Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Cutaneous Methotrexate-Related Epstein–Barr Virus-Positive Diffuse Large B-Cell Lymphoma in a Patient with Granulomatous Cutaneous T-Cell Lymphoma: A Case Report and Literature Review
Authors Kositkuljorn C, Rutnin S , Rattananukrom T , Puavilai T , Khiankaew B, Boonsakan P, Iamsumang W
Received 2 May 2023
Accepted for publication 3 August 2023
Published 15 August 2023 Volume 2023:16 Pages 2229—2235
DOI https://doi.org/10.2147/CCID.S419534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Chaninan Kositkuljorn,1 Suthinee Rutnin,1 Teerapong Rattananukrom,1 Teeraya Puavilai,2 Burana Khiankaew,3 Paisarn Boonsakan,3 Wimolsiri Iamsumang1
1Division of Dermatology, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Division of Hematology, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 3Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Wimolsiri Iamsumang, Division of Dermatology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Rajthevi, Bangkok, 10400, Thailand, Tel +662-201-1141, Fax +662-201-1211, Email [email protected]
Abstract: Methotrexate-related lymphoproliferative disorders (MTX-LPDs) are immunodeficiency diseases following methotrexate (MTX) administration, mainly occurring in rheumatoid arthritis patients. Although uncommon, MTX-LPDs have been reported in some patients with psoriasis, dermatomyositis, and cutaneous T-cell lymphoma (CTCL) who received MTX. Granulomatous mycosis fungoides (GMF) is a rare subtype of cutaneous T-cell lymphoma, where MTX is one of the treatment options in recalcitrant cases. Herein, we report a case of a 72-year-old female patient with GMF who additionally developed cutaneous Epstein–Barr virus (EBV)-positive diffuse large B-cell lymphoma (DLBCL) during MTX treatment. According to the 5th edition of the WHO classification of Haematolymphoid Tumors (WHO-HAEM), this condition is currently categorized as “lymphoma arising in immunodeficiency/dysregulation”. In this article, we also reviewed published literature on cutaneous MTX-LPDs in the setting of CTCL. This entity should be considered in cases of new, atypical skin nodules and/or plaques in CTCL patients receiving long-term MTX treatment.
Keywords: cutaneous T-cell lymphoma, granulomatous mycosis fungoides, methotrexate, methotrexate-related lymphoproliferative disorders
Introduction
Methotrexate (MTX) is widely used as a standard therapeutic medication for several inflammatory conditions including rheumatoid arthritis (RA) and psoriatic arthritis. It also appears to be an effective treatment for cutaneous T-cell lymphoma (CTCL). However, unfavorable side effects resulting from MTX have been occasionally reported.1 MTX-related lymphoproliferative disorders (MTX-LPDs) are among the life-threatening adverse reactions that occur in immunosuppressed patients using MTX.2 These diseases can present as benign lymphoid proliferation or malignant lymphoma and can affect either nodal or extranodal sites (eg, gastrointestinal tract, skin, oral cavity, and lungs), or both.3–5 MTX-LPDs have been found predominantly in RA patients treated with MTX; nonetheless, they have been sometimes reported in the context of CTCL.6–10 To raise awareness of MTX-LPDs, we herein report a rare case of cutaneous MTX-related Epstein–Barr virus (EBV)-positive diffuse large B-cell lymphoma (DLBCL) in a patient with known granulomatous mycosis fungoides (GMF). A literature review regarding cutaneous MTX-LPDs in individuals with CTCL was also performed to better characterize these rare conditions.
Case Presentation
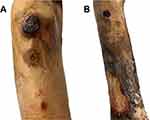
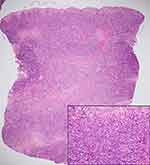
A 72-year-old female with type II diabetes and hypertension first presented in January 2020 with a 1-year duration of chronic progressive indurated erythematous plaques on both legs, with ulceration on the right leg (Figure 1A). Skin biopsy showed dermal atypical lymphoid infiltrate with granulomatous reaction (Figure 1B), positive on immunohistochemistry for CD2, CD3, CD4, CD5, and CD7 and negative for CD8, CD20, CD30, CD34, and CD56. In-situ hybridization showed the absence of Epstein–Barr virus-encoded small RNA (EBER) in atypical lymphoid cells. T-cell receptor gene rearrangement defined monoclonal proliferation. Complete staging work-up including computed tomography scan, biopsy of enlarged inguinal nodes, and bone marrow biopsy demonstrated no overt evidence of systemic lymphoma. GMF stage IB was therefore diagnosed. Although she received 10 sessions of radiotherapy in March 2020, the lesions progressed (GMF stage IIB). MTX, starting at 5 mg/week, and acitretin 10 mg/day were commenced. Interferon-α 3 million units 3 times/week was discontinued after 2 months due to intolerance of adverse effects. MTX was further increased to 25 mg/week without improvement.
