Back to Journals » Orthopedic Research and Reviews » Volume 14
Current Perspectives on the Management of Bone Fragments in Open Tibial Fractures: New Developments and Future Directions
Authors Farhan-Alanie MM , Ward J, Kelly MB, Al-Hourani K
Received 12 April 2022
Accepted for publication 2 August 2022
Published 12 August 2022 Volume 2022:14 Pages 275—286
DOI https://doi.org/10.2147/ORR.S340534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Muhamed M Farhan-Alanie,1 Jayne Ward,1 Michael B Kelly,2 Khalid Al-Hourani3
1Department of Trauma & Orthopaedics, University Hospital Coventry & Warwickshire, Coventry, UK; 2Department of Trauma & Orthopaedics, Southmead Hospital, Bristol, UK; 3Department of Trauma & Orthopaedics, Royal Infirmary of Edinburgh, UK
Correspondence: Muhamed M Farhan-Alanie, Email [email protected]
Abstract: Open tibial fractures may be associated with bone loss at the time of the injury or following surgical debridement of the fracture. This article discusses the various treatment options available and the latest developments surrounding the management of free bone fragments in open tibial fractures.
Keywords: tibia, open, fracture, bone, fragments, trauma, devitalized, devitalised
Introduction
Over the years significant progress has been achieved in the management of open tibial fractures as reflected by the temporal trend of improving outcomes.1–3 Nonetheless, open tibial fractures continue to remain one of the most challenging injuries to manage in orthopaedic traumatology. This is primarily due to the relative paucity of soft tissues around the tibia.4 These injuries can be further complicated by the presence of bony comminution and fragment devitalisation resulting from high energy mechanisms of injury.4 These fragments of bone may be extruded at the time of injury owing to the tibia’s relatively subcutaneous position although in most cases they remain in-situ.5,6 These fragments are encountered by the surgeon during the debridement stage of open fracture management. During this step, the viability of tissues in the zone of injury is evaluated and generally, all devitalised tissue is discarded to reduce the risk of infection and promote healing.7 Traditionally, this has included cortical segments of bone which fail the “tug test” – a subjective assessment of the bone fragment’s viability measured by the strength of soft tissues and periosteum connecting the fragment to the main bone. Loose bone fragments which detach with minimal force, such as with the use of surgical forceps or two fingers, are typically considered to be devitalised.8 Along with other forms of devitalised tissues, these are also believed to be a potential nidus of infection thus commonly discarded. Many believe discarding these fragments and managing the consequent deformity is preferable to treatment of potential osteomyelitis. However, discarding devitalised cortical bone fragments, particularly if large and mechanically relevant, may compromise fracture stability, alignment of the bone, and limb length which can negatively affect patient’s functional outcomes as well as necessitate additional surgical procedures to manage the resulting bone defect and any associated deformity.6,9–13 It is also worth mentioning that any further procedure is associated with an increased risk of patient morbidity13 and cost from both a health and societal perspective. Therefore, the retention of free bony fragments may offer several advantages to both patient and society.
Recently, the common practice of discarding devitalised cortical fragments is being increasingly challenged.14–17 Several methods of disinfecting bone fragments prior to their re-incorporation have previously been published.16,18–22 It is important to balance the effects of these techniques on reducing bacterial load and risk of infection with cell viability and the bone’s remaining osteogenic potential to support callus formation.
The management of free bone fragments in open tibial fractures continues to be a topic of debate. There is a paucity of reviews summarising the literature on the management of open tibial fractures involving free bone fragments. In this article, we discuss the most up-to-date management options of free bone fragments in the context of open tibial fractures, and highlight areas for future research. Studies included in this review have been selected based on a variety of factors including relevance to this article’s topic, material novelty, year of publication, participant sample size, duration of follow-up period, and those most cited for this topic.
Discard
It has long been postulated that retention of devitalised fragments may increase the risk of infection and are therefore discarded. This remains the consensus opinion in the orthopaedic community, albeit without clear high-quality evidence for this, or based on outdated evidence.9,10
It is clear that in severely contaminated or severely comminuted fractures (such as in ballistic injuries), fragment retention is not a suitable option, and therefore discarding said fragments remains the logical and perhaps only option available to the treating surgeon. However, further reconstructive procedures are then needed to correct any resulting segmental or non-segmental defects, which are associated with long treatment times and potential complications to patients.
Distraction Osteogenesis
As an alternative to circular frames, intramedullary nails can be used to facilitate distraction osteogenesis23–25 including for traumatic related bone defects.26 Although not a novel method, intramedullary nail devices have recently evolved to allow lengthening adjustment involving a magnetic mechanism operated via a remote control.27,28 Their application avoids the potential morbidity associated with the use of external fixators including pin tract infection, adjacent joint stiffness, soft tissue contractures and tethering,29 as well as reducing the risk of fracture following frame removal.30
Bone Defect <5cm
For a small defect of 3–5cm, acute shortening and compression-distraction osteogenesis can be achieved in a single stage procedure involving combination of internal fixation with a plate and an intramedullary lengthening nail. After approximating the fracture ends and stabilising the fracture site using plate fixation, an osteotomy in the metaphysis farthest from the site of the fracture is performed and a lengthening intramedullary nail is then inserted. Progressive distraction is enabled through the nail’s lengthening mechanism until the bone is restored to its original length.31 This technique avoids the need for docking sites which are often the rate limiting step to achieving union.32,33
Bone Defect >5cm
Vertical segment bone transport is recommended for the management of bone defects exceeding approximately 5cm.31,34 In addition to application of lengthening intramedullary nails to facilitate distraction osteogenesis in shortened limbs,23 these devices are also being used in combination with external fixators or internal plates to enable vertical segmental bone transport techniques. The application of a magnetic intramedullary nail compared to a conventional intramedullary nail for this technique helps reduce the total number of pins required if an external fixator is used, thereby reducing the risk of associated pin-site related infections. However, the combination of a magnetic nail and conventional internal plate enables control of the floating segment while maintaining limb alignment respectively, avoiding the requirement for external fixators altogether. This technique eliminates the risk of pin-site related infections as well as the possibility of pin tract infections communicating to the medullary canal and colonising the intramedullary nail.35 This is also highlighted by Simpson et al30 who cautioned against the use of external fixation along with intramedullary nailing in open fracture patients due to the possibly increased risk of deep infection. Furthermore, it is also technically more difficult to site the pins of the external fixator with an intramedullary nail in situ.
