Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Cutaneous plasmacytosis Characterized by Head Plaques: An Unusual Case Report of a 57-Year-Old Male
Authors Wei L , Zhang J, Di D, Wang D, Luo G
Received 25 April 2023
Accepted for publication 25 July 2023
Published 4 August 2023 Volume 2023:16 Pages 2021—2028
DOI https://doi.org/10.2147/CCID.S418750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Lu Wei,* Jialin Zhang,* Dake Di, Dongmei Wang, Guangpu Luo
Department of Traditional Chinese Medicine Dermatology, Dermatology Hospital of Southern Medical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guangpu Luo; Dongmei Wang, Department of Traditional Chinese Medicine Dermatology, Dermatology Hospital of Southern Medical University, No. 2, Lujing Road, Yuexiu District, Guangzhou City, Guangdong Province, 510091, People’s Republic of China, Tel +86 13926166004 ; +86 13431086997, Fax +86-20-87257353, Email [email protected]; [email protected]
Abstract: Cutaneous plasmacytosis (CP) is a rare disorder of uncertain etiology. We report an unusual and rare case of CP in a 57‑year‑old male who presented with popular nodules all over the body, accompanied by head plaques. Pathological biopsy of the skin revealed large infiltration of mature plasma cells within the dermis. Elevated serum IgG4 concentrations were found. Immunohistochemical analysis confirmed the polyclonal nature of the plasma cells. The diagnosis of CP was established. Steroid therapy was administered at a dose of 20 mg/day. After 1 month of treatment, the patient’s eruption showed regression. These findings remind dermatologists to include CP in their clinical differential diagnosis of patients with head plaques. Meanwhile, clinicians should carefully that individuals diagnosed with CP at risk for malignant transformation.
Keywords: cutaneous plasmacytosis, IgG4-related disease, plasma cell, IgG4
Introduction
Cutaneous plasmacytosis (CP) is a rare skin disorder. The etiology and exact pathogenesis of CP remain unknown, it may be related to genetic factors, environmental influence, and infectious triggers.1 Wu et al2 held that sunlight exposure may be one of the predisposing factors for cutaneous and systemic plasmacytosis (CSP). The plasma cell infiltrate may indicate an infectious agent as the cause of CSP, with syphilis or borrelia infections, in particular, being considered. Tuberculosis infection is an unusual but reported association with reactive plasmacytosis.3,4 Current evidence suggests that a deregulated production of interleukin (IL)-6 plays a critical role in the pathogenesis of CP.5 CP patients characteristically present with multiple diffuse, occasionally pruritic reddish-brown plaques, and nodules, primarily on the trunk and face. The histologic features most often show clusters of polyclonal plasma cell infiltrate. CP may be accompanied by extracutaneous involvement, superficial lymphadenopathy, or tumors. Potential malignant transformation has been reported in a few cases. To date, reported treatment modalities include topical/systemic steroids, topical calcineurin inhibitors, psoralen and UVA (PUVA) therapy, photodynamic therapy (PDT), radiotherapy, thalidomide, rituximab, and chemotherapy regimens with variable responses.6 However, there has been no single effective treatment described. Because of the rarity of this disease, many primary dermatologists lack sufficient knowledge about CP, which is prone to misdiagnosis and missed diagnosis. We aim to increase clinicians’ awareness of this disease.
Case Presentation
The patient is a 57-year-old male who presented with symmetrical papules and a scattering of indurated nodules on both upper limbs for the past ten years ago, accompanied by intense itching. The patient was diagnosed with “eczema” in the local hospital. He had been treated intermittently with topical and systemic corticosteroids for years without improvement. Five years ago, the lesions gradually spread all over the body, with indurated erythema and infiltrative plaques appearing on the head, aggravated itching and obvious exudation. A few years ago, atopic dermatitis (pruritic rash type) was diagnosed through a pathological biopsy of trunk and neck skin lesions. The patient denied any history of sexually transmitted disease contact.
