Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Cutaneous larva migrans: A One Health Perspective on Familial Infection Among Tourists Returning from Southeast Asia
Authors Sałamatin R , Knysz B, Paszta W , Lelonek E, Matos O , Wesołowska M
Received 4 July 2023
Accepted for publication 11 August 2023
Published 22 November 2023 Volume 2023:16 Pages 3375—3382
DOI https://doi.org/10.2147/CCID.S425885
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Rusłan Sałamatin,1,2,* Brygida Knysz,3 Wojciech Paszta,4 Edyta Lelonek,5 Olga Matos,6,7 Maria Wesołowska8,*
1Department of Microbiology and Parasitology, Faculty of Medicine. Collegium Medicum, Cardinal Stefan Wyszyński University in Warsaw, Warsaw, Poland; 2Department of General Biology and Parasitology, Medical University of Warsaw, Warsaw, Poland; 3Department of Infectious Diseases, Liver Diseases and Acquired Immune Deficiencies, Wrocław Medical University, Wrocław, Poland; 4Wrocław ZOO, Wrocław, Poland; 5Department of Dermatology, Venereology and Allergology, Wrocław Medical University, Wrocław, Poland; 6Medical Parasitology Unit, Group of Opportunistic Protozoa/HIV and Other Protozoa, Global Health and Tropical Medicine, Instituto de Higiene e Medicina Tropical, Universidade Nova de Lisboa, Lisboa, Portugal; 7Environmental Health Institute, Faculdade de Medicina da Universidade de Lisboa, Lisboa, Portugal; 8Department of Biology and Medical Parasitology, Wrocław Medical University, Wrocław, Poland
*These authors contributed equally to this work
Correspondence: Rusłan Sałamatin, Department of Microbiology and Parasitology, Faculty of Medicine. Collegium Medicum, Cardinal Stefan Wyszyński University in Warsaw, Wóycickiego 1/3, 01-938 Warsaw, Poland, Email [email protected] Maria Wesołowska, Department of Biology and Medical Parasitology, Wrocław Medical University, Mikulicza-Radeckiego 9, 50-345 Wrocław, Poland, Email [email protected]
Abstract: Cutaneous larva migrans (CLM) is a dermatosis caused by accidental infestation with animal hookworms and is widely distributed in tropical and subtropical regions. Humans become infected when their skin comes into contact with soil contaminated with dog faeces. The filariform larvae penetrate and burrow into human skin, causing a condition known as “creeping eruption”. We describe a case, well-documented by photos, of CLM infection in a family of three who returned from Thailand.
Keywords: Cutaneous larva migrans, CLM, nematoda, Ancylostoma, Poland, Thailand
Graphical Abstract:
Introduction
Cutaneous larva migrans (CLM) is a human dermatosis caused by infection with animal hookworms found in tropical and subtropical regions, particularly in Africa, South America, the Far East, and the South Pacific.1–3 These hookworms are absent from most arid zones and temperate climates, thus, in Europe, cases of CLM are reported in tourists returning from endemic areas, primarily from Malaysia, Thailand, Indonesia, Brazil, the Caribbean, and West Africa.4 CLM is caused by various animal hookworms, including Ancylostoma braziliense, A. caninum, and Uncinaria stenocephala. Dogs and cats defecate in soil or sand and excrete eggs, from which first-stage larvae (L1) hatch and later develop into the infective third-stage larvae (L3). This filariform larvae (L3) penetrate through human skin and cause CLM syndrome, which is characterized by visible tracks with red, painful, and swollen advancing ends, usually associated with intense itching.5,6 As the number of visitors to tropical countries increases, so does the number of tourists infected with hookworms.
We describe a case, well-documented by photos, of CLM infection in a family of three who returned from Thailand.
