Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Current Opinion on Prucalopride in Gastroparesis and Chronic Constipation Treatment: A Focus on Patient Selection and Safety
Authors Hong JT
Received 7 March 2021
Accepted for publication 27 May 2021
Published 8 June 2021 Volume 2021:17 Pages 601—615
DOI https://doi.org/10.2147/TCRM.S269330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Ji Taek Hong
Division of Gastroenterology, Department of Medicine, Ewha Womans University College of Medicine, Seoul, Korea
Correspondence: Ji Taek Hong
Division of Gastroenterology, Department of Medicine, Ewha Womans University College of Medicine, 1071, Anyangcheon-ro, Yangcheon-gu, Seoul, Republic of Korea
Tel +822-2650-5114
Fax +822-2650-6983
Email [email protected]
Abstract: Prucalopride is a third-generation, highly selective 5-hydroxytryptamine 4 (5-HT4) receptor agonist. Many recent studies indicate prucalopride may play an important role in various motility disorders. The aim of this study was to investigate safety and patient selection considerations when using prucalopride as gastroparesis and chronic constipation treatment. We systematically searched PubMed, Embase, the Cochrane Central Register and ClinicalTrials.gov, and we reviewed all studies that evaluated prucalopride for the treatment of gastroparesis and chronic idiopathic constipation in adults. Prucalopride is an effective and safe option based on all the studies currently conducted. Thus, it may be the first-line treatment in the future. Prucalopride has the potential to be useful in the treatment of functional constipation and other forms of gastrointestinal diseases (eg, gastroparesis). Through the research on this potential, prucalopride is expected to be a useful and versatile option for treating gastrointestinal diseases in the future.
Keywords: prucalopride, gastroparesis, constipation, safety
Introduction
Prucalopride is a third-generation, highly selective 5-hydroxytryptamine 4 (5-HT4) receptor agonist. Prucalopride stimulates colonic transit and is the underlying principle of action for chronic constipation.1,2 Thus, prucalopride provided clinicians with an option that could help treat conventional laxative refractory constipation. Many recent studies indicate prucalopride may play an important role in various motility disorders such as gastroparesis, which is often treated with prokinetic drugs. Moreover, symptom improvements can be expected by stimulating gastric motility and increasing the gastric emptying rate. However, these treatments are often ineffective for severe gastroparesis. Approved prokinetics are also limited in use due to significant side effects (central, extrapyramidal, anticholinergic, and cardiac arrhythmia, and so on). New prokinetic agents that are effective and safe are necessary to treat gastroparesis. Prucalopride improves gastric transit in canine models and healthy subjects as well as in patients with chronic constipation.2–4 Thus, studies are being conducted to determine the effect of prucalopride on gastroparesis treatment. Currently, available evidence suggests that using prucalopride may help, especially in patients suffering from both gastroparesis and chronic constipation. This study aims to investigate safety and patient selection considerations when using prucalopride as gastroparesis and chronic constipation treatment.
The Use of Prucalopride for Chronic Constipation and Potential for Gastroenterology
Lifestyle modifications, fiber supplementation, or bulk-forming agents are common treatments for chronic constipation. Basic osmotic laxatives (eg, polyethylene glycol (PEG)-based laxatives and lactulose) can be administered if the response is insufficient.5 Stimulant laxatives (eg, bisacodyl or senna) can be used if other treatments are ineffective. However, none of these treatments is sufficient for laxative refractory chronic constipation. Therefore, intestinal secretagogues (eg, lubiprostone and linaclotide) or prokinetic agents targeting 5-HT4 and motilin receptors have been considered as new therapeutic approaches.
The 5-HT4 receptors are members of the G-protein family coupled receptors that are widely expressed throughout the gastrointestinal tract of smooth muscle cells and intestinal neurons.6,7 Therefore, many studies have been conducted on these receptors as targets for prokinetic drugs. The activation of these receptors promotes gastrointestinal motility and mucosal secretion through inhibitory circular smooth muscle relaxation and cholinergic transmission enhancement acetylcholine release.8,9 Several types of 5-HT4 agonists were developed for the prokinetic treatment of multiple dysmotility diseases. Each agent had different degrees of affinity and selectivity for the 5-HT4 receptor.
Early 5-HT4 receptor agonists, represented by cisapride and tegaserod, were withdrawn from the market due to serious side effects (eg, cardiovascular and QT prolongation).10,11 These side effects are due to the effect on the human ether-à-go-go-related gene (hERG)-encoding potassium channel and not only 5-HT4 but also unrelated 5-HT1 or 5-HT2 receptors. This is because selectivity for only 5-HT4 receptors is insufficient.6,12 Unlike these drugs, the new highly selective 5-HT4 receptor agonists, including prucalopride, velusetrag, and naronapride, are known to be relatively safe. It did not show effects on the 5-HT receptor or hERG-encoding potassium channels known to be associated with QT prolongation and cardiovascular side effects.6,13,14
Chronic constipation is often alone or overlapped with functional gastrointestinal disorders (eg, irritable bowel syndrome, gastroesophageal reflux disease, and dyspepsia). Also, gastroparesis and chronic constipation seem to occur in the same patient frequently. An analysis of 206 patients by Zikos et al showed that patients with gastroparesis were more likely to have slow colon transit than patients with dyspeptic symptoms but normal gastric emptying. Moreover, patients with slow colon transit were more likely to have delayed gastric emptying.15 According to Hasler’s report, >40% of the 209 patients with suspected gastroparesis reported an extra gastric passage delay.16 Prucalopride showed a decrease in esophageal acid exposure and accelerated gastric emptying in healthy adult male volunteers, although whether the decrease in esophageal acid exposure is a secondary finding due to the improvement of esophageal movement is unclear.4 In gastro reflux patients with ineffective esophageal motility, prucalopride promoted the frequency of esophageal secondary peristalsis and significantly increased primary peristaltic wave amplitude.17 Prucalopride also accelerated gastrointestinal transit in patients with chronic constipation without a rectal evacuation disorder.2 Therefore, the effect on gastric emptying and reduction of esophageal acid exposure suggests that prucalopride may be effective in treating gastroparesis or reflux esophagitis. Thus, prucalopride may play an important role in several motor disorders, especially gastroparesis, and impaired esophageal motility. However, research on these diseases is officially lacking, and more studies are needed. Therefore, it is necessary to examine the subjects and the effects and side effects of prucalopride from the studies published to date.
