Back to Journals » OncoTargets and Therapy » Volume 11
CXCR2 is a novel cancer stem-like cell marker for triple-negative breast cancer
Authors Wang Y, Tu L, Du C, Xie X, Liu Y, Wang J, Li Z, Jiang M, Cao D, Yan X, Luo F
Received 16 May 2018
Accepted for publication 22 June 2018
Published 6 September 2018 Volume 2018:11 Pages 5559—5567
DOI https://doi.org/10.2147/OTT.S174329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Yuyi Wang,1,* Li Tu,1,* Chi Du,2,* Xiaoxiao Xie,1 Yanyang Liu,1 Jiantao Wang,1 Zhixi Li,1 Ming Jiang,1 Dan Cao,1 Xi Yan,1 Feng Luo1
1Lung Cancer Center, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Department of Oncology, The Second People’s Hospital of Neijiang, Neijiang, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Background: Breast cancer is the leading cause of mortality from cancer in women worldwide, and cancer stem-like cell (CSC) is responsible for failure treatment of breast cancer. It plays an important role in resistant disease and metastasis. CD44/CD24 and ALDH are well-accepted protein markers of breast CSC, and it was reported that distinct subtypes of breast CSC were identified by the 2 markers. It is possible that there are various kinds of breast CSC which could be identified by different markers, and CSC markers utilized at present are not enough to fully understand breast CSC. Finding out more novel CSC markers is necessary. CXCR2 is involved in breast cancer metastasis, treatment resistance, and recurrence and has positive cross-talk with known breast CSC protein markers. It can be concluded that CXCR2 is related to breast CSC, and further study is in need.
Results: In this study, we assessed expression of CXCR2 with immunohistochemistry in breast cancer tissues from 37 patients and discovered that level of CXCR2 was significantly lower in triple-negative breast cancer (TNBC) compared with non-TNBC. CXCR2 expression decreased in estrogen receptor-negative or HER2-negative breast cancer, but not progesterone receptor-negative counterparts. By immunofluorescence, we observed high coexpression rate of CXCR2 and CSC-related proteins, including NANOG and SOX2. To prove our speculation that CXCR2 was a novel CSC marker for TNBC, we used 4T1 cell, which is a TNBC cell line, to analyze CXCR2-positive subpopulations and observed that CXCR2-positive 4T1 cells showed characteristics of CSC, including resistance to cisplatinum, radiation, and hypoxia, low proportion (around 1%), much more tumor xenografts, tumor spherule formation, and higher levels of CSC-related mRNA compared with CXCR2-negative cells.
Conclusion: CXCR2 is an acceptable and newly discovered CSC marker for only TNBC.
Keywords: cancer stem-like cell, CXCR2, triple-negative breast cancer, marker
Introduction
Breast cancer is one of the leading causes of death in women worldwide, and it is the most commonly diagnosed cancer among women in People’s Republic of China.1 Development of chemotherapy, radiotherapy, and targeted therapy, such as trastuzumab, has decreased mortality of this disease, but millions of breast cancer patients will still die because of failure treatment. One of the well-known explanations for this is the presence of cancer stem-like cell (CSC) which is resistant to chemotherapeutics,2 radiation,3 and trastuzumab.4 In addition to resistance to therapies, CSC is also responsible for tissue invasion,5 metastasis, and recurrence.6 Moreover, CSC is even reported to mimic endothelial cells and form tumor blood vessels.7 Because of these properties, breast cancer with a high proportion of CSC is correlated with a poor overall survival (OS),8 and CSC thus becomes a promising target. Therefore, research on breast CSC was important, and many studies were conducted during the recent decade.
The first step in conducting research on breast CSC is the identification and differentiation of them from other “ordinary” tumor cells with protein markers, and breast cancer cells with high expression of these markers are reported to contain many more CSCs. Nowadays, CD44+CD24−,9,10 ALDH-positive10 subpopulations are considered as CSCs in breast cancer. The majority of studies on breast CSCs choose them as the research target. However, it was shown by a study that CD44+CD24− breast CSCs represent a quiescent, mesenchymal-like population, while ALDH+ CSCs represent a cycling, epithelial-like population, and this discovery suggests that CD44+CD24− and ALDH+ breast CSCs are distinct from each other.11 It is possible that several different types of breast CSC with different protein markers exist in breast cancer, and finding out more subtypes of markers is important for the study of breast CSCs.
