sided P-value <0.05 was considered statistically significant. GraphPad Prism 6 (https://secure.graphpad.com/) was used for survival analysis, the HR and 95% CI were calculated by univariate Cox proportional hazards model, multivariate Cox regression models were performed to compute HR and 95% CI, after adjusting for confounding factors.
Results
Characteristics of the patients and controls
The clinical and demographic characteristic of BC patients and controls were described in our previous studies.25,26 The cases and controls were matched by age (Student’s t-test, P=0.612). As shown in Table 1, there was no significant difference in the distribution of menopausal state between the two groups (χ2 test, P=0.716). However, the BMI was significantly different between BC patients and healthy controls (Student’s t-test, P=0.038), which we considered may be related to the fact that most BC patients underwent modified radical mastectomy.
Association between CYP17 polymorphisms and BC risk
The genotypes and allele frequencies of the CYP17 rs2486758 and rs743572 polymorphisms are shown in Table 2. The genotype frequencies of both SNPs in controls were in accordance with Hardy–Weinberg Equilibrium (HWE) (χ2 test, for rs743572, P=0.49; for rs2486758, P=0.95 respectively). Compared with the wild genotype of rs743572, we found a significantly reduced risk of BC associated with the variant genotypes in all genetic models except recessive model (χ2 test, heterozygote model: OR=0.69, 95% CI=0.53–0.89; homozygote model: OR=0.68, 95% CI=0.49–0.95; dominant model: OR=0.69, 95% CI=0.54–0.87; overdominant model: OR=0.78, 95% CI=0.62–0.98; allele model: OR=0.79, 95% CI=0.66–0.93). These results suggested that the CYP17 rs743572 polymorphism had a protective effect on BC risk. However, we did not observe a significant association between the CYP17 rs2486758 polymorphism and BC risk in any genetic model.
Stratified analysis of CYP17 polymorphisms and BC risk
Stratified analysis regarding the effect of rs743572 and rs2486758 polymorphisms on BC according to menopausal status are displayed in Table 3. The results indicated that rs743572 was associated with a decreased BC risk in both premenopausal and postmenopausal women (χ2 test, for premenopausal women, homozygote model: OR=0.40, 95% CI=0.23–0.71; dominant model: OR=0.69, 95% CI=0.48–0.96, and for postmenopausal women, heterozygote model: OR=0.62, 95% CI=0.43–0.89; dominant model: OR=0.70, 95% CI=0.50–0.97). However, there was no association between rs2486758 and BC risk in premenopausal patients or postmenopausal patients.
Association between CYP17 polymorphisms and clinical parameters of BC patients
In order to determine the effect of CYP17 polymorphisms on the different clinical features of BC patients, we then analyzed the associations between the CYP17 polymorphisms and a series of clinicopathological parameters, including tumor size, lymph node metastasis, estrogen receptor (ER) status, progesterone receptor (PR) status, and Her-2.
As shown in Table 4, we found that the mutational genotype frequency of rs743572 was significantly higher in patients with ER positive (χ2 test, heterozygote model: OR=2.13, 95% CI=1.47–3.08; homozygote model: OR=3.29, 95% CI=1.94–5.58; dominant model: OR=2.39, 95% CI=1.69–3.37) and PR positive (χ2 test, homozygote model: OR=3.17, 95% CI=1.82–5.55). However, no significant relation was detected in other clinical parameters of BC patients. For rs2486758, we did not find any associated clinical parameters of BC patients (Table 5).
Haplotype analysis of CYP17 polymorphisms and BC risk
Linkage disequilibrium (LD) tests were conducted to evaluate LD, the results – D`=0.997, r2=0.128 – showed that LD did not exist in the two SNPs. We further conducted haplotype analysis by using the Phase 2.1 software to explore whether the interaction of rs743572 and rs2486758 SNPs affected BC risk. Compared with the Ars743572Trs2486758 haplotype, Grs743572Crs2486758 haplotype showed a decreased risk of BC (χ2 test, OR=0.52, 95% CI=0.40–0.67, P<0.001, as shown in Table 6).