There are currently very limited published data in the literature on the combined application of magnetic nails and conventional plates in vertical segment bone transport for open fracture patients. In a case report involving an open tibial fracture patient (Gustilo-Anderson type IIIB) who developed non-union that led to implant failure approximately two years following his index procedure, revision surgery was performed using plate-assisted bone segment transport with the NuVasive PRECICE 2 intramedullary limb lengthening system.36 The bone defect measured to be 2.5 cm in size following removal of the failed metalwork. With the aid of a Synthes 16-hole variable-angle limited contact plate to span the bone defect, a corticotomy was made away from the non-union site following which a NuVasive PRECICE 2 intramedullary nail was inserted with the distal interlocking screw placed in the transport segment.36,37 Limb lengthening was commenced from post-operative days 7 to 32 at 0.75mm per day via the telescoping rod of the nail. A similar technique has also been described in a small case series which included three open fracture patients with tibial bone defect sizes ranging between 4.8 and 10 cm.34 This group of authors recommend the use of provisional external fixation to maintain limb length and alignment at the time of plating. Their study results reported that two patients achieved union at six and nine months post-operatively while the third patient was experiencing delayed union at the docking site at the time of publication.
Although certain lengthening nails such as the NuVasive PRECICE 2 are bidirectional enabling both push and pull bone transport, the former method can help reduce the consolidation time after lengthening through protection of the regenerate from collapse, deviation or fracture,30,38 and also allows relatively earlier full weight bearing.34 The bidirectional feature also allows alternating cycles of distraction and compression in the distracted bone defect gap, termed the accordion manoeuvre.39 This technique is believed to help enhance bone regeneration in cases involving absent or delayed callus formation in limb lengthening as well as help achieve union at the docking site.33 Furthermore, magnetic intramedullary nails can also be used to undertake trifocal bone transport with the assistance of cables40 which potentially has the advantage of shortening treatment time.41 However, there are also several limitations associated with the use of this described technique. The length of the plate may be a limiting factor and could influence the level where the corticotomy site is performed which should ideally be within the metaphyseal region of the bone.42 Another potential limitation is the stroke distance of the intramedullary nail implant which represents the lengthening capability, and reflects the maximum possible distance a bone segment can be transported without requiring to reshorten or relengthen the nail.34 In addition, lengthening intramedullary nails including those featuring magnetically operated mechanisms have been associated with a variety of complications themselves. Depending on their generation, these include nail bending or fracture,28,43 superficial infection around subcutaneous receivers,44 and failure of the lengthening mechanism to allow further distraction as well as acute over-distraction.45–48 Recently in early 2021, several lengthening intramedullary nails have been recalled and suspended from use in the United Kingdom and Republic of Ireland due to concerns relating to their unknown long-term biological safety profile as they have not undergone all biological assessments of the international standardised medical device evaluation process (ISO 10993–1:2018).49,50 Additional testing is currently ongoing to address the existing gaps and complete risk assessments relating to carcinogenicity, chronic toxicity, developmental toxicity, and reproductive toxicity. In addition, there have been reports of pain and bony abnormalities at the interface between the telescoping nail segments involving the PRECICE Stryde product. NuVasive also issued an urgent field safety notice in which they stated their devices were not indicated for individuals younger than 18 years of age, do not enable full weight bearing, and require removal within one year of implantation, in addition to other considerations and guidelines.51
3D Printing and Custom Implants
Advances in 3D printing technology have enabled the use of custom implants in orthopaedics for a variety of procedures including complex post-traumatic limb reconstruction for segmental bone loss.52–55 Data based on computed tomographic images obtained from the patient’s injured limb is processed to enable production of a custom implant. As the application of this technology is relatively new, there are limited studies currently present in the literature evaluating its effectiveness. The longest reported follow-up is five years and involves a patient who sustained an open tibial fracture and received a custom 3D-printed titanium cage truss as well as a standard intramedullary rod to facilitate ankle arthrodesis and salvage the limb in favour of amputation.55 Serial radiographs of this patient’s fracture site demonstrated bony ingrowth and lack of stress shielding. Due to the material used in their construction, these implants are mechanically robust and light weight permitting early motion and protected weight bearing.56,57 Furthermore, their construct configuration allows for each strut to be in compression and tension which promotes bone remodelling. The construct also features an open architecture which maximises the volume of bone graft that can be incorporated within the cage itself.58 Application of 3D-printed titanium cage trusses in conjunction with the Masquelet technique59 has recently been described for use in femoral defects,57 and helps tackle the associated potential complications of non-weight bearing.
Current research is now focusing on the use of bio-inks in 3D printing which allows inclusion and precise placement of cells, biomolecules, and biomaterials within the 3D structure.60 The resultant tissue engineered generated scaffold contains a microenvironment possessing osteogenic properties to support new bone formation. However, this technique is relatively more expensive than standard 3D printing given the additional tissue engineering processes involved. Also, the strength of biomaterials compared to titanium may not allow early weight bearing activities or provide the same degree of stability to enable bone regeneration, and further research in this area is required. Furthermore, there is typically an interim period for the design and production of custom implants before proceeding to surgery. However, most recently, in situ 3D bio-printing with the aid of a robotic arm containing a printing nozzle has been evaluated in an animal study where tibial bones were intentionally fractured with a resultant segmental bone defect prior to undergoing internal fixation with a titanium plate to maintain tibial strength during healing.61 Animals were sacrificed at 12 weeks post-operatively and significantly greater osteogenic effects were observed in the group which received a 3D bioprinted custom implant compared to controls. Post-operative CT scanning demonstrated that animals which received the 3D bioprinted custom implant possessed thick cortical bone tissues compared to the control group which had presence of gaps and cavities in the defect region, rough cortical bone surfaces and thin cortical bone tissues. The intervention group also had a significantly greater bone volume to total volume ratio implying large volume of newly-formed bone tissue. Spatial morphology of bone trabeculae was also analysed and showed higher numbers and thickness of trabeculae and lower separation implying this bone tissue had improved structure and mechanical strength, and more active osteogenesis.
