Back to Journals » International Journal of Nanomedicine » Volume 18
Current Knowledge and Future Perspectives of Exosomes as Nanocarriers in Diagnosis and Treatment of Diseases
Authors Zou Z , Li H, Xu G, Hu Y, Zhang W, Tian K
Received 4 May 2023
Accepted for publication 29 July 2023
Published 21 August 2023 Volume 2023:18 Pages 4751—4778
DOI https://doi.org/10.2147/IJN.S417422
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dongwoo Khang
Zaijun Zou,1,2 Han Li,1,2 Gang Xu,1,3 Yunxiang Hu,2 Weiguo Zhang,1,3 Kang Tian1,3
1Department of Sports Medicine, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, 116011, People’s Republic of China; 2School of Graduates, Dalian Medical University, Dalian, Liaoning, 116000, People’s Republic of China; 3Key Laboratory of Molecular Mechanism for Repair and Remodeling of Orthopaedic Disease, Dalian, Liaoning Province, 116011, People’s Republic of China
Correspondence: Kang Tian; Weiguo Zhang, Tel +86-18098871513 ; +86-18041191111, Email [email protected]; [email protected]
Abstract: Exosomes, as natural nanocarriers, characterized with low immunogenicity, non-cytotoxicity and targeted delivery capability, which have advantages over synthetic nanocarriers. Recently, exosomes have shown great potential as diagnostic markers for diseases and are also considered as a promising cell-free therapy. Engineered exosomes have significantly enhanced the efficacy and precision of delivering therapeutic agents, and are currently being extensively employed in targeted therapeutic investigations for various ailments, including oncology, inflammatory disorders, and degenerative conditions. Particularly, engineered exosomes enable therapeutic agent loading, targeted modification, evasion of MPS phagocytosis, intelligent control, and bioimaging, and have been developed as multifunctional nano-delivery platforms in recent years. The utilization of bioactive scaffolds that are loaded with exosome delivery has been shown to substantially augment retention, extend exosome release, and enhance efficacy. This approach has advanced from conventional hydrogels to nanocomposite hydrogels, nanofiber hydrogels, and 3D printing, resulting in superior physical and biological properties that effectively address the limitations of natural scaffolds. Additionally, plant-derived exosomes, which can participate in gut flora remodeling via oral administration, are considered as an ideal delivery platform for the treatment of intestinal diseases. Consequently, there is great interest in exosomes and exosomes as nanocarriers for therapeutic and diagnostic applications. This comprehensive review provides an overview of the biogenesis, composition, and isolation methods of exosomes. Additionally, it examines the pathological and diagnostic mechanisms of exosomes in various diseases, including tumors, degenerative disorders, and inflammatory conditions. Furthermore, this review highlights the significance of gut microbial-derived exosomes. Strategies and specific applications of engineered exosomes and bioactive scaffold-loaded exosome delivery are further summarized, especially some new techniques such as large-scale loading technique, macromolecular loading technique, development of multifunctional nano-delivery platforms and nano-scaffold-loaded exosome delivery. The potential benefits of using plant-derived exosomes for the treatment of gut-related diseases are also discussed. Additionally, the challenges, opportunities, and prospects of exosome-based nanocarriers for disease diagnosis and treatment are summarized from both preclinical and clinical viewpoints.
Keywords: exosomes, nanocarriers, diseases, diagnosis, treatment
Introduction
The fields of oncology, immunology, and regenerative medicine have consistently presented challenges and served as focal points for clinical management research. Notably, targeted therapies have made significant strides in the treatment of these diseases, particularly with the advancements in nanomedicine.1–4 Several artificial nanocarriers such as liposomes, nanotubes, micelles, dendrimers and self-assembled peptides have been developed for targeted delivery of therapeutic agents.5–9 However, with the clinical translation of nanocarriers, several challenges have emerged, such as cytotoxicity, immunogenicity, complex fabrication processes, and other drawbacks.10–12
Exosomes are also considered nanoparticles (NPs) owing to their nanoscale size and some properties similar to NPs, including passive targeting ability and enhanced permeability and retention effects.13 Typically, exosomes are highly stable, with low immunogenicity, and can be transported long distances in vivo across physiological barriers.14 Specific membrane proteins and carrier ligands such as tetraspanins and integrins on the exosome membrane surface mediate the specific binding of exosomes to target cells with specific cytophilic properties and homing selectivity.15 Therefore, exosomes are considered to be a natural nanocarrier. Additionally, exosomes can be secreted by all eukaryotic cells and bacteria, are the main mediators of extracellular communication and carry proteins, nucleic acids, lipids and other active components that can be detected in body fluids such as blood, urine, joint fluid, saliva and breast milk.16 Therefore, exosome as a liquid biopsy method can be used for disease diagnosis. Significantly, the emergence of gut microbiome analysis techniques has led to the identification of extracellular vesicles (EVs) derived from the gut microbiota, which have been detected in blood and urine samples of both healthy individuals and patients exhibiting characteristics strongly associated with the gut microbiome. These EVs may offer a novel approach for the diagnosis and prognosis of gut-related disorders.17
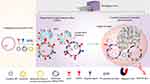