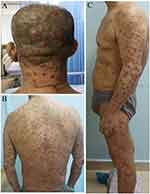
On physical examination, the patient was noted to have scattered 2.0–4.0-cm reddish or dark erythema and infiltrative plaques on the head, isolated and not fused (Figure 1A). The central hair of the plaques was missing. The trunk and limbs had numerous soybean bean- to broad-sized papules and nodules, which were well-demarcated, without fusion, and indurated (Figure 1B and C). No painful lymph nodes of 1.0–2.0-cm in diameter were palpable in the right cervical regions. No hepato-splenomegaly was found.
Laboratory values were as follows: white blood cell count was 12.785x109/L (range, 3.5–9.5 x109/L), absolute neutrophil count was 8.75x109/L (range, 1.8–6.3 x109/L), the amount of eosinophil was 1.40x109/L (range, 0–0.52 x109/L), the IgE level was 8988.0 IU/mL (range, 0–100IU/mL), the rheumatoid factor level was 55.80 IU/mL (range, 0–10 IU/mL), the level of IgG4 and IgG were 5682.6mg/L (range, 39.2–864.0mg/L) and 23.39 g/L (range, 6.5–16.00g/L), respectively. ANA quantification was positive, homogeneous nucleolus type, 1:80. The remaining hematological, biochemical investigations, tumor index were normal. Results of antinuclear antibodies, parasite antibody, treponema pallidum antibody test, anti-human immunodeficiency virus antibody, five items of hepatitis B test, and hepatitis C virus antibody test were all negative. In February 2017, a pathological biopsy was performed on the trunk showed that many lymphohistiocytes and eosinophils infiltrated the blood vessels and hair follicles in the superficial dermis. Serial sections: dermal edema, lymphatic tissue cells, eosinophils and plasma cells infiltrate blood vessels and around sebaceous glands. Acid-fast staining and love orchid dye were negative. In April 2021, the initial skin biopsy specimen obtained from a nodule on the posterior neck demonstrated focal parakeratosis of the epidermis, thickening of the spinous layer, pseudoepitheliomatous hyperplasia, focal cavernous edema, and a few lymphohistiocytes and eosinophils around the vessels in the superficial dermis. Prurigo nodularis was considered as the diagnosis. A pathological biopsy was performed on the head recently, and showed that no obvious change in the epidermis. Many plasma cells, histiocytes, lymphocytes, and eosinophils were infiltrated in the dermis in sheets or focal, showing small neutrophilic abscesses (Figure 2A and B). Direct immunofluorescence was negative. Immunohistochemical study showed that the infiltrating plasma cells were positive for CD3, CD4, CD68 and CD163, small foci positive for CD20 and CD30, partially positive for CD8, but negative for CD21 and CD23. There were a great number of CD38- and CD138-positive cells and a small number of CD79a-positive cells. The Ki67 index was 20–30%. The IgG4-positive cells were 23–33 per high-power field. The ratio of infiltration of IgG4-positive cells to total IgG-positive cells were low (<40%). Polyclonal nature was confirmed by positive staining for both kappa and lambda chains (Figure 3). Pathological diagnosis was CP. Serum immunoglobulin electrophoresis, IL-6 detection and bone marrow biopsy were not performed because of the patient’s mental anxiety.
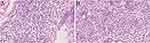 |
Figure 2 Histological findings of plaque on the head. (A and B) Prominent plasma cell infiltration of the dermis (HE, ×400). |
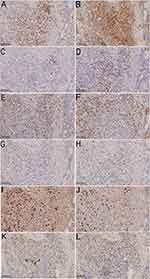 |
Figure 3 Immunohistochemistry results. (A–L) Infiltrating plasmacytes were CD3, CD4, CD20, CD8, CD68, CD163, CD138, Ki-67, Kappa, Lambda, IgG and IgG4 positive (×200). |
According to the histopathology and immunohistological study, a diagnosis of CP was made. Treatment with methylprednisolone 20mg per dose, once a day and methotrexate 10mg per dose, once a week was initiated. After 1 month of treatment, the skin lesions began to shrink, and no new skin lesions appeared. The therapeutic dose of the steroid was gradually reduced to 5mg per dose, once a day after 6 months of treatment. Reexamination showed that the serum IgG4 levels decreased after 1 month of treatment and 6 months, to 2189.6mg/L and 1721.1 mg/L, respectively. After 6 months of treatment, the skin lesions were dramatically diminished (Figure 4).