Induced Membrane Technique
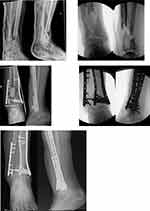
Pioneered over 30 years ago, this technique involves a two-stage procedure. Polymethyl methacrylate (PMMA) cement, typically antibiotic-loaded, is implanted at the site of the bone defect to act as a spacer for a period of approximately 6–8 weeks. A thick, well vascularised, pseudoperiosteum induced membrane then forms around the cement mantle which is carefully incised to allow extraction of the cement and substitution with bone graft62,63 (Figure 1). Indication for its application in trauma is typically for managing segmental bone defects ranging between 5 and 25 cm in length.64 Its advantages over vertical segment bone transport include dead space management and prevention of soft tissue invagination as well as reduction of bacterial counts where antibiotic-loaded cement is used.65,66 It may also be associated with potentially faster consolidation of the bone defect.63 Importantly, inconsistent clinical outcomes have been associated with this procedure67–70 which are likely due to differences in injury and host characteristics such as quality of the soft tissue envelope and patient comorbidities, as well as variations in performing the technique itself particularly in relation to spacer volume and composition (plain or antibiotic-loaded cement), fixation methods (plate, intramedullary nail, external fixator) and bone graft sources (iliac crest, reamer-irrigator-aspirator, nonautologous graft).71
Vascularised Bone Graft
Typically advocated for bone defect sizes exceeding approximately 5cm,72,73 vascularised bone grafting is another alternative surgical technique for managing traumatic bone defects. A number of vascularised bone grafts can potentially be harvested however the fibula is considered ideal for applications involving diaphyseal tibial bone defects. This is due to its dimensions, mechanical strength and potential for hypertrophy, as well as its predictable vascular supply which can be pedicled within the ipsilateral limb.74–76 Free vascularised bone grafts are relatively more technically challenging as the procedure involves microsurgical skills for the end-to-end anastomoses of both artery and vein at the recipient site.
The key advantage of this method for addressing bone defects is preservation of the graft’s blood supply. This enables the graft’s osteogenic potential to be maintained which allows it to incorporate at the recipient site by either primary or secondary bone healing rather than undergoing creeping substitution that is associated with nonvascularised bone graft.77 This distinction translates to relatively less graft resorption and initial loss of graft strength at the recipient site which is typically seen with nonvascularised bone graft78,79 thereby helping to reduce the risk of mechanical failure and infection.80 Limitations of this technique include graft stress fractures due to prematurely excessive weight bearing as well as anastomotic complications where a free vascularised bone graft is used.81 In a case series reporting on the outcomes of 21 patients following free vascularised fibular graft performed for Gustilo-Anderson III tibial fractures respectively, mean time to union was 19 and 20 weeks for the proximal and distal fibula, respectively.81
Retention
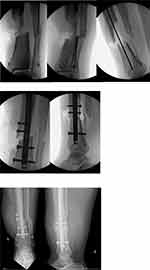
In the absence of severe contamination, devitalised fragments can be retained if these are deemed to be a mechanically relevant segment of bone by the surgeon. The pre-requisites to such a decision include orthoplastic involvement, a thorough debridement, and timely soft tissue coverage if required.82 Re-incorporating these fragments can help bridge bone defects, thereby allowing more circumferential bone on bone contact and subsequent increased construct stability (Figure 2). It is imperative that a combination of debridement and disinfection techniques are performed to reduce the bacterial load of these fragments prior to their re-incorporation at the fracture site. This can take place at an early or delayed stage (greater than one month16) either during the index or a subsequent procedure respectively.
Fragment Value
The merits of retaining each devitalised bone fragment must be carefully considered on an individual basis by the operating surgeon. The significance of a bone fragment is variable and its value lies along a spectrum. Although this assessment is relatively subjective, in general the significance of a fragment is associated with its characteristics as well as patient factors.6,83,84 The former focusses on the fragment’s value to biomechanical stability of the fracture whereas the latter relates to the patient’s biology. Patient factors are likely to include age, smoking status, and comorbidities such as peripheral vascular disease and diabetes mellitus. Biomechanical value of the fragments likely relate to size, cortical diameter involvement, and anatomical location from which it has detached from the tibia.85 Larger sized bone fragments are likely to have an integral role in providing mechanical stability of the fracture. Discarding these critical sized fragments is likely to substantially complicate any future reconstructive challenge.4 The location of the bone fragment is also relevant in judging the fragment’s value. Fragments which involve the metaphysis are usually considered high value as may contain critical ligamentous and tendinous attachments which serve important function. Similarly, osteoarticular fragments contain overlying cartilage which contributes to joint function and retention may help to reduce the risk of post-traumatic secondary osteoarthritis developing in the future.4 Fortunately, the most common site of involvement is the diaphysis.6
Bone defects have been subjectively and inconsistently described by surgeons using varying terms such as “massive defect” and “critical sized defect”.83–85 The currently accepted definition of a “critically-sized” defect is one which prevents spontaneous healing despite skeletal stabilisation, and further surgical intervention is required to achieve union.6,85 Although helpful, this definition is somewhat subjective and vague as the parameters which constitute a “critically-sized” defect in the tibia remain uncharacterised, and further investigation is required. Examples of previous parameters of “critical” size defects have included a bone defect greater than 1 cm in length and >50% cortical diameter involvement however applying these criteria to the subgroup of patients in the SPRINT study did not prove specific given 47% achieved union without further secondary intervention.84 In a different study of 40 open tibial fracture patients, the study authors attempted to determine the threshold size of a “critical bone defect”. Results demonstrated no patients achieved bony union if the fracture gap size exceeded 25mm, measured by calculating the average defect size on all four cortices on the radiograph.86
Recently, the OTA/AO fracture classification has been extended to include a classification scheme describing bone defects.87 In summary, bone defects can be broadly classified into one of three categories and then further classified into one of three subcategories giving a total of nine different possible combinations. In keeping with the existing alpha numeric scheme, categories are appended the suffix “D” followed by a number (1–3), and the three subcategories are then designated the letters A-C. This is described in further detail below.