Twenty-seven months following MTX initiation, at a dosage of 15 mg/week, the patient reported an eruption of a round erythematous plaque on her right leg and a nodule on the left leg, measuring 2–3 cm in diameter with ulceration and central necrotic crust (Figure 2A and B). This was in addition to preexisting large brownish ulcerative plaques on her lower legs (Figure 2B). She reported neither systemic symptoms nor fever. Oral administration of a retinoid derivative and systemic psoralen and ultraviolet A (RePUVA) was added but ineffective. A separate pathological process was suspected. Differential diagnoses included mycosis fungoides with large cell transformation, MTX-LPDs, primary cutaneous diffuse large B-cell lymphoma, leg type (PCLBCL-LT), anaplastic large cell lymphoma, squamous cell carcinoma, and cutaneous metastasis.
Biopsy from the new nodule on her left lower leg showed dense, diffuse atypical monomorphous large lymphoid infiltrate involving the entire dermis (Figure 3). The atypical cells were strongly positive for CD20 and EBER in-situ hybridization. Additional immunohistochemistry identified expression of CD30, MUM1, and Ki-67 (>90%), but not CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD56, LANA1, and BCL6 stains (Figure 4). Computed tomography imaging of the chest and abdomen revealed no evidence of systemic involvement. The diagnosis of cutaneous MTX-related EBV-positive DLBCL in a patient with GMF stage IIB was hence established. MTX treatment was promptly discontinued; however, new nodules continued developing in the following 2 weeks. Therefore, R-CHOP chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) was scheduled. Although partial remission was achieved, unfortunately, the patient passed away due to COVID-19 pneumonia with respiratory failure after the fifth cycle of chemotherapy.
Discussion
MTX-LPDs can involve nodal and/or extranodal sites. Approximately 50% of the cases are found to have an extranodal involvement (gastrointestinal tract, skin, oral cavity, and lungs among others).3,11 The diseases are predominantly B-cell driven, with DLBCL being the most common histologic subtype (50–66%), followed by Hodgkin lymphoma (10–30%).3,12,13 Other reported lymphoproliferative disorders include polymorphic B-cell LPD (20%), reactive lymphadenitis (10%), peripheral T-cell lymphoma (4%), follicular lymphoma (3%), and natural killer/T-cell LPD (2.1%).3,12,13 EBV is associated in nearly half of the reported cases (40%).14 Additionally, some studies also reported that EBV-positive MTX-LPDs correlated with an increased incidence of spontaneous regression after MTX discontinuation.13–15
The 5th edition of the WHO classification of Haematolymphoid Tumors (WHO-HAEM) currently categorizes MTX-LPDs as “lymphoid proliferations and lymphoma arising in immunodeficiency/dysregulation”. This standardized nomenclature refers to LPDs that arise in immunodeficiency or immunodysregulation from various causes. There are three criteria: 1) histological diagnosis of lymphoproliferative disorders; 2) presence or absence of one or more oncogenic viruses (EBV or Kaposi’s sarcoma herpesvirus/human herpesvirus 8); 3) immunodeficiency background, eg, primary immunodeficiencies, HIV infection, post transplantation, and other iatrogenic immunodeficiencies.16 The cutaneous presentation and histologic findings of B-cell lymphoproliferative disease in our patient could potentially be considered PCLBCL-LT, Epstein–Barr virus (EBV)-positive DLBCL, or MTX-LPDs. Nonetheless, due to the limited skin involvement and the patient’s immunosuppressive setting, the diagnosis of cutaneous MTX-related EBV-positive DLBCL is the most appropriate diagnosis.16
Although cutaneous MTX-LPDs have been best characterized in patients with RA, they have also been noted in patients with dermatomyositis, CTCL, and non-rheumatoid peripheral arthritis treated with MTX.17 Based on previous literature, cutaneous MTX-related EBV-positive DLBCL following a history of T-cell lymphoma is relatively rare as there are only 7 published cases (Table 1).6–10 The rarity could be explained by the fact that CTCL is a much less common disease than RA.18 All patients were diagnosed with erythrodermic CTCL (4 Sézary syndrome and 3 erythrodermic mycosis fungoides). To the best of our knowledge, this is the first reported case with GMF who secondarily developed another