Previous studies, including large randomized controlled trials, assessed the efficacy, tolerability, safety, and impact on prucalopride’s quality of life.18 In general, prucalopride reportedly had allowable safety and tolerability, especially regarding cardiovascular risks, and electrocardiography parameters. Commonly reported side effects are abdominal pain, diarrhea, nausea, and headache.19–23 Most of these side effects occurred in the study group within the first 24 h after initiation of prucalopride therapy, and the duration of side effects was relatively short.23 While using prucalopride for 24 months, gastrointestinal symptoms, and headache occurred in 3.3% and 1.0%, respectively.24 In the first 3 months, 2.5% of patients stopped using prucalopride due to side effects (ie, abdominal pain, diarrhea, nausea, and headache).24 Moreover, prucalopride showed relatively safe results, did not affect the QT interval, and did not show any association with adverse cardiovascular side effects.22,25
Prucalopride does not affect the P glycoprotein or cytochrome P450 functions and is not widely metabolized in the body. Thus, few clinically significant interactions with other drugs (eg, oral contraceptive bioavailability) exist.21 Prucalopride is also not significantly metabolized in the human body, does not significantly change, and is mainly excreted in the urine.26,27 Therefore, drug clearance can be reduced significantly in patients with severe renal impairment. Thus, dose reduction is required. Furthermore, it should not be used in patients on dialysis or with drug sensitization, intestinal obstruction, or toxic megacolon.28 Although liver metabolism is minimal, patients with advanced liver disease need similar dose reductions.27 Even in patients with constipation >65 years old, prucalopride was equally effective and safe when 1 or 2–4 mg was taken.29 Therefore, 2-mg prucalopride is recommended for once-a-day administration. However, 1-mg prucalopride is recommended for the elderly (>65 years old) patients, patients with severe liver impairment (Child–Pugh class C) or severe renal impairment (glomerular filtration rate, <30 mL/min/1.73 m2). It is also not recommended for use during lactation or pregnancy (category C drug).30 Several case reports exist on serious neuropsychiatric events or acute tubular necrosis. However, they do not appear to have a clear causal relationship.31,32
Patient Perspectives Focusing on the Critical Safety of Patients with Gastroparesis and Chronic Constipation
Gastroparesis
Gastroparesis refers to delayed gastric emptying without mechanical obstruction.33 It is caused by diabetes complications and can be defined as idiopathic cases occurring without a specific cause. Gastroparesis can be considered a pan-gastroenteric disorder due to various causes (eg, visceral hypersensitivity, impaired accommodation of gastric fundus/body, impaired pyloric relaxation, as well as lowering of gastric emptying).34 Prokinetics are generally considered as a gastroparesis treatment, but evidence for effectiveness is lacking.35 Furthermore, prokinetics cause gastric emptying too quickly and can cause gastric fundus/body accommodation dysregulation and other symptoms.35,36 It can be difficult to find a physiologically optimal point where gastric emptying is neither too fast nor too slow because the severity of the patient’s symptoms, the cause of gastroparesis, and the rate of gastric emptying are all different. Diabetic gastroparesis may respond differently to prokinetics compared to idiopathic gastroparesis. Due to these various factors, there is no consensus on the optimal access to treatment for gastroparesis.
The diagnosis of gastroparesis requires two conditions to be fulfilled: the identification of delayed gastric emptying using appropriate tools and the recognition of the cardinal symptoms. For symptom evaluation, a validated questionnaire (ie, PAGI-SYM-based Gastroparesis Cardinal Symptom Index [GCSI] and the recently revised GCSI-Daily Diary score) was developed to quantify the severity of gastroparesis and other digestive symptoms.37,38 In particular, two validated patient-reported outcomes for gastroparesis (the American Neurogastroenterology and Motility Society, GCSI daily diary, and Diabetic Gastroparesis Symptom Severity Diary) are being used in ongoing clinical trials.39,40 To measure GE, various measurement methods such as stable isotope breath testing and wireless motility capsule are available, but scintigraphic gastric emptying is the current gold standard.41,42
Prucalopride, as a novel and investigational medication, has been studied. For gastroparesis, a small number of small, randomized placebo-controlled crossover studies have been conducted in each patient with predominantly female idiopathic or diabetic gastroparesis to date. Therefore, summarizing the research and results, examining the adaptation of prucalopride to gastroparesis, and planning future research directions will be important.
Summary of Completed Research
Two randomized placebo-controlled crossover pilot trials are available to date (Table 1). In a study of 34 gastroparesis patients (28 idiopathic and 6 diabetics), Carbone et al reported significant improvement in the daily 2-mg prucalopride 4-week treatment group compared with the placebo group. Gastric half-emptying time (p < 0.050) and the severity of symptoms assessed with GCSI were significantly reduced (bloating/distension, p < 0.001; nausea/vomiting, p = 0.010; and fullness/satiety, p < 0.001) compared with the placebo group.43 Moreover, in Andrews’ study of 15 gastroparesis patients [13 diabetics and 2 connective tissue disease (CTD)], 4 mg of prucalopride accelerates gastric emptying and stool frequency, but does not appear to improve gastroparesis or diet-related symptoms.44
 |
Table 1 Characteristics of the Randomized Placebo-Controlled Crossover Pilot Trials About the Effect of Prucalopride on Gastroparesis |
Research Subject Analysis
The few pilot studies conducted to date have shown improved gastric emptying in idiopathic, diabetic, and CTD gastroparesis.43,44 Also, 2-mg daily prucalopride in the idiopathic group improved patient symptoms and quality of life. However, there was no association between symptom improvement and the gastric emptying rate.43 The correlation between symptoms and delayed gastric emptying in diabetes and idiopathic gastroparesis remains the subject of ongoing debate. In several studies, the relationship between symptom improvement and gastric half-emptying time was not good and did not show a significant correlation.35,45 This may be because gastric half-emptying time measurements cannot reflect all of these in evaluating response to treatment and postprandial motor function.