D1 – Incomplete defect: These types of bone defects are incomplete and involve a maximum of three out of four cortices. The subcategories are defined by the transverse extent or percentage of cortical bone (A: <25% bone loss, B: 25 to <75% bone loss, C: >75% to 99% bone loss).
D2 – Subcritical/minor defect (<2 cm): These defects are defined by bone loss of less than 2 cm calculated by determining the mean value of the maxima and minima longitudinal lengths of the bone defect on both AP and lateral radiographs. The three subcategories for this bone defect are distinguished by the shape of the fracture ends (A: 2 oblique ends; B: 1 oblique and 1 transverse end; C: 2 transverse ends, ie, segmental defect).
D3 – Segmental/critical size defect (≥2 cm): These defects are segmental in configuration and subcategories define the size of the defect (A: 2 to <4 cm; B: 4 to <8cm; C: ≥8cm). Size should be assessed using the aforementioned technique.
It is important to mention that the most accurate time to assess and classify bone loss is following debridement, fracture reduction, preliminary stabilisation, and restoration of length to its original state.
Development of a classification for defining bone defects is progress in helping to unify and standardise terminology used amongst surgeons. This has a particularly useful application in research enabling improved reporting on the details of the bone defects under investigation allowing improved applicability of the research findings to real-life. Now that nine types of bone defects have been defined, it is important to research the outcomes associated with each of these and determine how they compare. Furthermore, it will enable comparison of which reconstruction methods are better suited for each bone defect type and their success rates.
Methods of Decontamination
Chemical and Mechanical Technique
In the face of a contaminated bone fragment deemed to be high-value and preferable to retain, it is essential to thoroughly disinfect the bone fragment prior to re-implantation. We advocate a two-part sequence consisting of both chemical and mechanical decontamination to reduce bacterial load as much as possible. Chemical decontamination should be performed using either 10% povidone-iodine (Betadine) or 4% chlorhexidine (Hibiclens).22 Subsequently, adjuvant mechanical decontamination should be performed using either a saline rinse or scrub with bristled sponge.
Auto-Sterilisation
“Auto-sterilisation” has also been used as an adjuvant technique in preservation and biological decontamination of free bone fragments in patients sustaining open fractures. This technique involves storage of the free bone fragments within a subcutaneous pouch of non-traumatised tissue at a site distant from the initial injury. Sufficient time is then allowed for the body’s immune system to help decontaminate these fragments prior to re-implantation.16 The average time to reimplantation is variable for this technique however has ranged between 6 and 18 weeks in the literature.16,88 Various studies involving different orthopaedic procedures have confirmed that free fragments, typically referred to as being devitalised in the context of an open fracture injury, still retain osteoinductive and osteoconductive properties when tested at a future date following a banking period in subcutaneous tissues.89–91 Following re-incorporation of the bone fragment, creeping substitution is commenced and time to complete graft incorporation depends on a variety of local and systemic factors.78
Improved Stability
Fixation of free bone fragments can help to improve the mechanical integrity of the tibia. This contributes to increasing the fracture’s overall stability which is known to play a critical role in enhancing the fracture healing process.92 This is particularly relevant in open tibial fractures given their associated high risk of non-union which has been reported to be as high as approximately 15% in Gustilo-Anderson IIIB and IIIC (tibial) fractures.93,94 However, fracture union relies on many biological factors in addition to a satisfactory mechanical environment.95 The health state of the various tissues in and around the fracture which constitute the “bone-healing unit”96 may be reflected by the presence and amount of devitalised bone. Discarding bone fragments may therefore not be conducive to the healing process and potentially considered a double whammy due to the added detrimental effect this may have on achieving union. Improved fracture stability also allows patients to weight bear and mechanically load their tibia relatively earlier which further promotes fracture healing as well as the maintenance of muscle and bone mass.97–100 Earlier weight bearing will also help reduce the risk of joint stiffness, prolonged hospital stay, and other complications associated with immobilisation and recumbency including venous thromboembolism.
It is also important to mention that reduced stability has been proposed to increase the risk of infection.101 It is plausible and believed that all inserted implants are colonised by bacteria at the time of surgery, eventually forming an inert and inactive biofilm on the implants surface. Instability of the implant, which may be influenced by the mechanical integrity of the fracture, is thought to disrupt the existing biofilm and convert this from an inert to an active state.101
These principles and theories are supported by the findings of the retrospective comparative cohort study by Al-Hourani et al which showed relatively fewer events of non-union, deep infection, and infection-associated flap failure in the patient group who underwent orthoplastic re-incorporation of devitalised bone although these findings were not statistically significant (2.3% versus 7.2%; 1/44 patients versus 5/69 patients, and 2.3% versus 10.1%; 1/44 patients versus 7/69, and 0% versus 5.8%; 0/44 patients versus 4/69, respectively; p > 0.05).14 Also, the possibility of selection bias cannot be excluded given the non-randomised study design.102 In a follow-up study17 comparing health-related quality of life between these patient groups, no statistically significant differences were found at a median follow-up of 3.8 years – EQ-5D: 0.743 (IQR 0.195) versus 0.748 (IQR 0.285), p = 0.71; SF-36: 80 (IQR 34.5) versus 77.5 (IQR 58.75), p = 0.72.