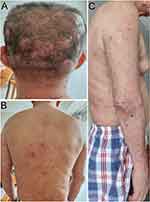 |
Figure 4 Clinical appearance after 6 months of treatment. |
Discussion
CP is a rare polyclonal plasmacytosis disorder, which mainly affects the skin. It was first reported by Yashiro in 1976 under the title “A kind of plasmacytosis” and later termed CP by Kitamura et al7,8 The disease is characterized by multiple skin patches, large numbers of mature plasma cells infiltrating the dermis accompanied by polyclonal hypergammaglobulinemia. Due to its rarity and most of the literature consisting of case reports and case series, the etiology and exact pathogenesis of CP remain to be elucidated, but it’s suggested that a deregulated production of IL-6 plays an important role in its pathogenesis.9 Some scholars speculate that CP may result directly from the mutation of genes coding for signaling molecules important in plasma cell regulation.10 Human herpes virus (HHV) 8 has not been implicated in CP.11
Others presume that it is related to typically increased levels of IL-6. IL-6 is responsible for the induction of terminal differentiation of B cells into plasma cells.12 There was a close link between the plasma IL-6 level and the disease activity. Patients may respond to pharmacologic therapy that interferes with IL-6 activity.5 IL‑6 genetic polymorphisms may explain the frequency of CP in individual regions.13 In recent years, several articles reported that in addition to the increase of plasma cells, there were abundant mast cells infiltration in the dermis.14,15 Studies have shown that mast cells enhance the proliferation of B lymphocytes and drive their differentiation toward plasma cells.16 It is reported that the perineural and intraneural distribution of plasma cells was recognized focally in six cases of CP.17 In the same case, the lesions affect only one side of the body and show neuronal pattern dermatoses. Suh JH et al18 suppose that the numerous plasma cells aggregated around the nerve fiber may correlate with the unilaterally neuronal pattern of the skin lesions. The typical symmetric pattern of CP on the chest or back may be a phenotypic expression reflecting its close relationship to the spinal nerve tract distribution. Brezinski et al19 found that perineural plasma cell infiltrates may be a histologic clue to the diagnosis of CP. Miyagawa-Hayashino et al20 observed that the three patients of CP showed high serum IgG4 levels and/or numerous IgG4 positive plasma cells in the affected tissues, and all of them were accompanied by related extracutaneous lesions (lymph nodes, lungs and bone marrow).
This patient’s rash is mainly composed of nodules, easily misdiagnosed as atopic dermatitis or prurigo nodosa. The only strange clinical manifestation is the infiltrative plaques on the head. Based on the characteristic pathological features, extremely high levels of serum IgG4 and immunohistological results, a diagnosis of CP was made. The clinical differential diagnosis for these head plaques included psoriasis and chronic actinic dermatitis, which can be differentiated through clinical features. To help dermatologists be aware of the CP when facing patients with head plaques, we make a figure to show the algorithm for head plaques finding (Figure 5). IgG4-related diseases are a chronic progressive systemic fibrosis disease. It is characterized by high levels of circulating IgG4 and tissue infiltration of IgG4+ plasma cells. It can cause multiple organs fibrosis, such as the pancreas, salivary glands, lacrimal glands, lungs, and skin.21 Research has shown that CP is a skin manifestation of IgG4-related diseases.22,23 However, it is uncertain whether there are fibroinflammatory changes involving multiple organs, such as the pancreas, gallbladder, salivary glands or lacrimal glands. CP patients with elevated serum IgG4 should exclude extracutaneous organ involvement. IgG4 analysis revealed an IgG4/IgG ratio lower than 40%, arguing against a diagnosis of IgG4-related diseases.