A recent report states that an optimal gastric emptying measurement for at least 3 h via scintigraphy or breathing tests shows the best correlation.41 Another study that analyzed the relationship between improved simultaneously measured symptoms and changes in gastric emptying rate after using prokinetics agents revealed no correlation between the two in the analysis of eight clinical trials, even in the closest apposition. The observed symptoms’ significant benefit may be attributable to effects other than enhanced gastric emptying.46 This result has been the subject of debate for some time.47 Therefore, other mechanisms may be involved in prucalopride’s therapeutic effect that is unrelated to the prokinetic effect. Prucalopride, which has an antinociceptive effect, may also improve symptoms by reducing the perception of luminal distension by gas.48 Thus, additional analysis, research on other measurements, or new methods is needed to confirm the therapeutic effect of prucalopride on symptoms.49–52 In Andrews’ study, the symptoms did not improve in the diabetic group. Too few CTD groups allow meaningful analysis to be performed.44 Moreover, patients with diabetic gastroparesis may have sensory neuropathy, which may confuse symptom evaluation, and more large-scale studies may need to be performed.33,53,54
It is unclear whether the studies to date, mainly targeting patients in tertiary care centers, can be applied to patients receiving different care levels. Similarly, the effect on gastroparesis is unclear because there have been no studies on patients with organic or drug-induced gastroparesis.
The Carbone et al and multicenter studies using velusetrag showed fewer symptom improvements than the placebo in a subset of diabetic patients, suggesting that idiopathic may have a better response than diabetic gastroparesis.43,55 Therefore, based on the studies to date, it is expected that gastroparesis, especially idiopathic gastroparesis, with symptoms that are being cared for in tertiary care centers can be used as an off-label. However, more studies are needed.
Capacity Analysis
The effective prucalopride dose for gastroparesis is currently unclear. Prokinetics increase gastric emptying through stimulation of gastric contractility. However, prokinetics may cause symptoms due to rapid food discharge by reducing fundic accommodation.35,36 The lack of correlation between changes in gastric emptying rates and symptomatic benefits of prucalopride suggests that enhanced gastric emptying is not necessarily the mechanism responsible for the majority of symptom improvement. Therefore, finding the optimal dose for which gastric emptying is neither too fast nor too slow and should be evaluated and studied differently according to gastroparesis etiology and symptom severity. A lower starting dose may be preferable in the off-label use for gastroparesis because the current diabetic group did not show symptom improvement in the 4-mg dose.44 Similarly, daily 2-mg prucalopride in the idiopathic group showed improved symptoms and quality of life.43
Analysis of Complications That Occurred
Both studies reported the side effects were diarrhea, headache, abdominal cramps, cystitis, and respiratory infection. Most of the side effects were temporary. In a study by Carbone et al, three patients in the prucalopride group dropped out due to small bowel volvulus, diarrhea, and headache (3/17). In Andrews’ study, abdominal cramping required dose reduction in one patient (1/15). In a study by Carbone et al, one serious side effect, small bowel volvulus, occurred.43 Therefore, more research is needed. However, caution may be exercised for use in patients with risk factors for volvulus (eg, enlarged colon or abdominal adhesions).56 Moreover, consideration should be given to the volvulus if any suspicious symptoms are noted during use.
Chronic Constipation
Table 2 shows seven Phase II studies, ten Phase III studies, and one Phase IV study. Table 2 summarizes the total, severe, and serious adverse events (AEs) and withdrawal rates from all Phase II, III, and IV trials in constipation. In particular, we have summarized the prucalopride stability in a specific population, cardiovascular safety, and side effects depending on the dose.
 |
Table 2 Characteristics and Safety Outcomes of the Randomized Placebo-Controlled Trials About the Effect of Prucalopride on the Constipation (Phase 2, 3, 4) |
 |
Table 2A Characteristics in Phase 2 Trials |
 |
Table 2B Characteristics in Phase 3 Trials |
 |
Table 2C Characteristics in Phase 4 Trials |
 |
Table 2D Safety Outcomes in Phase 2 Trials |
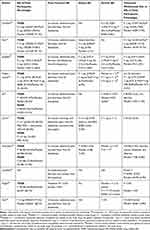 |
Table 2E Safety Outcomes in Phase 3 Trials |
 |
Table 2F Safety Outcomes in Phase 4 Trials |
Summary of Research Completed
Treatment-associated AEs were reported in 50–88% of the patients due to the safety assessment of phase II randomized controlled trial comparing the placebo group and the 0.5, 1, 2, or 4 mg prucalopride-treated group. The most common treatment-associated AEs were headache, nausea, abdominal pain, and diarrhea. Most AEs were transient and arose within the first 24 h of prucalopride treatment. Discontinuation rates due to AEs were reported in the placebo (10.6%) and the prucalopride treatment (4–50%) groups. Many discontinuations were the consequence of abdominal pain and discomfort, diarrhea, vomiting, headache, atypical chest discomfort, fecal incontinence, back pain, nausea, flatulence, malaise, edema sensation, dizziness, dysphagia, palpitations, insomnia, dry skin, and increased micturition frequency. No consistent, clinically relevant, or treatment-related differences were reported regarding PR, QT, QTcB, or QTCF intervals. A randomized controlled trial was conducted in elderly patients aged ≥65 years to investigate prucalopride’s safety compared to the placebo group and the 0.5, 1, or 2 mg daily prucalopride-treated group.22 Of the patients included in this study, 78% were being treated for cardiovascular disease, and 88% had a history of cardiovascular disease in the past. No group experienced life-threatening arrhythmias. Prolongation of QTc interval (QTcF of >450 and >470 ms in males and females, respectively) was reported in about 5% of the placebo, 0.5 mg prucalopride and 1 mg prucalopride groups. There were no differences between the groups. Severe AEs (eg, moderate diarrhea, melaena, colitis, and diverticulitis), all of which occurred in one patient in the prucalopride 0.5 mg group, were reported to be drug related. However, the side effects of most of the current phase II studies have been mild to moderate. Therefore, the phase II study results showed that the overall use of prucalopride was safe across the therapeutic dose range.
The most common treatment-associated AEs were headache followed by nausea, abdominal pain, and diarrhea at 25%–30%, 12%–24%, 16%–23%, and 12%–19%, respectively, in the prucalopride group and 12%–17%, 8%–14%, 11%–19%, and 3%–5%, respectively, in the placebo group.19,22,25,57–59 Most AEs were of mild to moderate severity, transient, and occurred within the first 24 h of prucalopride treatment. Excluding AEs reported on day 1, the difference between prucalopride and placebo recipients in the incidence of these AEs was weak.19,57,58,60 Discontinuation rates due to AEs were reported in the placebo (1.2–6.9%) and prucalopride treatment (2.5–15.1%) groups. Among the serious side effects reported related to the drug were GI disorder and vertigo that occurred in the 1-mg prucalopride group, which was severe and possibly related to study medication.61 Moreover, diarrhea was reported in 6–14% of patients in the daily 2- or 4-mg prucalopride-treated group. The Asian Pacific study cohorts were reported to have higher diarrhea occurrence (22.1%). In the prucalopride group, changes in QTcF from baseline were not significant. Similarly, the occurrence of the prolonged QT interval was not significant compared with the placebo group.19,25,29,57,61–64
Research Subject Analysis
Cardiovascular risks of prucalopride have been carefully examined for the safe use of prucalopride in chronic constipation patients, including cardiologically vulnerable patients (eg, elderly patients), especially because of concerns over the negative effects of 5-HT4 on the cardiovascular system. Additional safety, tolerability, and clinical effectiveness assessments in elderly patients are required for the routine use of prucalopride in conventional laxative-resistant chronic constipation patients.