Lastly, it is important to mention that definitive re-incorporation of devitalised bone fragments may not always be appropriatehowever temporary re-incorporation may be beneficial as a technique to help obtain a more anatomic reduction of the fracture during the definitive fixation procedure.103
Authors Practiced Surgical Technique
Most patients presenting to our centre with a type IIIB Gustilo-Anderson open tibial fracture undergo a two-stage orthoplastic approach.104 The first stage involves initial wound excision and thorough debridement and is essential in reducing infection.2,105 We retain significant (mechanically relevant) devitalized bone fragments of any size at the initial debridement. The fragment is cleared of any soft tissue, and then chemically and mechanically decontaminated. The bone ends are then refreshed and the segment re-incorporated into the fracture followed by temporary internal fixation using a 3.5mm dynamic compression plate to stabilise both fragment and fracture.14,106 We rarely utilise external fixation for open tibial fractures due to the risk of developing pin site infection.9 The wound is dressed with negative pressure wound therapy which is not disturbed until definitive fixation and soft-tissue coverage are commenced. During this second stage, the temporary internal fixator is removed and a further debridement of both the open fracture wound area and devitalised bone fragment are performed. Following fracture reduction and re-incorporation of the bone fragment, a fresh 3.5mm dynamic compression plate is applied and utilised in compression mode. Intramedullary nailing is then performed and simplified owing to the reduction already held by the plate. Definitive soft-tissue free-flap coverage is performed during this same theatre episode which is commonly referred to as “fix and flap”.107
Conclusion
Management of free fragments in open fractures is a contentious topic amongst orthopaedic surgeons likely associated with a lack of community equipoise and low case volume, which may preclude high-quality prospective research on this topic. Regardless of technique used to manage bone fragments, the principles of an effective orthoplastic set up, adequate debridement, early antibiotic administration and early soft tissue coverage must be maintained.
Retention and re-incorporation of bone fragments in open fractures, particularly of the tibia where there is poor soft-tissue coverage and vascularity, provides improved reduction and immediate fracture stability, however this technique, whilst providing encouraging early results, remains in its infancy and must be strictly considered within an effective and standardised orthoplastic set-up.
Discarding critical-sized free fragments may necessitate future additional reconstructive procedures which can be challenging and associated with an increased risk of patient morbidity as well as increased costs to healthcare and society. A variety of established reconstructive methods are available, and new techniques based on these principles have been developed in part due to extensive research activities and advancing technology such as magnetic intramedullary nails and 3D (bio)printing.
Decision-making regarding the management options described in this article should incorporate patient factors such as comorbidities, surgeon factors such as prerequisite skillset, and facilities and equipment available in their hospital or region. Where possible and deemed appropriate, thoroughly decontaminated and debrided critical-sized free fragments should be retained to aid in restoration of leg length and alignment, and preserve optimum function.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pape HC, Webb LX. History of open wound and fracture treatment. J Orthop Trauma. 2008;22(10 Suppl):S133–4.
2. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453–458.
3. Singh J, Dhillon MS, Dhatt SS. Single-stage “Fix and Flap” gives good outcomes in grade 3B/C open tibial fractures: a prospective study. Malays Orthop J. 2020;14(1):61–73.
4. Adamczyk A, Meulenkamp B, Wilken G, Papp S. Managing bone loss in open fractures. OTA Int. 2020;3(1):e059.
5. Kironde E, Sekimpi P, Kajja I, Mubiri P. Prevalence and patterns of traumatic bone loss following open long bone fractures at Mulago Hospital. OTA Int. 2019;2(1):e015.
6. Keating JF, Simpson AH, Robinson CM. The management of fractures with bone loss. J Bone Joint Surg Br. 2005;87(2):142–150.
7. Cross WW 3rd, Swiontkowski MF. Treatment principles in the management of open fractures. Indian J Orthop. 2008;42(4):377–386.
8. Mauffrey C, Bailey JR, Bowles RJ, et al. Acute management of open fractures: proposal of a new multidisciplinary algorithm. Orthopedics. 2012;35(10):877–881.
9. Edwards CC, Simmons SC, Browner BD, Weigel MC. Severe open tibial fractures. Results treating 202 injuries with external fixation. Clin Orthop Relat Res. 1988;1(230):98–115.
10. Blick SS, Brumback RJ, Lakatos R, Poka A, Burgess AR. Early prophylactic bone grafting of high-energy tibial fractures. Clin Orthop Relat Res. 1989;1(240):21–41.
11. Chan KM, Leung YK, Cheng JC, Leung PC. The management of type III open tibial fractures. Injury. 1984;16(3):157–165.
12. Gopal S, Giannoudis PV, Murray A, Matthews SJ, Smith RM. The functional outcome of severe, open tibial fractures managed with early fixation and flap coverage. J Bone Joint Surg Br. 2004;86(6):861–867.
13. Kadhim M, Holmes L, Gesheff MG, Conway JD. Treatment options for nonunion with segmental bone defects: systematic review and quantitative evidence synthesis. J Orthop Trauma. 2017;31(2):111–119.
14. Al-Hourani K, Stoddart M, Khan U, Riddick A, Kelly M. Orthoplastic reconstruction of type IIIB open tibial fractures retaining debrided devitalized cortical segments: the Bristol experience 2014 to 2018. Bone Joint J. 2019;101-B(8):1002–1008.
15. Sodhai V, Pradhan C, Sancheti P, Shyam A. Successful sterilization and immediate reimplantation of extruded femoral diaphyseal segment: a case report and review of literature. J Orthopaedics. 2015;1:2210491720963288.
16. Lindvall E, Martirosian A, Morshed S. Autosterilization of contaminated and devascularized bone fragments through a subcutaneous bone pouch. J Orthop Trauma. 2015;29(12):558–562.
17. Al-Hourani K, Pearce O, Stoddart M, Riddick A, Khan U, Kelly MB. Orthoplastic reconstruction of type IIIB open tibial shaft fractures utilising debrided devitalised cortical segments: health-related quality of life outcomes. J Orthop Trauma. 2022;1:845.