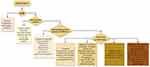 |
Figure 5 Algorithm for head plaques finding. |
Data on the case of CP, current treatments include oral and topical corticosteroids, glucocorticoids combined with cyclophosphamide, anti-CD20 monoclonal antibodies, and etc. Thalidomide may have inhibited the growth of plasma cells by decreasing the secretion of IL-6, thus slowing down the disease progression.13 Treatments for CP are limited. Although intralesional corticosteroids are moderately beneficial,24 other studied treatments with most either work in only certain cases or not at all. The clinical course of CP is usually chronic. A few cases are complicated or develop into malignant tumors. As for prognostic factors, it has been reported that high IgG levels of greater than 5000mg/dL and high plasma cell counts in bone marrow have been considered as poor prognostic indicators.25 If the patient is diagnosed with CP without systemic symptoms, the clinician is proposed to conduct a thorough evaluation for systemic disease and a close follow-up, to rule out any extracutaneous involvement or malignant transformation.
Conclusion
CP mainly presents a chronic course, but the recurrent rash seriously affects the patient’s quality of life. The lesions in our case are characterized by systemic nodules and head plaques, which are easily misdiagnosed as atopic dermatitis or prurigo nodularis, suggesting that when facing patients with head plaques, serum IgG4 should be checked to exclude CP.
Ethics Statement
The publications of images were included with the patient’s consent.
Consent Statement
The patient had given written informed consent for the publication of her clinical details. Institutional approval is not required for this case study.
Acknowledgments
The authors would like to thank the patients recruited for participation in this study. Lu Wei and Jialin Zhang are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Guangdong Medical Science and Technology Research Fund (NO. B2022220 and NO. A2020614).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wagner G, Rose C, Klapper W, et al. Cutaneous and systemic plasmocytosis. J Dtsch Dermatol Ges. 2013;11(12):1161–1167. doi:10.1111/ddg.12190
2. Wu J, Lu AD, Zhang LP, et al. 儿童核心结合因子相关性急性髓系白血病疗效及预后因素分析 [Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2019;40(1):52–57. Chinese. doi:10.3760/cma.j.issn.0253-2727.2019.01.010
3. Chantachaeng W, Chularojanamontri L. Cutaneous plasmacytosis: a case report and review of pulmonary findings. Dermatol Rep. 2011;3(3):e39. doi:10.4081/dr.2011.e39
4. Yetgin S, Uç A, Ozbek N, et al. Reactive plasmacytosis and plasmacytic skin infiltration in a patient. Eur J Haematol. 1995;55(2):131–132. doi:10.1111/j.1600-0609.1995.tb01823.x
5. Haque M, Hou JS, Hisamichi K, et al. Cutaneous and systemic plasmacytosis vs. cutaneous plasmacytic castleman disease: review and speculations about pathogenesis. Clin Lymphoma Myeloma Leuk. 2011;11(6):453–461. doi:10.1016/j.clml.2011.07.004
6. Krishnaram AS, Ck S, Sivakumar A. Diffuse cutaneous plasmacytosis masquerading as leonine facies with novel dermoscopic findings. Int J Dermatol. 2023;62(5):709–711. doi:10.1111/ijd.16654