Case Presentation
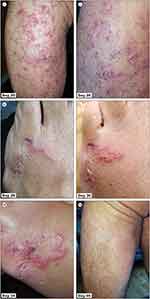
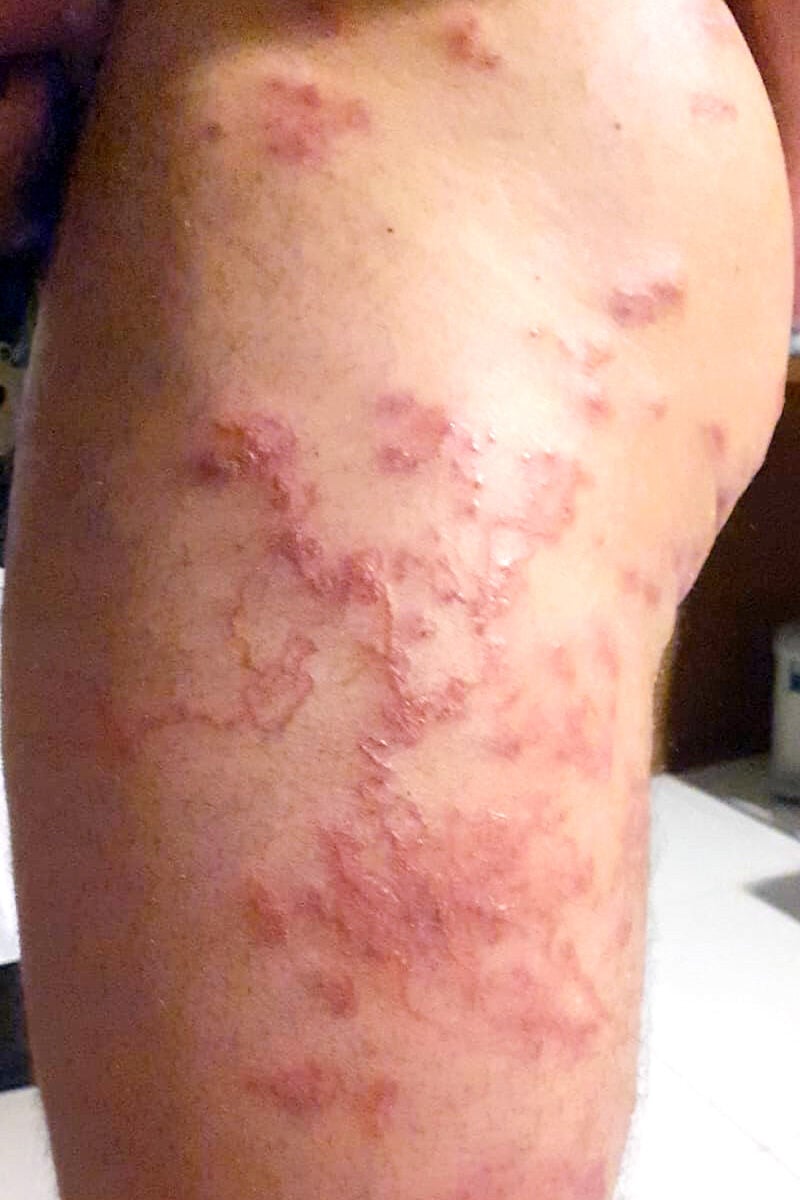
In January 2022, after a holiday in Thailand (Ko Phi Phi Island), a three-person family (male 44 years old, female 34, and a girl of 14 months) was admitted to the Department of Infectious Diseases in Wrocław, Poland, due to the presence of serpiginous cutaneous tracks several centimetres long. The first symptoms experienced by the family appeared in Thailand as a maculopapular rash, but which were, in fact, the places where the larvae had penetrated the skin. The first diagnosis in Thailand was dermatitis and allergy, with amoxicillin and antihistamines being prescribed. The most numerous changes occurred in the male (Figure 1). Three to seven days after the first symptoms, reddened bullous lumps followed by serpentine channels developed, accompanied by pruritus. The channels made by the larvae were filled with serum. The migration of the larvae caused intense itching and secondary bacterial infections due to intense scratching. After the larvae had died, the serpiginous tracks dried up and itchy scabs formed. The skin lesions in the woman and child, however, were significantly more limited (Figures 2 and 3).
Figure 1 Continued.
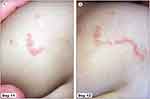 |
Figure 2 Characteristic skin lesions at Day 11 (A) and Day 12 (B) in 14-month-old girl. |
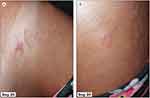 |
Figure 3 Typical serpiginous tracts with erythematous rash at Day 20 in a woman after a tourist trip to Thailand (A and B). |
After returning to Poland, on the eighteenth day of infection, the whole family received one dose each of pyrantel (the male received 1000 mg; the female, 750 mg; and the girl, 100 mg), followed by two courses of albendazole (1 × 400 mg/3 days) on day 19 and 33 of the invasion. On days 28 and 33 of the invasion, three new serpentine lesions appeared on the male’s thigh and foot as a result of the reactivation of dormant larvae. After two months, the lesions on the patients’ skin had completely disappeared. A chronology of the disease progression in the man is presented in the timeline in Figure 4.
 |
Figure 4 Timeline illustrating the chronology of the disease’s progression in the man. |
Routine blood tests were found to be normal, and no parasites were found in the stool samples of any of the family members. Based on the dermatological examination cutaneous larva migrans was diagnosed in all three of the family members.