18. Shanmuganathan R, Chandra Mohan AK, Agraharam D, Perumal R, Jayaramaraju D, Kulkarni S. Successful reimplantation of extruded long bone segments in open fractures of lower limb–a report of 3 cases. Injury. 2015;46(7):1389–1392.
19. Mazurek MT, Pennington SE, Mills WJ. Successful reimplantation of a large segment of femoral shaft in a type IIIA open femur fracture: a case report. J Orthop Trauma. 2003;17(4):295–299.
20. Moosazadeh K. Successful reimplantation of retrieved large segment of open femoral fracture: case report. J Trauma. 2002;53(1):133–138.
21. Kumar P, Shrestha D, Bajracharya S. Replacement of an extruded segment of radius after autoclaving and sterilising with gentamicin. J Hand Surg Br. 2006;31(6):616–618.
22. Bruce B, Sheibani-Rad S, Appleyard D, et al. Are dropped osteoarticular bone fragments safely reimplantable in vivo? J Bone Joint Surg Am. 2011;93(5):430–438.
23. Rozbruch SR, Birch JG, Dahl MT, Herzenberg JE. Motorized intramedullary nail for management of limb-length discrepancy and deformity. J Am Acad Orthop Surg. 2014;22(7):403–409.
24. Bost FC, Larsen LJ. Experiences with lengthening of the femur over n intramedullary rod. J Bone Joint Surg Am. 1956;38-A(3):567–584.
25. Guichet JM, Casar RS. Mechanical characterization of a totally intramedullary gradual elongation nail. Clin Orthop Relat Res. 1997;337:281–290.
26. Cole JD, Justin D, Kasparis T, DeVlught D, Knobloch C. The intramedullary skeletal kinetic distractor (ISKD): first clinical results of a new intramedullary nail for lengthening of the femur and tibia. Injury. 2001;32(Suppl 4):SD129–39.
27. Thaller PH, Furmetz J, Wolf F, Eilers T, Mutschler W. Limb lengthening with fully implantable magnetically actuated mechanical nails (PHENIX((R)))-preliminary results. Injury. 2014;45(Suppl 1):S60–5.
28. Lee DH, Kim S, Lee JW, Park H, Kim TY, Kim HW. A comparison of the device-related complications of intramedullary lengthening nails using a new classification system. Biomed Res Int. 2017;2017:8032510.
29. Aquerreta JD, Forriol F, Canadell J. Complications of bone lengthening. Int Orthop. 1994;18(5):299–303.
30. Simpson AH, Cole AS, Kenwright J. Leg lengthening over an intramedullary nail. J Bone Joint Surg Br. 1999;81(6):1041–1045.
31. Kähler Olesen U, Herzenberg JE. Bone transport with internal devices. Techniques in Orthopaedics. 2020;35(3):219–224.
32. Oh CW, Apivatthakakul T, Oh JK, et al. Bone transport with an external fixator and a locking plate for segmental tibial defects. Bone Joint J. 2013;95-B(12):1667–1672.
33. Giotakis N, Narayan B, Nayagam S. Distraction osteogenesis and nonunion of the docking site: is there an ideal treatment option? Injury. 2007;38(Suppl 1):S100–7.
34. Olesen UK, Nygaard T, Prince DE, Gardner MP, Singh UM, McNally MA, et al. Plate-assisted bone segment transport with motorized lengthening nails and locking plates: a technique to treat femoral and tibial bone defects. J Am Acad Orthop Surg Glob Res Rev. 2019;3(8):e064.
35. Liu Y, Yushan M, Liu Z, Liu J, Ma C, Yusufu A. Complications of bone transport technique using the Ilizarov method in the lower extremity: a retrospective analysis of 282 consecutive cases over 10 years. BMC Musculoskelet Disord. 2020;21(1):354.
36. Barinaga G, Beason AM, Gardner MP. Novel surgical approach to segmental bone transport using a magnetic intramedullary limb lengthening system. J Am Acad Orthop Surg. 2018;26(22):e477–e82.
37. NuVasive. NuVasive Precice Antegrade and Retrograde. Femur Operative Technique 2020. Available from: https://www.nuvasive.com/wp-content/uploads/2020/09/PRECICE-Technique-Guide-Femur.pdf.
38. Chaudhary M. Limb lengthening over a nail can safely reduce the duration of external fixation. Indian J Orthop. 2008;42(3):323–329.
39. Makhdom AM, Cartaleanu AS, Rendon JS, Villemure I, Hamdy RC. The Accordion Maneuver: a noninvasive strategy for absent or delayed callus formation in cases of limb lengthening. Adv Orthop. 2015;2015:912790.
40. Hwang J, Sems S, Yuan B. Trifocal tibial bone transport using a magnetic intramedullary nail: a case report. JBJS Case Connect. 2021;11:4.
41. Catagni MA, Azzam W, Guerreschi F, et al. Trifocal versus bifocal bone transport in treatment of long segmental tibial bone defects. Bone Joint J. 2019;101-B(2):162–169.
42. French Society of Orthopaedic S, Rigal S, Merloz P, Le nen D, et al. Bone transport techniques in posttraumatic bone defects. Orthop Traumatol Surg Res. 2012;98(1):103–108.
43. Schiedel FM, Vogt B, Tretow HL, et al. How precise is the PRECICE compared to the ISKD in intramedullary limb lengthening? Reliability and safety in 26 procedures. Acta Orthop. 2014;85(3):293–298.
44. Dincyurek H, Kocaoglu M, Eralp IL, Bilen FE, Dikmen G, Eren I. Functional results of lower extremity lengthening by motorized intramedullary nails. Acta Orthop Traumatol Turc. 2012;46(1):42–49.
45. Burghardt RD, Herzenberg JE, Specht SC, Paley D. Mechanical failure of the Intramedullary Skeletal Kinetic Distractor in limb lengthening. J Bone Joint Surg Br. 2011;93(5):639–643.
46. Mazeau P, Assi C, Louahem D, L’Kaissi M, Delpont M, Cottalorda J. Complications of Albizzia femoral lengthening nail: an analysis of 36 cases. J Pediatr Orthop B. 2012;21(5):394–399.
