Back to Journals » Open Access Emergency Medicine » Volume 12
Cyclosporine-A-Based Immunosuppressive Therapy-Induced Neurotoxicity: A Case Report
Authors Teimouri A , Ahmadi SR , Anavri Ardakani S , Foroughian M
Received 14 December 2019
Accepted for publication 18 April 2020
Published 28 April 2020 Volume 2020:12 Pages 93—97
DOI https://doi.org/10.2147/OAEM.S241501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Hans-Christoph Pape
Ali Teimouri,1 Sayyed Reza Ahmadi,2 Saeideh Anavri Ardakani,3 Mahdi Foroughian2
1Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran; 2Department of Emergency Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; 3Department of Neurology, Mashhad University of Medical Sciences, Mashhad, Iran
Correspondence: Mahdi Foroughian
Ghaem Hospital, Mashhad, Iran
Tel/Fax +98 51 38525312
Email [email protected]
Abstract: Cyclosporine-A (CsA) and mycophenolate mofetil are immunosuppressive drugs used for the prevention of transplant rejection. Various clinical studies have been performed on different forms of CsA neurotoxicity, including tremor, paresthesia, confusion, ataxia, neuralgia, hemiplegia, occipital seizures, and transient cortical blindness. Mycophenolate is associated with several neurological side effects including headache, insomnia, dizziness, depression, confusion, hypertonia, and paresthesia. A 31-year-old male with a history of kidney transplantation was treated with CsA and mycophenolate mofetil, for 18 years. He had been referred to the emergency department with complaints of generalized tonic-clonic seizure for 1 minute and 15 minutes of the post-ictal phase. Almost all laboratory tests including cerebrospinal fluid analysis were within normal limits. Brain MRI findings were compatible with CsA-based neurotoxicity. The patient’s symptoms and MRI findings improved on decreasing CsA to the minimum dose. CsA neurotoxicity is more common in intravenous therapy, early days of CsA administration, P450 inhibitors administration, and following liver transplantation. MRI findings in CsA neurotoxicity include signal changes in the cerebral cortex and juxtacortical white matter of the occipital lobes, temporal, parietal, and frontal lobes. Every year, many solid organ transplantations are performed. Many of these patients received CsA-based regimens for the prevention of rejection. Therefore, it is necessary to consider CsA neurotoxicity in suspected patients.
Keywords: kidney transplantation, mycophenolic acid, neurotoxicity, MRI, adverse effects
Introduction
Cyclosporine-A (CsA) is a potent immunosuppressive drug for organ transplantation that is capable of prolonging patient survival compared with other former immunosuppressants.1 The administration of CsA reduces mortality rate, graft rejection, and duration of hospitalization and also improves the quality of life and life expectancy of transplant recipients.2
The most important benefit of this drug is that it does not suppress the bone marrow. As a result, it is considered as the main drug in kidney, liver, heart, and lung transplantation.3 Nevertheless, the CsA is associated with several side effects, including nephrotoxicity, hypertension, gingival hyperplasia, hypertrichosis, infection, hyperkalemia, hypomagnesemia, hepatotoxicity, increased incidence of specific cancers, and neurotoxicity.4–6
The neurotoxicity is a common CsA-induced complication seen in up to 50% of patients, especially in the early days of drug use and in high doses.7
Various clinical studies have been performed on different forms of CsA neurotoxicity, including tremor, paresthesia, confusion, ataxia, neuralgia, hemiplegia, occipital seizures, and transient cortical blindness.8 However, most of these reports were on the cases of bone marrow and liver transplants and few cases reported CsA neurotoxicity following kidney transplantation.9 CsA neurotoxicity may be reversible by discontinuing the drug or temporarily reducing its dose.9 Despite the unclear pathogenesis of CsA neurotoxicity, neuropeptide-mediated ischemia, corticosteroids, hypomagnesemia, hypocholesteremia, or other neurotoxins are thought to cause this syndrome.7,8
Mycophenolate mofetil is another drug used for different organ transplantation. Mycophenolate is associated with several neurological side effects including headache, insomnia, dizziness, depression, confusion, hypertonia, and paresthesia.10
The present report introduces a kidney transplant recipient who has experienced the CsA-based immunosuppressive therapy-induced neurotoxicity.
Case Presentation
The patient was a 31-year-old male with kidney transplantation treated with the CsA at a dose of 5 mg/kg/day and mycophenolate mofetil 2 g/day orally, for 18 years. He had been referred to the emergency department with complaints of the generalized tonic-clonic seizure (GTCS) for 1 minute and post-ictal phase for about 15 minutes. The patient had no history of seizures, headaches, and mood changes, but he reported the vision problems a month before admission.