Discussion
CLM, also known as “creeping eruption”, ground itch, or sandworms, is one of the more common dermatological disorders reported in returning travellers who are ill. Studies showed that hookworm-related CLM syndrome occurred in 2–70% of tourists who returned from endemic areas; however, there is little information about it in the literature.7 We describe the course of CLM, with detailed photographic documentation, in a family who returned from Ko Phi Phi Island, Thailand. To our knowledge, no photos have so far been published showing the course of an entire invasion, from the beginning of infection to recovery.
The family in the described case became infected probably on the beach through skin coming into direct contact with moist sand contaminated with filariform larvae (L3). The man, who had the most severe symptoms, probably became infected while lying on a towel on wet beach sand under a tree, so most of the larvae were on his right thigh. Infection by direct skin contact with contaminated objects, such as clothing or towels, has been reported by other authors.8
After the larvae enter the human body, they migrate between the epidermis and dermis’ germinal layer.5,6 Since the zoonotic larvae are unable to penetrate deeper into the dermis because they lack the appropriate enzymes, they produce highly pruritic, advancing erythematous vesicular lines of serpiginous eruptions, 1 to 5 cm long.
CLM must be distinguished from the condition larva currens, the dermatologic manifestation of strongyloidiasis. Both are associated with migratory serpiginous lesions which are erythematous, raised, and pruritic.9 The conditions can usually be clinically distinguished based on the speed of advancement; larva currens can progress at approximately 5 cm10 to 10 cm11 per hour, while the larval track of CLM progresses at approximately 1 to 2 cm per day.12
If pyogenic bacteria, such as Staphylococcus aureus or streptococci, enter along with the larvae, there may also be burning, erythema, and oedema.4 Humans are a dead-end host for zoonotic hookworms; that is, the larvae cannot complete their life cycle. During the course of the infection, some larvae remain dormant in the human subcutaneous tissue; this can lead to a reactivation of the infection after a few weeks or months. In our patient’s case, the dormant larvae became active after four weeks, producing new, serpentine tracts on the patient’s foot and thigh.
Zoonotic canine hookworms include Ancylostoma braziliense, A. caninum, A. ceylanicum, Uncinaria stenocephala; while feline A. braziliense, A. ceylanicum, U. stenocephala are primarily responsible for causing CLM.4,13 Stray dogs and cats are widespread in Thailand and they are potential reservoirs for zoonotic hookworms. Animals come in close contact with people in touristic areas, such as beaches or resort surroundings, and can contaminate the soil and sand with faeces containing the hookworms’ eggs.7,14–16 Larvae hatch in the soil in one to two days, moult, and then become infective filariform (third-stage) larvae. These larvae can survive three to four weeks in the soil and are able to penetrate human skin.
Reports have revealed that thousands of cats were abandoned on Ko Phi Phi Island in Krabi Province in Thailand during the COVID pandemic.17,18
Studies have shown that in Thailand 84.0% of tested soil samples were contaminated with parasite eggs/cysts, including 36% with Ancylostoma eggs.19 Kladkempetch et al, in a study carried out in Thailand, showed that the prevalence rate of hookworm in dogs was 26.4%, with the most prevalent species being A. ceylanicum (96.55%) and A. caninum (3.45%).13 Others authors have also confirmed that A. ceylanicum is the most common zoonotic hookworm in Thailand and in other parts of Asia.1,13
Conclusions
Tourists should be aware of how they can become infected with cutaneous larva migrans (CLM) and avoid direct bare skin exposure to damp soil and sand. Initial symptoms may not be characteristic and may therefore be misdiagnosed, delaying appropriate treatment, which can then lead to symptoms of the disease becoming exacerbated. Our case highlights the importance of proper diagnosis for suitable treatment. Patients need long-term observation.
Ethics and Consent
Written informed consent was provided by the patients to have the details of the case and accompanying images published. An ethical review and approval were not required to publish the case details, in accordance with local legislation and institutional requirements.