Although prucalopride’s efficacy and safety have been demonstrated in several randomized clinical trials, trials in elderly populations with generally associated heart, kidney, liver, and lung comorbidities and prevalent incidence of chronic constipation are lacking. However, based on the current research results, prucalopride was shown to be safe in elderly patients.22,29 A previous study investigated a 0.5–2 mg dose escalation of prucalopride. This study had 89 participants in a nursing home. History of cardiovascular disease was reported in 89% of the participants. Headache and diarrhea were the most common adverse effects of the escalation and any electrocardiographic changes or cardiac events were not reported.22
Prucalopride use was recommended for women because 87% of the patients in this study were women. Thus, the effect in men was not sufficiently reviewed.65 From the clinical experience of real experts and the results of a randomized controlled study, the use of prucalopride can be used effectively and safely to treat male constipation.63 In a phase III trial with 370 men, the occurrence of at least one treatment-emergent AE (TEAE) was not significantly higher in the prucalopride (42.4%) than the placebo group (34.4%). The most common TEAEs regarding prucalopride were diarrhea, nausea, abdominal pain, and headache. One and four serious TEAEs were reported in the prucalopride and placebo groups, respectively. Moreover, mild or moderate TEAEs are the majority. Treatment discontinuation due to AEs was similar between the two groups at 3.3% and 3.8% in the prucalopride and placebo groups, respectively. One and two cardiovascular AEs were reported in the prucalopride (coronary artery occlusion) and placebo (one angina and one myocardial ischemia) groups showing low cardiovascular AE occurrence in both groups, respectively. One patient in the prucalopride group had prolonged QT at week 4 but it normalized at week 12. Prucalopride treatment was continued subsequently.
In the integrated analysis of phase III clinical trials regarding prucalopride, the Asian and non-Asian subgroups of the prucalopride group showed a significant difference in the number of patients reporting at least one TEAE (p < 0.001). More patients in the Asian subgroup experienced diarrhea, but other TEAEs, including headache, dizziness, abdominal pain, upper abdominal pain, flatulence, nausea, and vomiting, were lower than the non-Asian subgroup.23,59 Numeric differences regarding efficacy and TEAEs were found among the Asian and non-Asian subgroups. The differences are thought to result partly from the concurrence of the functional gastrointestinal disorders in the non-Asian subgroup.
Prucalopride was effective in patients with a defecatory disorder or constipation-predominant irritable bowel syndrome and slow-transit constipation.66 In addition to typical constipation symptoms (eg, straining, residual stool, and hard stool), abdominal symptoms (eg, bloating and discomfort) were improved.67 Therefore, prucalopride can be used in patients with chronic constipation of various subtypes if no response to traditional laxatives is noted.
Chronic opioid therapy could commonly cause opioid-induced constipation (OIC) in patients suffering from cancer- or noncancer-related pain.68 OIC is defined in Rome IV criteria as an atypical change in bowel habits after beginning opioid therapy with any of the following characteristics: bowel frequency reduction, development or exacerbation of tension at stools, incomplete evacuation sensation, or a patient’s feeling of discomfort regarding bowel habits.69 New OIC treatments have been approved by the Food and Drug Administration including methylnaltrexone, naloxegol, and naloxone with the recent approval of naldemedine and lubiprostone. In the case of prucalopride, two placebo-controlled double-blind trials have been conducted for the efficacy in OIC patients.70,71 However, one trial was discontinued prematurely due to the safety of unrelated business decision priority of the sponsor.72 The primary endpoint was set as the percentage of patients with >1 CSBM/week increase from the baseline. The prucalopride group showed modest results compared with the placebo group in these trials (RR = 0.74; 95% confidence interval (CI), 0.58–0.96; NNTT = 6).73 However, the therapeutic value, compared with the placebo, was lower in the prucalopride trial than in the methylnaltrexone and naloxegol trials.74 The total scores of Patient Assessment of Constipation-Symptom and Patient Assessment of Constipation-Quality of Life were improved in the prucalopride trial.70 The patients who remained constipated with active treatment were lower in the prucalopride trial (31%) compared with the methylnaltrexone trials (42%) and significant AEs were not reported.70,75
No published prucalopride trial is believed to exist regarding constipation-predominant IBS (IBS-C) even though the European Agency of Medicinal Products approved prucalopride as the treatment for laxative-resistant chronic constipation. An integrated data analysis from three double-blind trials, consisting of 936 constipated female patients, demonstrated favorable outcomes of prucalopride regarding typical IBS abdominal symptoms (eg, bloating, abdominal discomfort, and abdominal pain in these patients).67 A recent study48 using an intestinal gas transit test reported that prucalopride decreased the perception of abdominal symptoms in a small number of IBS-C patients (mean score, 2.3 ± 0.5) compared with the placebo group (mean score, 3.5 ± 0.3; p = 0.045) with no modification in the gas evacuation rate from the bowel. These results justify the conduct of a new trial investigating the use of prucalopride in IBS-C patients. However, no randomized controlled trial regarding prucalopride use in IBS-C patients is yet published. Since the stimulation of 5-HT4 receptors leads to visceral antinociceptive effects and prucalopride decreases abdominal symptoms (eg, abdominal pain and discomfort) without accelerating gas transit and modifying gas retention, the efficacy of prucalopride in IBS-C patients is expected.48,67 Moreover, prucalopride was reportedly used rather frequently in clinical practice among Canadian and Italian gastroenterologists for IBS-C patients in two recently conducted surveys.76,77 Further studies regarding this crucial clinical issue are required.