  | Table 7 Primers used in this study Abbreviation: SNP, single-nucleotide polymorphism. |
Survival analysis of patients with CYP17 rs743572 and triple-negative breast cancer (TNBC)
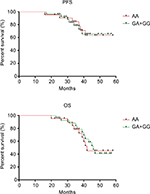
Compared to other molecular portraits of BC, TNBC patients have a poorer prognosis. Thus, we wanted to explore the relationship between SNPs and the prognosis of TNBC patients. A total of 48 TNBC patients were recruited, with mean age of 48.02. Patients underwent modified radical mastectomy or breast-conserving surgery, and received chemotherapy or radiotherapy after surgery. As shown in Figure 1, up to the follow-up time, there was no difference between the TNBC patients with CYP17 rs743572 GA/GG (56.25%) and CYP17 rs743572 AA (43.75%) in terms of the progression-free survival (PFS) (log-rank test, P=0.976; HR=0.98, 95% CI=0.33–2.92). Similar results were obtained in terms of overall survival (OS) (log-rank test, P=0.867; HR=1.08, 95% CI=0.45–2.57).
Discussion
CYP17 is a crucial estrogen-signaling regulatory enzyme, which mediates a variety of physiological and pathological processes. Several studies have demonstrated the association of CYP17 rs743572 and rs2486758 polymorphisms with increased risk of various cancers,12–16 and these two SNPs are the most common type of variation mutation. Related studies showed that rs743572 A>G SNP in CYP17 may change the binding characteristics of the promoter region and then modify the gene’s function.27 This could lead to a change of estrogen levels and risk of BC. In addition, the rs2486758 minor allele has been reported to be associated with higher serum 17β-estradiol levels in premenopausal women.11 In our study, we observed that variant genotypes of CYP17 rs743572 were associated with decreased BC risk, but rs2486758 was not related to BC risk. In further stratification analysis by clinical characteristics, results showed that rs743572 was associated with ER and PR status, but there was no association between rs2486758 and any clinical characteristics of BC.
According to several published studies, rs743572 is the most commonly studied CYP17 SNP. A significantly increased relationship was found with CYP17 rs743572 and BC in Caucasian populations,28 Feigelson et al also found an increased risk of advanced BC in women carrying an A2 allele in Caucasian populations.10 However, Sangrajrang et al considered that there was no relationship between rs743572 and BC in Thai women.17 In addition, in a study of a Chinese population, no evidence of relation was detected.29 Tan et al, Hu et al, and Sakoda et al found a similar result.20–22 In contrast, Zhang et al and Wang et al obtained the opposite result.23,24 Our results have demonstrated the negative association between CYP17 rs743572 SNP and BC risk in a Northwest Chinese population. The differences may be attributed to the geographical and lifestyle differences in different regions of the People’s Republic of China, which may have led to differences in the frequencies of genetic variations. It was also indicated that CYP17 rs743572 was a key site in the process of estrogen biosynthesis and metabolism, and thus may affect the development of various malignant tumors, which may offer evidence for clinical treatment and prognosis evaluation.
rs2486758 is another frequently studied CYP17 SNP, which is localized in the intergenic section near the 5′ of CYP17. rs2486758 minor allele affects the CYP17 gene’s splicing and transcription, leading to an increase in the expression of CYP17.12 Iversen et al found that CYP17 rs2486758 minor allele was related to higher 17β-estradiol levels, modification of the minor allele of CYP17 rs2486758 may have significant implications for the prevention of BC in women.11 Another cohort study did not support any evidence about the association between CYP17 rs2486758 and BC.30 As shown in our study, there was also no association between rs2486758 and any clinical characteristics of BC, which is consistent with the previously mentioned study. Mechanistically, the risk of prostate cancer is based on the location of rs2486758 in the promoter region of CYP17A1,31 while there was no other study to prove the relation between CYP17 rs2486758 and BC, we considered CYP17 rs2486758 was not affect the transcription of genes.
TNBC is a unique subtype of BC with poor survival, which is not affected by hormone metabolism.32,33 As CYP17 is a key enzyme in estradiol synthesis,5 and the occurrence and development of TNBC does not depend on estrogen levels, we hypothesized that CYP17 polymorphisms and TNBC survival are not directly related, which was confirmed by our results.