CXCR2 is a G-protein-coupled cell surface chemokine receptor, which has been found to be overexpressed in various cancers including breast cancer;12–16 in addition, CXCR2 with its binding partners, such as IL-8, is implicated in growth, proliferation, metastasis, and resistance to chemotherapeutic agents of breast cancer.17–21 A higher level of CXCR2 ligand is observed in patients with advanced stages of breast cancer and is linked to poor prognosis.22–26 Our previous study showed that CXCL1, a ligand of CXCR2, was able to increase the population of ALDH+ breast CSCs in 4T1 breast cancer cell line, and it was also shown that inhibition of CXCR2 decreased the volume of breast cancer and the proportion of breast CSCs (unpublished). These discoveries suggest that CXCR2-related signaling pathways are tightly related to breast CSCs and play an important role in breast cancer.
In this study, we assessed expression of CXCR2 in human breast cancers and explored the relationship of CXCR2 and breast CSCs by experiments in vitro and in vivo, trying to find out a novel treatment target for breast cancer.
Materials and methods
Cancer cell culture and treatment
The 4T1 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM (Life Technologies, Bedford, MA, USA) containing 10% FBS and 1% penicillin/streptomycin and kept in a humid chamber at 37°C with 5% CO2. In vitro studies were performed as follows: 6-well plates with the same volume of 4T1 cells were prepared. About 200 μmol/L cobalt chloride (CoCl2) and 10 ng/mL cisplatinum were used, respectively, to treat 4T1 cells for 48 hours. About 8 Gy radiation, once every other day for twice (16 Gy in total) was used for radiotherapy of 4T1 cells.
Murine model and in vivo tumor formation assay
Female BALB/C mice, which were 6–8 weeks old (Sichuan University, Chengdu, People’s Republic of China), were maintained in the Laboratory for Animal Experiments under specified pathogen-free conditions. Indicated numbers of sorted CXCR2+ or CXCR2− cells were implanted subcutaneously in the breast pad of mice. The number of palpable tumors was counted after 12 weeks. All procedures involving animals were approved by the Animal Care and Use Committee of Sichuan University. The experimental manipulation of mice was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Flow cytometry
For the assessment of CXCR2 expression in single cells from 4T1 tumors, cells were washed and counted to make sure that the number of cells in each test was between 3×105 and 1×106. The primary antibody, PE-CXCR2 (149609, BioLegend, San Diego, CA, USA), was added. Cells were then placed at room temperature and incubated in the dark for 30 minutes in accordance with the manufacturers’ instructions. Cells were rewashed with PBS and resuspended in 400–500 μL PBS, then analyzed or separated in a cell sorter.
Sphere formation assay
4T1 cells or separated CXCR2+/CXCR2− 4T1 cells were plated in ultra-low attachment 6-well plates (Corning, Lowell, MA, USA) and grown in serum-free DMEM/F12 (1:1) (Invitrogen, Carlsbad, CA, USA). 1X-N2 supplement (Invitrogen), 10 ng/mL EGF (Peprotech, Rocky Hill, NJ, USA), and 10 ng/mL bFGF (Peprotech) were added into the culture medium. These cells were cultured for 60 days. Images of tumor spheres were taken with a phase contrast microscope (Nikon, Tokyo, Japan).
Reverse-transcription PCR and quantitative real-time PCR
Expression of mRNA of ALDH, ABCG2, NOTCH1, SOX2, and NANOG were analyzed by applying the quantitative reverse-transcription PCR (Takara, Kyoto, Japan) and SYBR green detection (Takara). Expression of β-actin was used as the control. The primers are listed in Table 1.
  | Table 1 Primers used in RT-PCR |
Immunofluorescence and immunohistochemical analysis
A total of 37 paraffin-embedded tissue sections from 37 patients with breast cancer were provided by the West China Hospital. CXCR2 expression was determined using rabbit anti-human CXCR2 antibody (GR132442-8, Abcam, Cambridge, MA, USA) immunostaining. NANOG expression was determined using mouse anti-human NANOG antibody (0710165 G-1, Novus Biologicals, Littleton, CO, USA) immunostaining. Sox2 expression was determined using mouse anti-human Sox2 antibody (0726153 H-8, Novus Biologicals) immunostaining. ALDH and CD44 expressions were determined using mouse anti-human ALDH (600162, ZenBio, Chengdu, People’s Republic of China) and mouse anti-human CD44 antibody (600211, ZenBio), respectively. Paraffin sections were dewaxed and blocked with 5% BSA. The sections were then incubated with the relevant antibodies, optimally diluted, at 4°C overnight. The sections were washed with PBS. For immunohistochemical analysis, sections were incubated with goat anti-rabbit or goat anti-mouse antibody (diluted 1:500). Peroxidase activity was visualized by utilizing 3,3′diaminobenzidine substrate according to the kit instructions (Beyotime Bioscience, Shanghai, People’s Republic of China). Sections were then counterstained with hematoxylin. Slides were examined using a microscope (Eclipse E600; Nikon). For immunofluorescence analysis, slides were incubated with fluorescein isothiocyanate goat anti-rabbit or tetramethylrhodamine goat anti-mouse antibody (diluted 1:500). Positive expression cells were observed with an Eclipse E600 light microscope (Nikon).