Analysis of Complications That Occurred
Cardiovascular Safety
The first-generation 5-HT4 receptor agonist’s cisapride and tegaserod were removed from the market due to associated cardiovascular risk. Arrhythmia was thought to be associated with QTc prolongation by cisapride, and an ischemic event of uncertain was caused by tegaserod. However, prucalopride works selectively only on 5-HT4 receptors, unlike cisapride and tegaserod. Thus, no risk of cardiovascular side effects was noted.78 The QT interval was not affected even when prucalopride was administered at a therapeutic dose (2 mg) and a dose exceeding the treatment dose (10 mg) in healthy volunteers.79 Palpitations have been reported during the first 2 days of taking prucalopride. However, the incidence is low, the incidence of other cardiovascular complications is very low, and no statistically significant difference exists compared with the placebo group.24,80 According to a multinational population-based cohort study by Gilsenan et al, the pooled adjusted incidence rate ratio of major adverse cardiovascular events in patients with chronic constipation using prucalopride was 0.64 when compared to PEG (95% CI, 0.36–1.14).81 In the post hoc analysis of major adverse cardiac events (MACE) from prucalopride’s clinical studies, 19 double-blind, placebo-controlled studies and 9 open-label studies for chronic idiopathic constipation (CIC) patients were noted. Moreover, the number of MACE in clinical trials with prucalopride in adults with CIC was low and similar to that for placebo.82 Therefore, attention should focus on using prucalopride in patients at risk of cardiovascular disease. However, the incidence of significant complications is very low. Thus, it can relatively be used safely.
Analysis of Side Effects According to Dose
A meta-analysis by Sajid showed an increasing trend in the number of patients with side effects after using 4 and 2 mg compared with 1 mg prucalopride. In patients with chronic constipation, the side effects of profiles identified by the OR of 1, 2, and 4 mg doses of prucalopride were 2.02, 1.76, and 1.52, respectively, indicating an increased prucalopride effect without increasing the side effects. However, the generally recommended dosage of prucalopride is 2 mg once daily.83 Exceeding a prucalopride dosage of 2 mg once daily is not expected to increase efficacy. The results of the Sajid study are only for a group of patients who have failed basic laxative treatments, lifestyle modifications, and potent laxative treatments. Therefore, generalizing and applying the conclusions to a group of patients for which all types of constipation and early treatment were not optimally tested is not possible.
Expert Opinion
In summary, it seems likely that prucalopride may help, especially in patients suffering from both gastroparesis and chronic constipation. According to a recent post hoc analysis analyzing six phases 3 and 4 randomized, double-blind, placebo-controlled studies of patients with significant abdominal bloating by Lembo et al, treatment with prucalopride improved symptoms compared with placebo, irrespective of baseline bloating severity, and was most effective in women and patients <65 years old with CIC.84 Thus, prucalopride had a beneficial effect on bloating, and the only item in the Carbone study that significantly improved in terms of quality of life was how patients’ clothing fit. Bloating is also often associated with lower gastrointestinal disorders and may originate from something other than the upper GI origin. Large effect sizes (>0.8) were seen on bloating and incomplete bowel movements according to a study by Tack et al.67 Thus, the clinical benefit of prucalopride in gastroparesis may be primarily related to a decrease in the perception of bloating, not as a result of a more intuitive prokinetic effect. In future clinical trials, a study investigating the increase of the therapeutic effect in patients with concurrent gastroparesis and chronic constipation may be conducted.
The long-term effectiveness of prucalopride is yet undisclosed.85 Prucalopride reportedly lost its efficacy gradually after the first few weeks of favorable and beneficial response in some patients. Tachyphylaxis, the development of tolerance, could cause this incomprehensible phenomenon, leading to drug dose escalation of reaching the same result. This often reversible mechanism could result either from an acceleration of the drug catabolism (inactivation) or reduced pharmacological activity due to receptor desensitization, which is more likely with prucalopride. The escalation of the prucalopride dose (up to 4 mg/day) does not provide sufficient efficacy in patients with tachyphylaxis. However, 2–4 weeks of delay followed by subsequent readministration of the previous effective dose of prucalopride seems to be more advantageous.
Prucalopride is currently recommended for use after failure of common laxatives, according to the guidelines. Moreover, prucalopride is not a first-line drug due to its relatively high price.86,87 The gradual decrease of previous laxatives, rather than abrupt stops, was recommended while waiting for the advent of prucalopride’s effect. Consequently, the gastroenterologist could adjust the therapeutic prucalopride scheme as needed without compromising the clinical efficacy because the drug’s efficacy is dose-dependent, and the drug may partially show its efficacy even below the commonly recommended dose. After the desired therapeutic response is achieved, the prucalopride administration schedule can be adjusted to determine each patient’s minimum effective dose. Even though no studies yet investigate the efficacy of the therapeutic schemes of prucalopride other than the daily 2 mg administration, this study could observe at least in some patients from the experience of this study that the therapeutic efficacy was maintained at a daily dose of 1 mg or with the administration of every other day or even with greater intervals. These therapeutic schemes may benefit the patients with minor AEs.
Conclusion: Analyzing the Data Presented in the Review
Prucalopride is an effective and safe option based on all the studies currently conducted. Its side effects are usually found on the first day of administration and are usually self-limiting and mostly mild to moderate. Its high selectivity for 5-HT4 receptor has a very favorable stability profile, and no concern exists for cardiovascular problems. Thus, it may be the first-line treatment for constipation or other gastrointestinal diseases in the future. Where possible, prucalopride should be considered when managing adult patients with constipation who have not obtained relief from laxatives, regardless of subtype or symptom pattern.66 Prucalopride also has the potential to be useful in the treatment of functional constipation and other forms of gastrointestinal diseases (eg, gastroparesis). Through the research on this potential, prucalopride is expected to be a useful and versatile option for treating gastrointestinal diseases in the future.
Funding
No external sources of funding were received for this study.
Disclosure
The author declares no conflicts of interests.