Our study had some limitations. First, it had a single-center design that only recruited Northwest Han Chinese women, which may preclude application of our conclusion in other ethnic populations. Second, survival analysis study was conducted only on patients with TNBC, thus, the effect of CYP17 rs743572 and rs2486758 on the prognosis of other molecular portraits of BC patients needs further study. Third, our sample size was relatively small, which may have limited the power to detect associations. Thus, we need to conduct a large, well-designed study to verify the associations between CYP17 polymorphisms and BC risk.
In summary, our case–control study indicates that the CYP17 rs743572 polymorphism may reduce BC susceptibility in Chinese Han women. Further functional studies and large, well-designed studies are still required to further elucidate the impact of CYP17 polymorphisms on BC.
Acknowledgments
This study was supported by National Natural Science Foundation, People’s Republic of China (No. 81471670), the Key Research And Development Plan, Shaanxi Province, People’s Republic of China (2017ZDXM-SF-066), Science and Technology Branch Project of Xinjiang Uygur Autonomous Region, People’s Republic of China (2017E0262).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152(10):950–964. | ||
McGrath M, Lee IM, Buring J, Hunter DJ, De Vivo I. Novel breast cancer risk alleles and endometrial cancer risk. Int J Cancer. 2008;123(12):2961–2964. | ||
Kristensen VN, Borresen-Dale AL. Molecular epidemiology of breast cancer: genetic variation in steroid hormone metabolism. Mutat Res. 2000;462(2–3):323–333. | ||
Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer. The role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Mutat Res. 2003;544(1):9–41. | ||
Kristensen VN, Kure EH, Erikstein B, Harada N, Børresen-Dale A. Genetic susceptibility and environmental estrogen-like compounds. Mut Res. 2001;482(1–2):77–82. | ||
Rai R, Sharma KL, Misra S, Kumar A, Mittal B. CYP17 polymorphism (rs743572) is associated with increased risk of gallbladder cancer in tobacco users. Tumour Biol. 2014;35(7):6531–6537. | ||
Guli MR, Wang JQ, Zhang JX, Deng G. [Association between the polymorphism of CYP17 gene and risk of prostate cancer in chinese vigurs men]. Zhonghua Nan Ke Xue. 2006;12(2):120–122. Chinese. | ||
Feigelson HS, Coetzee GA, Kolonel LN, Ross RK, Henderson BE. A polymorphism in the CYP17 gene increases the risk of breast cancer. Cancer Res. 1997;57(6):1063–1065. | ||
Iversen A, Thune I, McTiernan A, et al. Genetic polymorphism CYP17 rs2486758 and metabolic risk factors predict daily salivary 17beta-estradiol concentration in healthy premenopausal Norwegian women. The EBBA-I study. J Clin Endocrinol Metab. 2012;97(5): E852–857. | ||
Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363(2):166–176. | ||
Chakraborty A, Murthy NS, Chintamani C, et al. CYP17 gene polymorphism and its association with high-risk north Indian breast cancer patients. J Hum Genet. 2007:52(2):159–165. | ||
Yazici H, Tigli H, Kadehci Z, et al. Are CYP17 genotypes a biomarker for ovarian cancer in patients with cancer history in their family? Oncol Res. 2006;16(1):43–47. | ||
Surekha D, Sailaja K, Rao DN, et al. Association of a CYP17 gene polymorphism with development of breast cancer in India. Asian Pac J Cancer Prev. 2010;11(6):1653–1657. | ||
Hou L, Xu J, Gao YT, et al. CYP17 MspA1 polymorphism and risk of biliary tract cancers and gallstones: a population-based study in Shanghai, China. Int J Cancer. 2006;118(11):2847–2853. | ||
Sangrajrang S, Sato Y, Sakamoto H, et al. Genetic polymorphisms of estrogen metabolizing enzyme and breast cancer risk in Thai women. Int J Cancer. 2009;125(4):837–843. | ||
Sun J, Zhang H, Gao M, et al. Association between CYP17 T-34C rs743572 and breast cancer risk. Oncotarget. 2017;9(3):4200–4213. | ||