Acknowledgments
We extend our sincere thanks to the patients for generously sharing their photos, as their contribution has enriched our article and knowledge, and we are truly grateful for their cooperation. This work was partially financed by grants from Wrocław Medical University (SUBZ.A060.22.054) for one of the co-authors (MW). Additionally, we express our appreciation to Laurence Taylor for diligently proofreading the article, ensuring its accuracy and clarity.
Funding
This research received no external funding.
Disclosure
The authors report no conflicts of interest relating to this work.
References
1. Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354(2):119–130. doi:10.1056/NEJMoa051331
2. Maxfield L, Crane JS. Cutaneous Larva Migrans. StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507706/.
3. Stufano A, Foti C, Lovreglio P, et al. Occupational risk of cutaneous larva migrans: a case report and a systematic literature review. PLoS Negl Trop Dis. 2022;16(5):e0010330. doi:10.1371/journal.pntd.0010330
4. Traub RJ, Zendejas-Heredia PA, Massetti L, Colella V. Zoonotic hookworms of dogs and cats – lessons from the past to inform current knowledge and future directions of research. Int J Parasitol. 2021;51(13–14):1233–1241. doi:10.1016/j.ijpara.2021.10.005
5. Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351(8):799–807. doi:10.1056/NEJMra032492
6. Hochedez P, Caumes E. Hookworm-related cutaneous larva migrans. J Travel Med. 2007;14(5):326–333. doi:10.1111/j.1708-8305.2007.00148.x
7. Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8(5):302–309. doi:10.1016/S1473-3099(08)70098-7
8. Wesołowski R, Mila-Kierzenkowska C, Pawłowska M, Szewczyk-Golec K, Kałużna L, Wozniak A. Cutaneous larva migrans imported from a tropical trip – case report and literature review. Anna Agricul Environ Med. 2021;28(4):709–712. doi:10.26444/aaem/131600
9. Leder K, Weller PF. Strongyloidiasis. Available from: https://www.uptodate.com/contents/strongyloidiasis.
10. Fülleborn F. Hautquaddeln und “Autoinfektion” bei Strongyloidesträgern. Archiv für Schiffs. 1926;30:12.
11. Arthur RP, Shelley WB. Larva currens. AMA Arch Derm. 1958;78(2):186–190. doi:10.1001/archderm.1958.01560080044007
12. Liu LX, Weller PF, Strongyloidiasis and other intestinal nematode infections. Infect Dis Clin North Am. 1993;7(3):655–682. doi:10.1016/S0891-5520(20)30548-1
13. Kladkempetch D, Tangtrongsup S, Tiwananthagorn S. Ancylostoma ceylanicum: the neglected zoonotic parasite of community dogs in Thailand and its genetic diversity among Asian countries. Animals. 2020;10(11):2154. doi:10.3390/ani10112154
14. Mohd Zain SN, Rahman R, Lewis JW. Stray animal and human defecation as sources of soil-transmitted helminth eggs in playgrounds of Peninsular Malaysia. J Helminthol. 2015;89(6):740–747. doi:10.1017/S0022149X14000716
15. George S, Levecke B, Kattula D, et al. Molecular identification of hookworm isolates in humans, dogs and soil in a tribal area in Tamil Nadu, India. PLoS Negl Trop Dis. 2016;10(8):e0004891. doi:10.1371/journal.pntd.0004891
16. Esme. Stray dogs in Thailand: everything you need to know. Available from: https://www.jtgtravel.com/asia/thailand/stray-dogs-in-thailand/.
17. Maneechote P. Phi Phi cats “getting all the help they need” after plight goes viral. Available from: https://www.thaienquirer.com/32215/phi-phis-abandoned-cats-getting-all-the-help-they-need-after-plight-goes-viral/.
18. Anonymous. Thousands of cats stranded on Thailand’s Koh Phi Phi during pandemic. Available from: https://www.thaipbsworld.com/thousands-of-cats-stranded-on-thailands-koh-phi-phi-during-pandemic/.
19. Pinyopanuwat N, Kengradomkij C, Kamyingkird K, Chimnoi W, Suraruangchai D, Inpankaew T. Stray animals (dogs and cats) as sources of soil-transmitted parasite eggs/cysts in temple grounds of Bangkok Metropolitan, Thailand. J Trop Med Parasitol. 2018;41(2):15–20.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.