Statistical analysis
Data were expressed as mean±standard error of the mean. Statistical analyses were performed with the SPSS (version 16.0; SPSS Inc., Chicago, IL, USA). Student’s t-test was used to determine between-group statistical significance, and log-rank statistics were used to test for differences among subgroups. Statistical significance was set at P<0.05.
Ethical approval
This study was approved by Ethics and Scientific Committees of West China Hospital of Sichuan University, and written informed consent was obtained from all patients.
Results
CXCR2 expression in tissue sections of human breast cancer from 37 patients
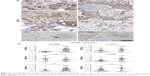
We assessed the expression of CXCR2 in human breast cancer tissue sections from 37 patients with breast cancer using immunohistochemistry (Figure 1A), and the information of these 37 patients is shown in Table 2. The immunohistochemistry findings showed that the expression of CXCR2 was significantly lower in TNBC (a subtype of breast cancer which has negative expression for estrogen receptor [ER], progesterone receptor [PR], and Her2) when compared with non-TNBC. The expression of CXCR2 was related with the expression of Her2 protein and ER, but not PR. Expression of CXCR2 was lower in Her2-negative or ER-negative breast cancer patients and was similar between PR-positive and PR-negative cases. CXCR2 expression has limited relationship with Ki67. Also, advanced (stage III–IV, according to National Comprehensive Cancer Network guidelines for breast cancer) and nonadvanced (stage I–II) breast cancer showed similar expression of CXCR2 (Figure 1B).
  | Table 2 Characteristics of the 37 patients with breast cancer |
High rate of coexpression of CXCR2 and CSC-related proteins
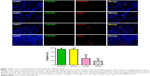
A quite low expression of CXCR2 in TNBC had been observed. So, the proportion of cells coexpressing CXCR2 and NANOG, SOX2, ALDH, and CD44, respectively, was measured to test the “stemness” of CXCR2-positive cells in TNBC, and the result of this study showed that the expression levels of CXCR2 and NANOG were relevant, as nearly 100% of CXCR2-positive cells also expressed NANOG. Among SOX2-positive cells, there were on average 89% (17/19) that also expressed CXCR2. There were 29% (15/51) ALDH-positive cells and 15% CD44-positive cells (17/114) that also coexpressed CXCR2 (Figure 2). These results support the relationship between CXCR2 and NANOG, CXCR2, and SOX2, and the possibility that CXCR2-positive cells in TNBC were CSCs. The findings also excluded the possibility that CXCR2 could be used as a marker of CSC in non-TNBC, because CSC is considered to be a small subpopulation of cells within the tissue, typically ≤1%.27
CXCR2+ cells were enriched with CSCs in 4T1 cell line
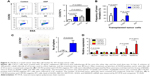
We utilized 4T1 breast cell, a TNBC cell line for further studies. CXCR2-positive and CXCR2-negative 4T1 cells were identified and sorted by flow cytometry to detect “stemness-related phenotypes,” including resistance to chemotherapy, radiation, and hypoxia, spherule formation, tumorigenic ability, and mRNA levels of CSC-related proteins. A low proportion of CXCR2-positive cells was observed in the 4T1 cell line (about 1%), which is consistent with the result that we observed in TNBC tissues from patients. Flow cytometry results showed that proportion of CXCR2-positive cells increased after treatment with cisplatinum, radiation, and hypoxia (Figure 3A), which indicated their resistance to traditional cancer treatment methods. CXCR2-positive 4T1 cells were capable of forming tumor spherules and transplanted tumors with a very low cell number (20,000, 2,000, and 200 in this study). CXCR2-negative 4T1 cells were also able to form tumors with a low cell number, but the success rate is much lower than CXCR2-positive ones and they quickly died in stem cell medium and could not form spherules (Figure 3B and C). The mRNA levels of several proteins were evaluated that are closely related with CSCs, including ALDH, ABCG2, NOTCH1, SOX2, and NANOG. The results showed that ALDH, SOX2, and NANOG were increased in CXCR2-positive cells (Figure 3D). These properties supported the hypothesis that CXCR2 could be used as a novel TNBC CSC marker.