References
1. Bouras EP, Camilleri M, Burton DD, McKinzie S. Selective stimulation of colonic transit by the benzofuran 5HT4 agonist, prucalopride, in healthy humans. Gut. 1999;44:
2. Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:
3. Prins N, Grijn AVD, Lefebvre R, Akkermans LM, Schuurkes JA. 5‐HT4 receptors mediating enhancement of contractility in canine stomach; an in vitro and in vivo study. Br J Pharmacol. 2001;132:1941–1947. doi:10.1038/sj.bjp.0703985
4. Kessing B, Smout A, Bennink R, Kraaijpoel N, Oors JM, Bredenoord AJ. Prucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjects. Neurogastroenterol Motil. 2014;26:1079–1086. doi:10.1111/nmo.12359
5. Roque MV, Bouras EP. Epidemiology and management of chronic constipation in elderly patients. Clin Interv Aging. 2015;10:919–930. doi:10.2147/CIA.S54304
6. De Maeyer J, Lefebvre R, Schuurkes J. 5‐HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99–112. doi:10.1111/j.1365-2982.2007.01059.x
7. Prins N, Shankley N, Welsh N, et al. An improved in vitro bioassay for the study of 5‐HT4 receptors in the human isolated large intestinal circular muscle. Br J Pharmacol. 2000;129:1601–1608. doi:10.1038/sj.bjp.0703254
8. Hegde SS, Eglen RM. Peripheral 5‐HT4 receptors. FASEB J. 1996;10:1398–1407. doi:10.1096/fasebj.10.12.8903510
9. Briejer MR, Akkermans LM, Schuurkes JA. Gastrointestinal prokinetic benzamides: the pharmacology underlying stimulation of motility. Pharmacol Rev. 1995;47:631–651.
10. Deenadayalu VP, Rex DK. Colon polyp retrieval after cold snaring. Gastrointest Endosc. 2005;62:253–256. doi:10.1016/S0016-5107(05)00376-7
11. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300. doi:10.1001/jama.2018.19323
12. Mohammad S, Zhou Z, Gong Q, January CT. Blockage of the HERG human cardiac K+ channel by the gastrointestinal prokinetic agent cisapride. Am J Physiol Heart Circulat Physiol. 1997;273:H2534–H2538. doi:10.1152/ajpheart.1997.273.5.H2534
13. Smith J, Beattie D, Marquess D, Shaw JP, Vickery RG, Humphrey PP. The in vitro pharmacological profile of TD-5108, a selective 5-HT 4 receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Archiv Pharmacol. 2008;378:125–137. doi:10.1007/s00210-008-0282-y
14. Camilleri M, Vazquez‐Roque M, Burton D, et al. Pharmacodynamic effects of a novel prokinetic 5‐HT4 receptor agonist, ATI‐7505, in humans. Neurogastroenterol Motil. 2007;19:30–38. doi:10.1111/j.1365-2982.2006.00865.x
15. Zikos TA, Kamal AN, Neshatian L, et al. High prevalence of slow transit constipation in patients with gastroparesis. J Neurogastroenterol Motil. 2019;25:267. doi:10.5056/jnm18206
16. Hasler W, May K, Wilson L, et al. Relating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesis. Neurogastroenterol Motil. 2018;30:e13196. doi:10.1111/nmo.13196
17. Lei WY, Hung JS, Liu TT, Yi CH, Chen CL. Influence of prucalopride on esophageal secondary peristalsis in reflux patients with ineffective motility. J Gastroenterol Hepatol. 2018;33:650–655. doi:10.1111/jgh.13986
18. Camilleri M, Piessevaux H, Yiannakou Y, et al. Efficacy and safety of prucalopride in chronic constipation: an integrated analysis of six randomized, controlled clinical trials. Dig Dis Sci. 2016;61:2357–2372. doi:10.1007/s10620-016-4147-9
19. Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009;58:
20. Tack J, Quigley E, Camilleri M, Vandeplassche L, Kerstens R. Efficacy and safety of oral prucalopride in women with chronic constipation in whom laxatives have failed: an integrated analysis. United Eur Gastroenterol J. 2013;1:
21. Tack J, Corsetti M. Prucalopride: evaluation of the pharmacokinetics, pharmacodynamics, efficacy and safety in the treatment of chronic constipation. Expert Opin Drug Metab Toxicol. 2012;8:1327–1335. doi:10.1517/17425255.2012.719497
22. Camilleri M, Beyens G, Kerstens R, Robinson P, Vandeplassche L. Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study. Neurogastroenterol Motil. 2009;21:
23. Leelakusolvong S, Ke M, Zou D, et al. Factors predictive of treatment-emergent adverse events of prucalopride: an integrated analysis of four randomized, double-blind, placebo-controlled trials. Gut Liver. 2015;9:
24. Camilleri M, Van Outryve MJ, Beyens G, Kerstens R, Robinson P, Vandeplassche L. Clinical trial: the efficacy of open-label prucalopride treatment in patients with chronic constipation - Follow-up of patients from the pivotal studies. Aliment Pharmacol Ther. 2010;32:
25. Tack J, Camilleri M, Chang L, et al. Systematic review: cardiovascular safety profile of 5-HT4 agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35:745–767. doi:10.1111/j.1365-2036.2012.05011.x
26. Flach S, Scarfe G, Dragone J, et al. A phase I study to investigate the absorption, pharmacokinetics, and excretion of [14C]prucalopride after a single oral dose in healthy volunteers. Clin Ther. 2016;38:2106–2115. doi:10.1016/j.clinthera.2016.08.003
27. Wong BS, Manabe N, Camilleri M. Role of prucalopride, a serotonin (5-HT4) receptor agonist, for the treatment of chronic constipation. Clin Exp Gastroenterol. 2010;3:49–56. doi:10.2147/ceg.s8091
28. Smith WB, Mannaert E, Verhaeghe T, Kerstens R, Vandeplassche L, Van de Velde V. Effect of renal impairment on the pharmacokinetics of prucalopride: a single-dose open-label phase I study. Drug Des Devel Ther. 2012;6:407. doi:10.2147/DDDT.S36142
29. Müller-Lissner S, Rykx A, Kerstens R, Vandeplassche L. A double-blind, placebo-controlled study of prucalopride in elderly patients with chronic constipation. Neurogastroenterol Motil. 2010;22:
30. Mearin F, Ciriza C, Mínguez M, et al. Clinical practice guideline: irritable bowel syndrome with constipation and functional constipation in the adult. Rev Esp Enferm Dig. 2016;108:332–363. doi:10.17235/reed.2016.4389/2016
31. Carnovale C, Pellegrino P, Perrone V, et al. Neurological and psychiatric adverse events with prucalopride: case report and possible mechanisms. J Clin Pharm Ther. 2013;38:524–525. doi:10.1111/jcpt.12087