47. Kirane YM, Fragomen AT, Rozbruch SR. Precision of the PRECICE internal bone lengthening nail. Clin Orthop Relat Res. 2014;472(12):3869–3878.
48. Paley D. PRECICE intramedullary limb lengthening system. Expert Rev Med Devices. 2015;12(3):231–249.
49. Health Products Regulatory Authority. Summary of Field Safety Notices; 2021. Available from: http://www.hpra.ie/homepage/medical-devices/safety-information/safety-notices/item?t=/summary-of-field-safety-notices—january-2021&id=24e70e26-9782-6eee-9b55-ff00008c97d0.
50. Medicines & Healthcare products Regulatory Agency. Device recall and supply suspended to the UK: all PRECICE Systems by NuVasive Specialized Orthopedics Inc; 2021. Available from: https://www.boa.ac.uk/static/552dea10-c795-4c9f-b1a08177bf9151c1/NuVasive-MHRA-notice.pdf.
51. NuVasive. Urgent Field Safety Notice - Precice System 2021; 2022. Available from: https://www.boa.ac.uk/asset/1B8D2C68-B020-4C0C-BDF4CBB66B23D583/.
52. Attias N, Thabet AM, Prabhakar G, Dollahite JA, Gehlert RJ, DeCoster TA. Management of extra-articular segmental defects in long bone using a titanium mesh cage as an adjunct to other methods of fixation: a multicentre report of 17 cases. Bone Joint J. 2018;100-B(5):646–651.
53. Hsu AR, Ellington JK. Patient-specific 3-dimensional printed titanium truss cage with tibiotalocalcaneal arthrodesis for salvage of persistent distal tibia nonunion. Foot Ankle Spec. 2015;8(6):483–489.
54. Hamid KS, Parekh SG, Adams SB. Salvage of severe foot and ankle trauma with a 3D printed scaffold. Foot Ankle Int. 2016;37(4):433–439.
55. Nwankwo EC, Chen F, Nettles DL, Adams SB. Five-year follow-up of distal tibia bone and foot and ankle trauma treated with a 3D-printed titanium cage. Case Rep Orthop. 2019;2019:7571013.
56. Tetsworth K, Block S, Glatt V. Putting 3D modelling and 3D printing into practice: virtual surgery and preoperative planning to reconstruct complex post-traumatic skeletal deformities and defects. SICOT J. 2017;3:16.
57. Tetsworth K, Woloszyk A, Glatt V. 3D printed titanium cages combined with the Masquelet technique for the reconstruction of segmental femoral defects: preliminary clinical results and molecular analysis of the biological activity of human-induced membranes. OTA Int. 2019;2(1):e016.
58. LifeHealthcare Orthopaedics. Patient specific implants 2015. Available from: http://www.lifehealthcare.com.au/wp-content/uploads/2016/05/057-LHC-Ortho-Brochure_r9.pdf.
59. Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41(1):27–37.
60. Ramiah P, Toit LC, Choonara YE, Kondiah PPD, Pillay V. Hydrogel-Based Bioinks for 3D Bioprinting in Tissue Regeneration. Frontiers in Materials. 2020;1:7.
61. Li L, Shi J, Ma K, et al. Robotic in situ 3D bio-printing technology for repairing large segmental bone defects. J Adv Res. 2021;30:75–84.
62. Giannoudis PV, Faour O, Goff T, Kanakaris N, Dimitriou R. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury. 2011;42(6):591–598.
63. Giannoudis PV. Treatment of bone defects: bone transport or the induced membrane technique? Injury. 2016;47(2):291–292.
64. Masquelet AC, Fitoussi F, Begue T, Muller GP. [Reconstruction of the long bones by the induced membrane and spongy autograft]. Ann Chir Plast Esthet. 2000;45(3):346–353. Norwegian
65. Klein C, Monet M, Barbier V, et al. The Masquelet technique: current concepts, animal models, and perspectives. J Tissue Eng Regen Med. 2020;14(9):1349–1359.
66. Chadayammuri V, Hake M, Mauffrey C. Innovative strategies for the management of long bone infection: a review of the Masquelet technique. Patient Saf Surg. 2015;9:32.
67. Apard T, Bigorre N, Cronier P, Duteille F, Bizot P, Massin P. Two-stage reconstruction of post-traumatic segmental tibia bone loss with nailing. Orthop Traumatol Surg Res. 2010;96(5):549–553.
68. Karger C, Kishi T, Schneider L, French Society of Orthopaedic S, et al. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop Traumatol Surg Res. 2012;98(1):97-102
69. Olesen UK, Eckardt H, Bosemark P, Paulsen AW, Dahl B, Hede A. The Masquelet technique of induced membrane for healing of bone defects. A review of 8 cases. Injury. 2015;46(Suppl 8):S44–7.
70. Morris R, Hossain M, Evans A, Pallister I. Induced membrane technique for treating tibial defects gives mixed results. Bone Joint J. 2017;99-B(5):680–685.
71. Hsu CA, Chen SH, Chan SY, Yu YH. The induced membrane technique for the management of segmental tibial defect or nonunion: a systematic review and meta-analysis. Biomed Res Int. 2020;2020:5893642.
72. Bumbasirevic M, Stevanovic M, Bumbasirevic V, Lesic A, Atkinson HD. Free vascularised fibular grafts in orthopaedics. Int Orthop. 2014;38(6):1277–1282.
73. Houdek MT, Bayne CO, Bishop AT, Shin AY. The outcome and complications of vascularised fibular grafts. Bone Joint J. 2017;99-B(1):134–138.
74. Hattori Y, Doi K, Sakamoto S, Satbhai N, Kumar KK. Pedicled vascularised fibular grafting in a flow-through manner for reconstruction of infected non-union of the tibia with preservation of the peroneal artery: a case report. J Orthop Surg. 2015;23(1):111–115.