Helzlsouer KJ, Huang HY, Strickland PT, et al. Association between CYP17 polymorphisms and the development of breast cancer. Cancer Epidemiol Biomarkers Prev. 1998;7(10):945–949. | ||
Tan W, Qi J, Xing DY, et al. [Relation between single nucleotide polymorphism in estrogen-metabolizing genes COMT, CYP17 and breast cancer risk among Chinese women]. Zhonghua Zhong Liu Za Zhi. 2003;25(5):453–456. | ||
Hu MB, Xie W, Xiong B, et al. [Study on the relationship between polymorphisms of genes (CYP17, CYP19 and SULT1A1) and susceptibility to breast cancer in Chinese women]. Zhonghua Liu Xing Bing Xue Za Zhi. 2006;27(4):351–355. | ||
Sakoda LC, Blackston C, Doherty JA, et al. Polymorphisms in steroid hormone biosynthesis genes and risk of breast cancer and fibrocystic breast conditions in Chinese women. Cancer Epidemiology Biomarkers Prev. 2008;17(5):1066–1073. | ||
Zhang L, Gu L, Qian B, et al. Association of genetic polymorphisms of ER-alpha and the estradiol-synthesizing enzyme genes CYP17 and CYP19 with breast cancer risk in Chinese women. Breast Cancer Res Treat. 2009;114(2):327–338. | ||
Wang YP, Li H, Li JY, et al. [Relationship between estrogen-biosynthesis gene (CYP17, CYP19, HSD17beta1) polymorphisms and breast cancer]. Zhonghua Zhong Liu Za Zhi. 2009;31(12):899–903. | ||
Wang M, Wang X, Fu SW, et al. Single-nucleotide polymorphisms in PSCA and the risk of breast cancer in a Chinese population. Oncotarget. 2016;7(19):27665–27675. | ||
Lin S, Wang M, Liu X, et al. FEN1 gene variants confer reduced risk of breast cancer in chinese women: a case-control study. Oncotarget. 2016;7(47):78110–78118. | ||
Carey AH, Waterworth D, Patel K, et al. Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum Mol Genet. 1994;3(10):1873–1876. | ||
Cribb AE, Joy Knight M, Guernsey J, et al. CYP17, catechol-o-methyltransferase, and glutathione transferase M1 genetic polymorphisms, lifestyle factors, and breast cancer risk in women on Prince Edward Island. Breast J. 2011;17(1):24–31. | ||
Han DF, Zhou X, Hu MB, et al. Polymorphisms of estrogen-metabolizing genes and breast cancer risk: a multigenic study. Chin Med J. 2005;118(18):1507–1516. | ||
Setiawan VW, Schumacher FR, Haiman CA, et al. CYP17 genetic variation and risk of breast and prostate cancer from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium (BPC3). Cancer Epidemiol Biomarkers Prev. 2007;16(11):2237–2246. | ||
Binder M, Zhang BY, Hillman DW, et al. Common genetic variation in CYP17A1 and response to abiraterone acetate in patients with metastatic castration-resistant prostate cancer. Int J Mol Sci. 2016;17(7):E1097. | ||
Wang J, Gildea JJ, Yue W. Aromatase overexpression induces malignant changes in estrogen receptor alpha negative MCF-10A cells. Oncogene. 2013;32(44):5233–5240. | ||
Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78(2):161–170. | ||
Gao J, Kang HF, Ma XB, et al. Functional promoter -765 G > C variant in COX-2 gene is associated with the susceptibility of breast cancer in Chinese Han women. Cancer Cell Int. 2014;14:38. | ||
Li S, Jin T, Zhang J, et al. Polymorphisms of TREH, IL4R and CCDC26 genes associated with risk of glioma. Cancer Epidemiol. 2012;36(3):283–287. | ||
Ren HT, Li YM, Wang XJ, et al. PD-1 rs2227982 polymorphism is associated with the decreased risk of breast cancer in northwest Chinese women: a hospital-based observational study. Medicine (Baltimore). 2016;95(21):e3760. | ||
Liu X, Wang X, Fu SW, et al. Genetic association of deleted in colorectal carcinoma variants with breast cancer risk: a case-control study. Oncotarget. 2016;7(22):32765–32773. | ||
Xia P, Li B, Geng T, et al. FGFR2 gene polymorphisms are associated with breast cancer risk in the Han Chinese population. Am J Cancer Res. 2015;5(5):1854–1861. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.