cutaneous lymphoma related to MTX treatment. The association between CTCL and B-cell lymphoproliferative disorders after MTX therapy still needs to be elucidated. It is believed that abnormal proliferation of malignant T-cells combined with an immunosuppressive state associated with MTX might contribute to promoting B-cell proliferation.6 This can be supported by the hypothesis that MTX would reduce host immunosurveillance of EBV-infected B-cells.5 Additionally, due to the patient’s age, immune senescence could potentially also explain the development of MTX-LPDs in this case.16 However, another possibility that should be kept in mind is the fact that patients with mycosis fungoides themselves are at increased risk of developing secondary B-cell malignancies.19
 |
Table 1 Clinical and Laboratory Characteristics of Cutaneous Methotrexate-Related Diffuse Large B-Cell Lymphoma in the Patients with Known Cutaneous T-Cell Lymphoma |
Of 8 CTCL patients with cutaneous MTX-LPDs, including ours, the mean age of onset was 72.6 years old. The mean duration of MTX introduction to the MTX-LPDs diagnosis was 23.8 months (range 2–66 months). MTX dosage at diagnosis ranged from 15 to 40 mg weekly. The most frequent cutaneous morphology was nodule/tumor (n = 7), followed by plaque (n = 2) and papule (n = 1). Lesions can be either multiple (n = 5) or solitary (n = 3), while most were ulcerated (6/8). The most common anatomical location was the lower limb (n = 3). Histological analysis compatible with DLBCL was found in all patients, and EBER expression was demonstrated in 87.5% (n = 7). Once diagnosed, MTX was immediately withdrawn in all previous seven cases. Four patients experienced spontaneous regression, while 3 patients required further treatment including R-CHOP chemotherapy (n = 2) and rituximab (n = 1). After treatment, all patients achieved clinical remission without relapse, with a mean follow-up time of 42.4 months (6–96 months). In the present case, we had a limitation of a short follow-up period as the patient expired after the fifth cycle of chemotherapy, thus the treatment outcome cannot be completely evaluated.
In terms of management, there are still no consensus guidelines for cutaneous MTX-LPDs. Nevertheless, treatment is primarily based on MTX withdrawal, with spontaneous remission observed in 60% to 88% of the cases.10,17,20 In most cases, the lesions significantly improved within 1 to 3 months (ranging from 15 days to 5 months) after MTX discontinuation.10,17,20 Chemotherapy regimens with or without rituximab (CHOP or R-CHOP) are recommended for patients with treatment failure or progressive disease.
To conclude, we report a case of cutaneous MTX-related EBV-positive DLBCL in a setting of GMF. When CTCL patients receiving an immunosuppressant, particularly MTX, develop new ulcerated nodule(s) and/or plaque(s) following apparent response to treatment, investigations including skin biopsy are crucial to exclude MTX-LPDs. Early diagnosis is clinically important since modifying the treatment regimen with MTX discontinuation would allow a favorable outcome.
Abbreviations
CTCL, cutaneous T-cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone; COVID-19, Coronavirus disease 2019; DLBCL, diffuse large B-cell lymphoma; EBER, Epstein–Barr virus-encoded small RNA; EBV, Epstein–Barr virus; GMF, granulomatous mycosis fungoides; HIV, human immunodeficiency virus; LPDs, lymphoproliferative disorders; MTX, methotrexate; MTX-LPDs, methotrexate-related lymphoproliferative disorders; PCLBCL-LT, primary cutaneous diffuse large B-cell lymphoma, leg type; RA, rheumatoid arthritis; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone; RePUVA, retinoid derivative and systemic psoralen and ultraviolet A; WHO-HAEM, WHO classification of Haematolymphoid Tumors.
Ethics Approval and Informed Consent
The patient had given written informed consent for the publication of her clinical details and accompanying images. Institutional approval is not required for this case study.
Funding
The authors received no financial support for this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–516. doi:10.1016/j.ejmech.2018.09.027
2. Solomon DH, Glynn RJ, Karlson EW, et al. Adverse effects of low-dose methotrexate: a randomized trial. Ann Intern Med. 2020;172(6):369–380. doi:10.7326/M19-3369
3. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322–331.