32. Sivabalasundaram V, Habal F, Cherney D. Prucalopride-associated acute tubular necrosis. World J Clin Cases. 2014;2:380–384. doi:10.12998/wjcc.v2.i8.380
33. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113–133. doi:10.1111/j.1365-2982.2009.01434.x
34. Siegfried WY, Rattanakovit K, Mack A, Rao S. Su1454 Is gastroparesis a pan-enteric neuropathic disorder: investigation with wireless motility capsule. Gastroenterology. 2015;148:
35. Janssen P, Harris SM, Jones M, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108:1382–1391. doi:10.1038/ajg.2013.118
36. Tack J, Goelen N, Carbone F, et al. Prokinetic effects and symptom relief in the pharmacotherapy of gastroparesis. Gastroenterology. 2020;158:1841–1842. doi:10.1053/j.gastro.2019.06.049
37. Revicki D, Rentz A, Dubois D, et al. Development and validation of a patient‐assessed gastroparesis symptom severity measure: the gastroparesis cardinal symptom index. Aliment Pharmacol Ther. 2003;18:141–150. doi:10.1046/j.1365-2036.2003.01612.x
38. Revicki D, Camilleri M, Kuo B, Szarka LA, McCormack J, Parkman HP. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index‐Daily Diary (GCSI‐DD). Neurogastroenterol Motil. 2012;24:456–463. doi:10.1111/j.1365-2982.2012.01879.x
39. Revicki D, Gleeson S, Speck R, et al. The American Neurogastroenterology and Motility Society Gastroparesis Cardinal Symptom Index-Daily Diary (ANMS GCSI-DD): assessing the content validity in patients with idiopathic or diabetic gastroparesis. Value Health. 2018;21:S86. doi:10.1016/j.jval.2018.04.588
40. Fehnel S, Nelson L, DiBenedetti D, DiBenedetti D, Spence S, Carson RT. Development and psychometric evaluation of the diabetic gastroparesis symptom severity diary. Gastroenterology. 2017;152:S517. doi:10.1016/S0016-5085(17)31904-2
41. Vijayvargiya P, Jameie-Oskooei S, Camilleri M, et al. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut. 2019;68:804–813. doi:10.1136/gutjnl-2018-316405
42. Pasricha PJ, Camilleri M, Hasler WL, Parkman HP. White paper AGA: gastroparesis: clinical and regulatory insights for clinical trials. Clin Gastroenterol Hepatol. 2017;15:1184–1190. doi:10.1016/j.cgh.2017.04.011
43. Carbone F, Van Den Houte K, Clevers E, et al. Prucalopride in gastroparesis: a randomized placebo-controlled crossover study. Am J Gastroenterol. 2019;114:1265–1274. doi:10.14309/ajg.0000000000000304
44. Andrews CN, Woo M, Buresi M, et al. Prucalopride in diabetic and connective tissue disease‐related gastroparesis: randomized placebo‐controlled crossover pilot trial. Neurogastroenterol Motil. 2020;e13958.
45. Vijayvargiya P, Camilleri M, Chedid V, Mandawat A, Erwin PJ, Murad MH. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta-analysis. Gastroenterology. 2019;156:1650–1660. doi:10.1053/j.gastro.2019.01.249
46. Carbone F, Van den Houte K, Schol J, Schol J, Goelen N, Tack J. The relation between changes in gastric emptying rate and improvement of simultaneously measured symptoms with prokinetic agents; an analysis of 8 treatment trials. Gastroenterology. 2020;158:S624–S624. doi:10.1016/S0016-5085(20)32259-9
47. Rayner CK, Jones KL, Horowitz M. Is making the stomach pump better the answer to gastroparesis? Gastroenterology. 2019;156:1555–1557. doi:10.1053/j.gastro.2019.03.030
48. Malagelada C, Nieto A, Mendez S, et al. Effect of prucalopride on intestinal gas tolerance in patients with functional bowel disorders and constipation. J Gastroenterol Hepatol. 2017;32:
49. Tack J, Van den Houte K, Carbone F. The unfulfilled promise of prokinetics for functional dyspepsia/postprandial distress syndrome. Am J Gastroenterol. 2019;114:204–206. doi:10.14309/ajg.0000000000000072
50. Carbone F, Goelen N, Porters K, Van Loock J. Impaired gastric distribution of a meal is associated with impaired intragastric pressure measurement and satiation in FD. Gastroenterology. 2017;152:S304.
51. Carbone F, Vanuytsel T, Tack J. Analysis of postprandial symptom patterns in subgroups of patients with Rome III or Rome IV functional dyspepsia. Clin Gastroenterol Hepatol. 2020;18:838–846. e3. doi:10.1016/j.cgh.2019.07.053
52. Goelen N, de Hoon J, Morales JF, et al. Codeine delays gastric emptying through inhibition of gastric motility as assessed with a novel diagnostic intragastric balloon catheter. Neurogastroenterol Motil. 2020;32:e13733. doi:10.1111/nmo.13733
53. Tack J. Prokinetics and fundic relaxants in upper functional GI disorders. Curr Opin Pharmacol. 2008;8:690–696. doi:10.1016/j.coph.2008.09.009
54. Stanghellini V, Tack JJG. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972–1978. doi:10.1136/gutjnl-2013-306084
55. Abell T, Kuo B, Esfandyari T, et al. 784–Velusetrag improves Gastoparesis both in symptoms and gastric emptying in patients with diabetic or idiopathic gastroparesis in a 12-week global Phase 2B study. Gastroenterology. 2019;156:S–164.
56. Bauman ZM, Evans CH. Volvulus. Surg Clin North Am. 2018;98:973–993. doi:10.1016/j.suc.2018.06.005
57. Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:
58. Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation–a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29:
59. Ke M, Tack J, Quigley EMM, et al. Effect of prucalopride in the treatment of chronic constipation in Asian and non-Asian women: a pooled analysis of 4 randomized, placebo-controlled studies. J Neurogastroenterol Motil. 2014;20:458–468. doi:10.5056/jnm14029
60. Mugie SM, Korczowski B, Bodi P, et al. Prucalopride is no more effective than placebo for children with functional constipation. Gastroenterology. 2014;147:
61. Cinca R, Chera D, Gruss HJ, Halphen M. Randomised clinical trial: macrogol/PEG 3350+electrolytes versus prucalopride in the treatment of chronic constipation – a comparison in a controlled environment. Aliment Pharmacol Ther. 2013;37:
62. Quigley EMM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation - A 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29:315–328.