Discussion
In this study, we observed low expression of CXCR2 in TNBC tissues from patients and assessed the rate of coexpression of CXCR2 with CSC-related proteins, including NANOG, ALDH, CD44, or SOX2.28 The corresponding mRNAs were mostly highly expressed in CXCR2-positive 4T1 cells in the present study. Results of immunofluorescence assays showed that nearly all CXCR2 cells expressed NANOG at the same time and most SOX2-positive cells also expressed CXCR2, suggested the “stemness” of CXCR2-positive cells. The coexpression of CXCR2 and ALDH, CXCR2, and CD44 in TNBC cells was also relatively common but obviously lower than CXCR2 and NANOG, or CXCR2 and SOX2. During further studies with a TNBC cell line, we observed a series of characteristics of CSC in CXCR2-positive 4T1 cells. First, the proportion of CXCR2-positive cells is quite low (around 1%), and it was surprising that so few cells were responsible for the development of ALDH-positive CSCs (according to our unpublished data). On review of related studies published in the literature, the expression of CXCR2 was also previously reported to be low in LM2-4175 lung cancer cell,29 which was derived from the MDA-MB-231 human breast cancer cell line (also a TNBC cell line). Other studies on CXCR2 suggested a role for CXCR2 in breast cancer metastasis, angiogenesis, and recurrence.30,31 One study also reported positive cross-talk between CXCR2 and CXCR4,32 which is a well-known CSC marker and a protein that is associated with metastasis. Therefore, we believed that CXCR2 might be a novel CSC marker for TNBC. Next, the present study tested the resistance of CXCR2-positive 4T1 cells to cisplatinum, radiation, and hypoxia, followed by the use of 4T1 tumor xenografts and assessment of tumor spherule formation and measurement of the levels of CSC-related mRNA. The findings from these study approaches all supported the possibility that CXCR2-positive 4T1 cells were a group of CSCs. Together with properties of CXCR2 observed in breast cancer tissues from patients, we assumed that CXCR2 might be a potential novel marker for TNBC.
At the present time, no studies have reported specific CSC markers for certain molecular subtype of breast cancer. In this study, for the first time, we identified CXCR2 as a novel CSC marker only for TNBC, using a TNBC cell line model and breast cancer sections from patients. Clinically, TNBC accounts for 15% of breast cancers, which have a more aggressive clinical course, with a higher risk of death and an early pattern of metastasis compared with non-TNBCs, particularly in the first 5 years.33,34 However, the major therapeutic approaches for breast cancers, including antihormone therapy or trastuzumab therapy, are not suitable for patients with TNBC due to the lack of a corresponding therapy target, which means that chemotherapy and radiation therapy are the main treatment choices for patients with TNBC. As we identified CXCR2 as a CSC marker for TNBC, treatment targeting CXCR2 may be a promising future treatment approach, but requires further study. Currently, a Phase IIa study to evaluate the safety and tolerability of AZD5069, a selective CXCR2 antagonist, in patients with moderate to severe chronic obstructive pulmonary disease was completed in 2015 (NCT01233232). The compound was well tolerated overall in those patients who completed the study treatment.35 Therefore, drugs such as AZD5069, which are in development and have been shown to have good safety profiles, may be studied in future in controlled clinical trials in patients with TNBC.
Conclusion
The findings of this study suggest that CXCR2 can be used as a novel CSC marker for TNBC.
Acknowledgment
This work was supported by the National Natural Sciences Foundation of China (81372506) (grantee: Feng Luo), and Sichuan Society of Medicine, The Youth Medical Research Creative Project (Q16042) (grantee: Chi Du).