4. Kojima M, Itoh H, Hirabayashi K, et al. Methtrexate-associated lymphoproliferative disorders. A clinicopathological study of 13 Japanese cases. Pathol Res Pract. 2006;202(9):679–685. doi:10.1016/j.prp.2006.05.007
5. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943–1949. doi:10.1200/JCO.1996.14.6.1943
6. Curry JL, Prieto VG, Jones DM, Vega F, Duvic M, Diwan AH. Transient iatrogenic immunodeficiency-related B-cell lymphoproliferative disorder of the skin in a patient with mycosis fungoides/Sezary syndrome. J Cutan Pathol. 2011;38(3):295–297. doi:10.1111/j.1600-0560.2009.01459.x
7. Rausch T, Cairoli A, Benhattar J, Spring P, Hohl D, de Leval L. EBV+ cutaneous B-cell lymphoproliferation of the leg in an elderly patient with mycosis fungoides and methotrexate treatment. APMIS. 2013;121(1):79–84. doi:10.1111/j.1600-0463.2012.02939.x
8. Ingen-Housz-Oro S, Ortonne N, Cordel N, et al. Epstein-Barr virus-associated B-cell lymphoproliferative disorder in a patient with Sezary syndrome treated by methotrexate. Br J Dermatol. 2016;175(2):430–433. doi:10.1111/bjd.14602
9. Maderal AD, Malone JC, Callen JP. Methotrexate-Associated B-Cell Lymphoproliferative Disease in a Patient With Cutaneous T-Cell Lymphoma. JAMA Dermatol. 2018;154(4):490–492. doi:10.1001/jamadermatol.2017.6062
10. Delaleu J, Maubec E, Rodrigues F, et al. Methotrexate-induced Primary Cutaneous Diffuse Large B-cell Lymphoma in Patients with Erythrodermic Cutaneous T-cell Lymphoma. Acta Derm Venereol. 2020;100(15):adv00226. doi:10.2340/00015555-3554
11. Gion Y, Iwaki N, Takata K, et al. Clinicopathological analysis of methotrexate-associated lymphoproliferative disorders: comparison of diffuse large B-cell lymphoma and classical Hodgkin lymphoma types. Cancer Sci. 2017;108(6):1271–1280. doi:10.1111/cas.13249
12. Ichikawa A, Arakawa F, Kiyasu J, et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol. 2013;91(1):20–28. doi:10.1111/ejh.12116
13. Tokuhira M, Saito S, Okuyama A, et al. Clinicopathologic investigation of methotrexate-induced lymphoproliferative disorders, with a focus on regression. Leuk Lymphoma. 2018;59(5):1143–1152. doi:10.1080/10428194.2017.1369073
14. Kaji D, Kusakabe M, Sakata-Yanagimoto M, et al. Retrospective analyses of other iatrogenic immunodeficiency-associated lymphoproliferative disorders in patients with rheumatic diseases. Br J Haematol. 2021;195(4):585–594. doi:10.1111/bjh.17824
15. Ohkura Y, Shindoh J, Haruta S, et al. Primary Adrenal Lymphoma Possibly Associated With Epstein-Barr Virus Reactivation Due to Immunosuppression Under Methotrexate Therapy. Medicine. 2015;94(31):e1270. doi:10.1097/MD.0000000000001270
16. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: lymphoid Neoplasms. Leukemia. 2022;36(7):1720–1748. doi:10.1038/s41375-022-01620-2
17. Shimizu S, Inokuma D, Murata J, et al. Cutaneous manifestations of methotrexate-associated lymphoproliferative disorders: report of two cases and a review of the literature. Acta Derm Venereol. 2015;95(3):366–367. doi:10.2340/00015555-1951
18. Dobos G, Pohrt A, Ram-Wolff C, et al. Epidemiology of Cutaneous T-Cell Lymphomas: a Systematic Review and Meta-Analysis of 16,953 Patients. Cancers. 2020;12(10):2921. doi:10.3390/cancers12102921
19. Barzilai A, Trau H, David M, et al. Mycosis fungoides associated with B-cell malignancies. Br J Dermatol. 2006;155(2):379–386. doi:10.1111/j.1365-2133.2006.07346.x
20. Satou A, Banno S, Kohno K, et al. Primary cutaneous methotrexate-associated B-cell lymphoproliferative disorders other than EBV-positive mucocutaneous ulcer: clinical, pathological, and immunophenotypic features. Pathology. 2021;53(5):595–601. doi:10.1016/j.pathol.2020.10.019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.