75. Parmaksizoglu F, Cansu E, Unal MB, Yener Ince A. Acute emergency tibialization of the fibula: reconstruction of a massive tibial defect in a type IIIC open fracture. Strategies Trauma Limb Reconstr. 2013;8(2):127–131.
76. Beris AE, Lykissas MG, Korompilias AV, et al. Vascularized fibula transfer for lower limb reconstruction. Microsurgery. 2011;31(3):205–211.
77. Hak DJ. The use of osteoconductive bone graft substitutes in orthopaedic trauma. J Am Acad Orthop Surg. 2007;15(9):525–536.
78. Khan SN, Cammisa FP, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13(1):77–86.
79. Flynn J. Fracture repair and bone grafting. OKU. 2011;10:11–21.
80. Allsopp BJ, Hunter-Smith DJ, Rozen WM. Vascularized versus nonvascularized bone grafts: what is the evidence? Clin Orthop Relat Res. 2016;474(5):1319–1327.
81. Ozaksar K, Sugun TS, Toros T, Gurbuz Y, Kayalar M, Ozerkan F. Free vascularized fibular grafts in Type 3 open tibia fractures. Acta Orthop Traumatol Turc. 2012;46(6):430–437.
82. Al-Hourani K, Pearce O, Bott A, Riddick A, Trompeter A, Kelly MB. Three-vessel view debridement of the open tibial fracture: a surgical technique. Eur J Orthop Surg Traumatol. 2021;1:87.
83. Obremskey W, Molina C, Collinge C, et al. Current Practice in the Management of Open Fractures Among Orthopaedic Trauma Surgeons. Part B: management of Segmental Long Bone Defects. A Survey of Orthopaedic Trauma Association Members. J Orthop Trauma. 2014;28(8):e203–7.
84. Sanders DW, Bhandari M, Guyatt G, et al. Critical-sized defect in the tibia: is it critical? Results from the SPRINT trial. J Orthop Trauma. 2014;28(11):632–635.
85. Nauth A, Schemitsch E, Norris B, Nollin Z, Watson JT. Critical-size bone defects: is there a consensus for diagnosis and treatment? J Orthop Trauma. 2018;32(Suppl 1):S7–S11.
86. Haines NM, Lack WD, Seymour RB, Bosse MJ. Defining the lower limit of a “critical bone defect” in open diaphyseal tibial fractures. J Orthop Trauma. 2016;30(5):e158–63.
87. Tetsworth KD, Burnand HG, Hohmann E, Glatt V. Classification of bone defects: an extension of the Orthopaedic Trauma Association Open Fracture Classification. J Orthop Trauma. 2021;35(2):71–76.
88. Harper MC. Storage of an autogenous cortical bone graft in a subcutaneous pouch with subsequent transplantation. Clin Orthop Relat Res. 1982;163:113–119.
89. Urist MR, Silverman BF, Buring K, Dubuc FL, Rosenberg JM. The bone induction principle. Clin Orthop Relat Res. 1967;53:243–283.
90. Hing CB, Ball RY, Tucker JK. Autobanking of femoral heads for revision total Hip replacement, a preliminary report of a new surgical technique. Surgeon. 2004;2(1):37–41.
91. Heslop BF, Zeiss IM, Nisbet NW. Studies on transference of bone. I. A comparison of autologous and homologous bone implants with reference to osteocyte survival, osteogenesis and host reaction. Br J Exp Pathol. 1960;41:269–287.
92. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093–1110.
93. Saddawi-Konefka D, Kim HM, Chung KC. A systematic review of outcomes and complications of reconstruction and amputation for type IIIB and IIIC fractures of the tibia. Plast Reconstr Surg. 2008;122(6):1796–1805.
94. Singh A, Jiong Hao JT, Wei DT, et al. Gustilo IIIB open tibial fractures: an analysis of infection and nonunion rates. Indian J Orthop. 2018;52(4):406–410.
95. Glatt V, Evans CH, Tetsworth K. A Concert between biology and biomechanics: the influence of the mechanical environment on bone healing. Front Physiol. 2016;7:678.
96. Elliott DS, Newman KJ, Forward DP, et al. A unified theory of bone healing and nonunion: BHN theory. Bone Joint J. 2016;98-B(7):884–891.
97. Wolff J. Das Gesetz der Transformation der Knochen, Berlin, A. Hirchwild. The Law of Bone Remodeling; 1892.
98. Rueff-Barroso CR, Milagres D, Do Valle J. Bone healing in rats submitted to weight-bearing and non-weight-bearing exercises. Med Sci Monit. 2008;14(11):BR231–6.
99. Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29(2):197–206.
100. Minaire P. Immobilization osteoporosis: a review. Clin Rheumatol. 1989;8(Suppl 2):95–103.
101. Rossiter ND, Trompeter AJ. Personal theories on non-union: it’s all mechanics! Orthopaedics Trauma. 2021;35:68–75.
102. Delgado-Rodríguez M. Bias LJ. J Epidemiol Community Health. 2004;58(8):635.
103. Barei DP, Taitsman LA, Beingessner D, Dunbar RP, Nork SE. Open diaphyseal long bone fractures: a reduction method using devitalized or extruded osseous fragments. J Orthop Trauma. 2007;21(8):574–578.
104. Mathews JA, Ward J, Chapman TW, Khan UM, Kelly MB. Single-stage orthoplastic reconstruction of Gustilo-Anderson Grade III open tibial fractures greatly reduces infection rates. Injury. 2015;46(11):2263–2266.
105. Hull PD, Johnson SC, Stephen DJ, Kreder HJ, Jenkinson RJ. Delayed debridement of severe open fractures is associated with a higher rate of deep infection. Bone Joint J. 2014;96-B(3):379–384.
106. Fowler T, Whitehouse M, Riddick A, Khan U, Kelly M, Retrospective Comparative A. Cohort study comparing temporary internal fixation to external fixation at the first stage debridement in the treatment of Type IIIB open diaphyseal tibial fractures. J Orthop Trauma. 2019;33(3):125–130.
107. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959–966.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.