Author contribution
YW, LT, and CD performed experiments; YW, XX, and YL designed experiments, and JW and ZL analyzed data; MJ and DC wrote the manuscript; XY and FL revised the paper and supervised the project. All authors read and approved the final manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology. 2014;287(12):1101–1107, 1110. | ||
Chen X, Liao R, Li D, Sun J. Induced cancer stem cells generated by radiochemotherapy and their therapeutic implications. Oncotarget. 2017;8(10):17301–17312. | ||
Rodríguez CE, Berardi DE, Abrigo M, Todaro LB, Bal de Kier Joffé ED, Fiszman GL. Breast cancer stem cells are involved in Trastuzumab resistance through the HER2 modulation in 3D culture. J Cell Biochem. 2018;119(2):1381–1391. | ||
Liu S, Cong Y, Wang D, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2014;2(1):78–91. | ||
Agliano A, Calvo A, Box C. The challenge of targeting cancer stem cells to halt metastasis. Semin Cancer Biol. 2017;44:25–42. | ||
Ping YF, Bian XW. Consice review: contribution of cancer stem cells to neovascularization. Stem Cells. 2011;29(6):888–894. | ||
Zhou L, Jiang Y, Yan T, et al. The prognostic role of cancer stem cells in breast cancer: a meta-analysis of published literatures. Breast Cancer Res Treat. 2010;122(3):795–801. | ||
Chen HC, Joalland N, Bridgeman JS, et al. Synergistic targeting of breast cancer stem-like cells by human γδ T cells and CD8+ T cells. Immunol Cell Biol. 2017;95(7):620–629. | ||
Sulaiman A, Sulaiman B, Khouri L, et al. Both bulk and cancer stem cell subpopulations in triple-negative breast cancer are susceptible to Wnt, HDAC, and ERα coinhibition. FEBS Lett. 2016;590(24):4606–4616. | ||
Liu S, Cong Y, Wang D, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2014;2(1):78–91. | ||
Miller LJ, Kurtzman SH, Wang Y, Anderson KH, Lindquist RR, Kreutzer DL. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res. 1998;18(1A):77–81. | ||
Richards BL, Eisma RJ, Spiro JD, Lindquist RL, Kreutzer DL. Coexpression of interleukin-8 receptors in head and neck squamous cell carcinoma. Am J Surg. 1997;174(5):507–512. | ||
Norgauer J, Metzner B, Schraufstätter I. Expression and growth-promoting function of the IL-8 receptor β in human melanoma cells. J Immunol. 1996;156(3):1132–1137. | ||
Takamori H, Oades ZG, Hoch OC, Burger M, Schraufstatter IU. Autocrine growth effect of IL-8 and GROα on a human pancreatic cancer cell line, Capan-1. Pancreas. 2000;21(1):52–56. | ||
Venkatakrishnan G, Salgia R, Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem. 2000;275(10):6868–6875. | ||
Lev DC, Ruiz M, Mills L, Mcgary EC, Price JE, Bar-Eli M. Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Mol Cancer Ther. 2003;2(8):753–763. | ||
Sharma B, Nawandar DM, Nannuru KC, Varney ML, Singh RK. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Mol Cancer Ther. 2013;12(5):799–808. | ||
de Larco JE, Wuertz BR, Manivel JC, Furcht LT. Progression and enhancement of metastatic potential after exposure of tumor cells to chemotherapeutic agents. Cancer Res. 2001;61(7):2857–2861. | ||
De Larco JE, Wuertz BR, Rosner KA, et al. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001;158(2):639–646. | ||
Maxwell PJ, Gallagher R, Seaton A, et al. HIF-1 and NF-κB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26(52):7333–7345. | ||
Benoy IH, Salgado R, Van Dam P, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10(21):7157–7162. | ||
Kozłowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. | ||
Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256(2):137–165. | ||
Addison CL, Daniel TO, Burdick MD, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165(9):5269–5277. | ||
Heidemann J, Ogawa H, Dwinell MB, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278(10):8508–8515. | ||
Da Cruz Paula A, Lopes C. Implications of different cancer stem cell phenotypes in breast cancer. Anticancer Res. 2017;37(5):2173–2183. | ||
Sun M, Zhang N, Wang X, et al. Hedgehog pathway is involved in nitidine chloride induced inhibition of epithelial-mesenchymal transition and cancer stem cells-like properties in breast cancer cells. Cell Biosci. 2016;6:44. | ||
Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165–178. | ||
Saintigny P, Massarelli E, Lin S, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res. 2013;73(2):571–582. | ||
Franz JM, Portela P, Salim PH, et al. CXCR2+1208 CT genotype may predict earlier clinical stage at diagnosis in patients with prostate cancer. Cytokine. 2017;97:193–200. | ||
Xiang Z, Zhou ZJ, Xia GK, et al. A positive crosstalk between CXCR4 and CXCR2 promotes gastric cancer metastasis. Oncogene. 2017;36(36):5122–5133. | ||
Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. | ||
Stockmans G, Deraedt K, Wildiers H, Moerman P, Paridaens R. Triple-negative breast cancer. Curr Opin Oncol. 2008;20(6):614–620. | ||
Kirsten AM, Förster K, Radeczky E, et al. The safety and tolerability of oral AZD5069, a selective CXCR2 antagonist, in patients with moderate-to-severe COPD. Pulm Pharmacol Ther. 2015;31:36–41. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.