He had an axillary temperature of 39°C and cervical lymphadenopathy, but other examinations including neurological tests were normal. Serum creatinine level was 0.98 (normal: 0.6–1.3 mg/dL) and serum urea level was 46.5 (normal: 15–45 mg/dL). Other tests, including bilirubin, magnesium, and other electrolytes, and blood cholesterol levels were within the normal range.
No pathological findings were observed on computed tomography scan (CT) and electroencephalography (EEG).
According to history and physical examination such as fever, a lumbar puncture was performed. Also, no pathological findings were observed in the analysis of cerebrospinal fluid (CSF).
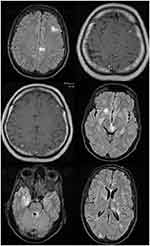
Magnetic resonance imaging (MRI) revealed a bilateral hyperintense patch of white matter in the supratentorial and infratentorial territories as well as changes in gray matter in the areas mentioned, which could justify the patient’s symptoms (Figure 1).
Given the presence of lymphadenopathy and cerebral involvement on the MRI, the findings of prescribed excisional biopsy from the cervical lymph nodes rolled out the lymphoma.
By suspecting the CsA-based neurotoxicity for the patient, the dose of CsA was reduced to the minimum possible, thereby ameliorating the patient’s symptoms. The radiologic imaging showed improvement in the examined territories during the follow-up duration.
The patients signed an informed consent containing the agreement to the publication of this case report, details, and images. Based on our consult with the ethics committee and vice chancellor for research at Mashhad University of Medical Sciences, we did not need institutional approval for this case report.
Discussion
The complication of CsA neurotoxicity is not well understood. In general, it consists of three grades including Grade 1 (mental status change, tremor, and headache) Grade 2 (vision problems and cortical blindness), and Grade 3 (seizures and coma). The seizures occur in approximately 1.8%, 5.5%, and 25% of the kidney, bone marrow, and liver transplant recipients, respectively. In our reported case, the symptoms started from Grade 1, progressed to Grade 3, and improved with a decrease in the dose of CsA.11
Although the mechanism of CsA neurotoxicity remains unclear, it is associated with intravenous administration and high doses. Moreover, this complication may even occur with oral administration of CsA and even serum levels in the therapeutic range of CsA.11
Many drugs increased CsA serum levels by inhibiting cytochrome P450 including calcium channel blockers, antifungal, methylprednisolone, warfarin, and many other drugs.9
Seizures have been seen in several patients receiving CsA following the liver or kidney transplantation.9 However, other factors such as urinary retention, hypertension, dysfunction, high dose of steroids, and demyelination may contribute to this disorder as well.7
Hypomagnesemia as a complication of CsA, Hypercholesterolemia, and neurotoxic substances such as bilirubin, BUN, and ammonia may also predispose a patient to seizures. However, in our patient, serum magnesium, cholesterol, and neurotoxic substance levels were within normal limits.7
Mycophenolate mofetil did not increase CsA serum levels but it could be responsible for many neurological side effects including headache, insomnia, dizziness, depression, confusion, hypertonia, and paresthesia.10
Several case reports show that mycophenolate mofetil could cause Tonic-clonic seizures.12
In our case, we could not measure the serum level of CsA or mycophenolate mofetil but after a reduced dosage of CsA patient’s symptoms improved and also radiologic imaging showed improvement in the examined territories during the follow-up duration.
Posterior reversible encephalopathy syndrome (PRES) is the most important neurological complication following the CsA administration.13 The syndrome is characterized by headaches, altered mental function, seizures, cortical blindness, and bilateral white-matter abnormalities as leukoencephalopathy predominantly in posterior brain regions (including the Pareto-occipital and temporal lobes) and in the pons, thalamus, and cerebellum.13
In our patient, the clinical signs were similar to the PRES, and the levels of cholesterol, magnesium, and blood pressure were normal. Also, the white matter involvement site on the MRI was similar to the PRES. Since the patient’s symptoms were improved after CsA dose reduction, his neurological symptoms can be attributed to the PRES.13
The CsA causes the release of potent vasoconstrictors, such as Endothelin 1 (ET-1) and thromboxane A2 (TXA2), from the vascular endothelium. The ET-1 induces vasoconstriction and vasospasm of the cerebral arteries, followed by ischemia and reversible white matter edema.14
The CsA-induced vascular injury releases and accumulates the cytokines causing vascular injury and disrupting the blood-brain barrier, which in turn increases vascular injury.14 Also, vascular injuries can cause thrombotic microangiopathy and thus the neurological disorders.14
Furthermore, posterior involvement of the brain in the CsA neurotoxicity is greater due to less adrenergic receptors in the vertebrobasilar system. CsA-associated hypertension stimulates peripheral sympathetic nerves, which in turn increases vascular resistance to protect the brain from hypertension.14 Since these receptors are lower in the posterior part of the brain, increased blood flow to the posterior part of the brain, impaired autoregulation and impaired blood-brain barrier, and vascular extravasation cause vasogenic edema.14
The clinical management and diagnosis of CsA-induced cerebral edema have been greatly improved using new MRI techniques such as fluid-attenuated inversion recovery and diffusion-weighted. Hence, diffusion-weighted MRI provides valuable information in distinguishing between cytotoxic edema and interstitial edema.8
Both CT scan and MRI are important in assessing radiologic abnormalities in patients with CsA neurotoxicity but more prominent in MRI; thus, the MRI is of more diagnostic value if the CT scan is normal.8
Although the clinical signs of CsA neurotoxicity are very different, MRI findings have almost diagnostic value, including signal changes within the cerebral cortex and juxtacortical white matter of the occipital lobes, posterior temporal, parietal, and frontal lobes.8 In our patient, there were bilateral pathological findings on MRI of the white matter of the frontal and parietal lobes, which improved rapidly after lowering the CSA dose during follow-up with the reduced dosage of CsA.