7. Yashiro A. A kind of plasmacytosis: primary cutaneous plasmacytoma? Jpn J Dermatol. 1976;86:910.
8. Kitamura K, Tamura N, Hatano H, et al. A case of plasmacytosis with multiple peculiar eruptions. J Dermatol. 1980;7(5):341–349. doi:10.1111/j.1346-8138.1980.tb01981.x
9. Kanbe N, Kurosawa M, Akimoto S, et al. Systemic plasmacytosis with deposition of interleukin (IL)-6 and elevated expression of IL-6 mRNA in the skin lesions. Br J Dermatol. 1998;138(4):721–723. doi:10.1046/j.1365-2133.1998.02206.x
10. Leonard AL, Meehan SA, Ramsey D, et al. Cutaneous and systemic plasmacytosis. J Am Acad Dermatol. 2007;56(2 Suppl):S38–S40. doi:10.1016/j.jaad.2006.05.019
11. Jayaraman AG, Cesca C, Kohler S. Cutaneous plasmacytosis: a report of five cases with immunohistochemical evaluation for HHV-8 expression. Am J Dermatopathol. 2006;28(2):93–98. doi:10.1097/01.dad.0000181107.08791.87
12. Kodama A, Tani M, Hori K, et al. Systemic and cutaneous plasmacytosis with multiple skin lesions and polyclonal hypergammaglobulinaemia: significant serum interleukin-6 levels. Br J Dermatol. 1992;127(1):49–53. doi:10.1111/j.1365-2133.1992.tb14827.x
13. Fang S, Shan K, Chen AJ. Cutaneous and systemic plasmacytosis on the face: effective treatment of a case using thalidomide. Oncol Lett. 2016;11(3):1923–1925. doi:10.3892/ol.2016.4140
14. Jain S, Hede RV, Khopkar US. Cutaneous plasmacytosis with mast cell infiltration. Indian J Dermatol Venereol Leprol. 2020;86(1):91–95. doi:10.4103/ijdvl.IJDVL_716_17
15. Han XD, Lee SSJ, Tan SH, et al. Cutaneous plasmacytosis: a clinicopathologic study of a series of cases and their treatment outcomes. Am J Dermatopathol. 2018;40(1):36–42. doi:10.1097/dad.0000000000000907
16. Merluzzi S, Frossi B, Gri G, et al. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood. 2010;115(14):2810–2817. doi:10.1182/blood-2009-10-250126
17. Honda R, Cerroni L, Tanikawa A, et al. Cutaneous plasmacytosis: report of 6 cases with or without systemic involvement. J Am Acad Dermatol. 2013;68(6):978–985. doi:10.1016/j.jaad.2012.11.031
18. Suh JH, Kim HY, Lee JH, et al. Cutaneous plasmacytosis showing a neuronal involvement in a 35-year-old female. Ann Dermatol. 2020;32(2):174–176. doi:10.5021/ad.2020.32.2.174
19. Brezinski EA, Fung MA, Fazel N. Cutaneous plasmacytosis with perineural involvement. Case Rep Dermatol Med. 2014;2014:840845. doi:10.1155/2014/840845
20. Miyagawa-Hayashino A, Matsumura Y, Kawakami F, et al. High ratio of IgG4-positive plasma cell infiltration in cutaneous plasmacytosis--is this a cutaneous manifestation of IgG4-related disease? Hum Pathol. 2009;40(9):1269–1277. doi:10.1016/j.humpath.2009.01.013
21. Tokura Y, Yagi H, Yanaguchi H, et al. IgG4-related skin disease. Br J Dermatol. 2014;171(5):959–967. doi:10.1111/bjd.13296
22. Yamaguchi H, Moriki M, Ito T, et al. Cutaneous plasmacytosis as a skin manifestation of IgG4-related disease. Eur J Dermatol. 2013;23(4):560–562. doi:10.1684/ejd.2013.2113
23. Kato K, Satoh T, Tanaka-Fujimoto T, et al. IgG4-positive cells in skin lesions of cutaneous and systemic plasmacytosis. Eur J Dermatol. 2013;23(2):255–256. doi:10.1684/ejd.2013.1962
24. Lu PH, Shih LY, Yang CH, et al. Cutaneous plasmacytosis: a clinicopathologic study of 12 cases in Taiwan revealing heterogeneous underlying causes. Int J Dermatol. 2015;54(10):1132–1137. doi:10.1111/ijd.12694
25. Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22(1):21–30. doi:10.1007/s10165-011-0571-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.