63. Yiannakou Y, Piessevaux H, Bouchoucha M, et al. A randomized, double-blind, placebo-controlled, Phase 3 trial to evaluate the efficacy, safety, and tolerability of prucalopride in men with chronic constipation. Am J Gastroenterol. 2015;110:741. doi:10.1038/ajg.2015.115
64. Ke M, Zou D, Yuan Y, et al. Prucalopride in the treatment of chronic constipation in patients from the Asia-Pacific region: a randomized, double-blind, placebo-controlled study. Neurogastroenterol Motil. 2012;24:
65. Quigley EM. Prucalopride: safety, efficacy and potential applications. Therap Adv Gastroenterol. 2012;5:23–30. doi:10.1177/1756283X11423706
66. Jadav A, McMullin C, Smith J, Chapple K, Brown SR. The association between prucalopride efficacy and constipation type. Tech Coloproctol. 2013;17:555–559. doi:10.1007/s10151-013-1017-8
67. Tack J, Stanghellini V, Dubois D, Joseph A, Vandeplassche L, Kerstens R. Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterol Motil. 2014;26:
68. Müller-Lissner S, Bassotti G, Coffin B, et al. Opioid-induced constipation and bowel dysfunction: a clinical guideline. Pain Med. 2017;18:1837–1863.
69. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393–1407. e5. doi:10.1053/j.gastro.2016.02.031
70. Sloots CEJ, Rykx A, Cools M, Kerstens R, De Pauw M. Efficacy and safety of prucalopride in patients with chronic noncancer pain suffering from opioid-induced constipation. Dig Dis Sci. 2010;55:2912–2921. doi:10.1007/s10620-010-1229-y
71. Forrest JH, Finlayson N, Shearman D. Endoscopy in gastrointestinal bleeding. Lancet. 1974;304:394–397. doi:10.1016/S0140-6736(74)91770-X
72. Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi:10.1016/j.cell.2007.01.029
73. Luthra P, Burr NE, Brenner DM, Ford AC. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and network meta-analysis. Gut. 2019;68:434–444. doi:10.1136/gutjnl-2018-316001
74. Nee J, Zakari M, Sugarman M, et al. Efficacy of treatments for opioid-induced constipation: a systematic review and meta-analysis. Gastroenterology. 2018;154:
75. Sonu I, Triadafilopoulos G, Gardner JD. Persistent constipation and abdominal adverse events with newer treatments for constipation. BMJ Open Gastroenterol. 2016;3:e000094. doi:10.1136/bmjgast-2016-000094
76. Tse Y, Armstrong D, Andrews CN, et al. Treatment algorithm for chronic idiopathic constipation and constipation-predominant irritable bowel syndrome derived from a Canadian national survey and needs assessment on choices of therapeutic agents. Can J Gastroenterol Hepatol. 2017;2017:8612189. doi:10.1155/2017/8612189
77. Soncini M, Stasi C, Satta PU, et al. IBS clinical management in Italy: the AIGO survey. Digest Liver Dis. 2019;51:782–789. doi:10.1016/j.dld.2018.10.006
78. Hong KS, Jung KW, Lee TH, et al. Current issues on the treatment of chronic constipation. Korean J Gastroenterol= Taehan Sohwagi Hakhoe Chi. 2014;64:148–153. doi:10.4166/kjg.2014.64.3.148
79. Mendzelevski B, Ausma J, Chanter DO, et al. Assessment of the cardiac safety of prucalopride in healthy volunteers: a randomized, double-blind, placebo- and positive-controlled thorough QT study. Br J Clin Pharmacol. 2012;73:203–209. doi:10.1111/j.1365-2125.2011.04088.x
80. Chaplin S, Blaker P, Wilkinson M. Prucalopride (Resolor): new treatment for chronic constipation. Prescriber. 2010;21:24–29. doi:10.1002/psb.639
81. Gilsenan A, Fortuny J, Cainzos-Achirica M, et al. Cardiovascular safety of prucalopride in patients with chronic constipation: a multinational population-based cohort study. Drug Saf. 2019;42:1179–1190. doi:10.1007/s40264-019-00835-0
82. Hinson J, Achenbach H, Kerstens R. Sa1719 evaluation of major adverse cardiac events from clinical studies of prucalopride in patients with chronic idiopathic constipation. Gastroenterology. 2020;158:S–396. doi:10.1016/S0016-5085(20)31681-4
83. Keating GM. Prucalopride: a review of its use in the management of chronic constipation. Drugs. 2013;73:1935–1950. doi:10.1007/s40265-013-0140-1
84. Lembo A, Hinson J, Kerstens R, et al. Efficacy of prucalopride in patients with chronic idiopathic constipation: an analysis of patients with significant abdominal bloating. Gastroenterology. 2020;158:
85. Omer A, Quigley EM. An update on prucalopride in the treatment of chronic constipation. Therap Adv Gastroenterol. 2017;10:877–887. doi:10.1177/1756283X17734809
86. Serra J, Mascort-Roca J, Marzo-Castillejo M, et al. Clinical practice guidelines for the management of constipation in adults. Part 2: diagnosis and treatment. Gastroenterología y Hepatología (English Edition). 2017;40:303–316.
87. Bassotti G, Gambaccini D, Bellini M. Prucalopride succinate for the treatment of constipation: an update. Expert Rev Gastroenterol Hepatol. 2016;10:291–300. doi:10.1586/17474124.2016.1129897
88. Krogh K, Jensen MB, Gandrup P, et al. Efficacy and tolerability of prucalopride in patients with constipation due to spinal cord injury. Scand J Gastroenterol. 2002;37:
89. Emmanuel A, Roy A, Nicholls T, Kamm M. Prucalopride, a systemic enterokinetic, for the treatment of constipation. Aliment Pharmacol Ther. 2002;16:1347–1356. doi:10.1046/j.1365-2036.2002.01272.x
90. Sloots CE, Poen AC, Kerstens R, et al. Effects of prucalopride on colonic transit, anorectal function and bowel habits in patients with chronic constipation. Aliment Pharmacol Ther. 2002;16:
91. Coremans G, Kerstens R, De Pauw M, Stevens M. Prucalopride is effective in patients with severe chronic constipation in whom laxatives fail to provide adequate relief. Results of a double-blind, placebo-controlled clinical trial. Digestion. 2003;67:
92. Piessevaux H, Camilleri M, Yiannakou Y, et al. Efficacy and safety of prucalopride in adults with chronic constipation: an integrated analysis of six randomized controlled clinical trials. United Eur Gastroenterol J. 2015;3:A459–A460.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