Conclusion
Although neurotoxicity of CsA following kidney transplantation is not very rare, according to WHO, only in 2015, a total of 84,347 kidney transplantations and 27,759 liver transplantations have been performed. Many patients who received solid organ transplantation received CsA-based regimens for the prevention of rejection. Therefore, it is necessary to consider the risk of CsA neurotoxicity following kidney transplantation, which needs further investigation into the mechanism of CsA neurotoxicity.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Benvenuto LJ, Anderson MR, Arcasoy SM. New frontiers in immunosuppression. J Thorac Dis. 2018;10(5):3141–3155. doi:10.21037/jtd.2018.04.79
2. Black CK, Termanini KM, Aguirre O, Hawksworth JS, Sosin M. Solid organ transplantation in the 21(st) century. Ann Transplant Medi. 2018;6(20):409. doi:10.21037/atm.2018.09.68
3. Tedesco D, Haragsim L. Cyclosporine: a review. J Transplant. 2012;2012:230386. doi:10.1155/2012/230386
4. Pal P, Giri PP, Sinha R. Cyclosporine in resistant systemic arthritis - A cheaper alternative to biologics. Indian J Pediatr. 2019;86(7):590–594. doi:10.1007/s12098-019-02912-9
5. Arslansoyu Camlar S, Soylu A, Kavukcu S. Cyclosporine in pediatric nephrology. Iran J Kidney Dis. 2018;12(6):319–330.
6. Shin HS, Grgic I, Chandraker A. Novel targets of immunosuppression in transplantation. Clin Lab Med. 2019;39(1):157–169. doi:10.1016/j.cll.2018.10.008
7. Rezzani R. Cyclosporine A and adverse effects on organs: histochemical studies. Prog Histochem Cytochem. 2004;39(2):85–128. doi:10.1016/j.proghi.2004.04.001
8. Trullemans F, Grignard F, Van Camp B, Schots R. Clinical findings and magnetic resonance imaging in severe cyclosporine-related neurotoxicity after allogeneic bone marrow transplantation. Eur J Haematol. 2001;67(2):94–99. doi:10.1034/j.1600-0609.2001.t01-1-00440.x
9. Wijdicks EFM. Neurotoxicity of immunosuppressive drugs. Liver Transplant. 2001;7(11):937–942. doi:10.1053/jlts.2001.27475
10. Anghel D, Tanasescu R, Campeanu A, Lupescu I, Podda G, Bajenaru O. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica. 2013;8(2):170–175.
11. Straathof K, Anoop P, Allwood Z, et al. Long-term outcome following cyclosporine-related neurotoxicity in paediatric allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(1):159–162. doi:10.1038/bmt.2016.232
12. Lee KM, Kim MK, Wee WR, Lee J. Tonic-clonic seizure following combined treatment of mycophenolate mofetil and acyclovir. Graefes Arch Clin Exp Ophthalmol. 2011;249(7):1107–1108. doi:10.1007/s00417-010-1521-8
13. de Oliveira RA, Fechine LM, Neto FC, Nicodemus JM, Silva GBJ, Silva LSV. Posterior reversible encephalopathy syndrome (PRES) induced by cyclosporine use in a patient with collapsing focal glomeruloesclerosis. Int Urol Nephrol. 2008;40(4):1095–1098. doi:10.1007/s11255-008-9431-y
14. Hauben M. Cyclosporine neurotoxicity. Pharmacotherapy. 1996;16(4):576–583. doi:10.1002/j.1875-9114.1996.tb03639.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.