Properties of natural exosomes make them an alternative to cellular therapies with increasing applications in anti-tumor, immunomodulatory and regenerative medicine. To further improve efficacy, engineered exosomes have gained popularity recently, such as loading schemes for different therapeutic agents, engineered membrane modifications, and engineered hybridization with artificial NPs based on exosomes (Figure 1). These engineered strategies have achieved remarkable results in terms of enhanced drug utilization, enhanced targeting, controlled release, and bioimaging.18–20 However, several studies have shown that exosomes tend to accumulate in the liver, lungs, and spleen and are rapidly eliminated by the mononuclear phagocytic system (MPS).21–23 In addition, phosphatidylserine from the phospholipid bilayer of exosomes membranes can be recognized by macrophages, leading to exosome clearance.24 Therefore, in recent years, researchers have again performed bionic optimization of exosomes, including membrane coating techniques, and design of targeted ligands that block MPS phagocytosis to circumvent this phenomenon.25,26 Based on the integration of these approaches, a series of multifunctional nano-delivery platforms have been developed successively.27 On the other hand, several new techniques have been developed successively in recent years to overcome the challenges of loading large molecules of RNA and proteins as well as large-scale loading.27–29 In addition, with the development of tissue engineering, bioactive scaffolds loaded with exosomes can enable precise delivery, avoid rapid clearance, and effectively improve retention, such as hydrogels.30 In recent years, advances in nanomaterials technology have facilitated the development of nanocomposite hydrogel and nanofiber hydrogel, which possess excellent mechanical properties, porosity and biocompatibility, improving the shortcomings of natural hydrogels.31,32 The role of exosomes in the pathological mechanisms of various diseases has been extensively reported.33–35 Recently, an increasing number of studies have shown that gut microbial-derived vesicles mediate microbiota function through the transport and delivery to host cells of effector molecules that regulate host signaling pathways and cellular processes.36,37 Gut microbiota-derived exosomes possess the potential to exert a substantial influence on health and disease. Conversely, orally-administered exosomes, such as those derived from plants, exhibit anti-inflammatory and protective characteristics towards intestinal tissues, and can also contribute to the restructuring of the intestinal microbiota. These exosomes are deemed to be optimal delivery vehicles for treating ailments associated with intestinal and microbiota dysbiosis.38
 |
Figure 1 An overview of the main sources and applications of natural exosomes, as well as the main delivery strategies and engineering modification strategies for exosomes. |
In light of the benefits of exosomes as natural nanocarriers over synthetic nanocarriers, as well as their growing importance as nanocarriers in the diagnosis and treatment of clinical disease, this paper firstly discusses exosome composition, biogenesis, uptake, and isolation techniques. The role of exosomes in disease pathological processes, including the recent popularity of gut microbial-derived exosomes, is also discussed. More importantly, the potential of exosomes as natural nanocarriers in disease diagnosis and treatment is demonstrated using latest research. Strategies and recent advances in engineered exosomes and bioactive scaffold-loaded exosomes delivery are also summarized, particularly multifunctional nano-delivery platforms and nanomaterial hydrogels. In addition, studies related to plant-derived exosomes and their advantages in gut microbial remodeling are also described. Through continued investigation into exosomes, the comprehension of their function as nanocarriers in the diagnosis and treatment of diseases will be enhanced. It is our aspiration that this article will aid in the clinical implementation and utilization of exosomes as nanocarriers for both therapeutic and diagnostic objectives in the foreseeable future.
Structure and Composition of Exosomes
Exosomes belong to EVs, approximately 30–150 nm in diameter, which are spherical lipid bilayer vesicles.39 The exosome membrane is a bilayer lipid membrane, mainly composed of unsaturated lipids, including cholesterol, phosphatidylserine and sphingomyelin. Generally, the unsaturated lipids and sphingolipids are believed to protect exosomes and maintain membrane rigidity, which is essential for maintaining exosome stability in vivo.40 Also, they are involved in cargo sorting and exosome secretion.41 The exosomal protein components include mainly the tetraspanin family (CD9, CD63 and CD81), membrane transport and fusion proteins (GTPases, annexins and flotillin), integrins, heat shock proteins (HSPs), endosomal sorting complex proteins required for transport (Alix, TSG101), cytoskeletal proteins and some proteins in specific types of exosomes such as transferrin receptors and MHC I and II molecules (Figure 2).42,43 In addition to proteins and lipids, exosomes contain large amounts of DNA, mRNA and non-coding RNA, which are the main cargo of intercellular communication and reflect the state of parental cells while influencing the biological processes of target cells.44
 |
Figure 2 Mechanisms of exosome biogenesis and uptake, and the main structure and composition of exosomes. |
Biogenesis and Uptake of Exosomes
Biogenesis
Exosomes originate from the endosomal system (Figure 2). Extracellular components, including proteins and lipids, can enter the cell along with cell surface proteins via endocytosis and plasma membrane invagination to form early endosomes, which further mature into late endosomes.45 The endosomal membrane buds inward to form luminal vesicles (ILVs), and late endosomes containing a large number of small vesicles are called multivesicular bodies (MVBs), during this process components derived from cytoplasmic proteins, nucleic acids and lipids are sorted into these vesicles.46 These MVBs are either fused to lysosomes and degraded or transported to the plasma membrane via the cytoskeleton and microtubule network to release ILVs extracellularly as exosomes.47 The fate of MVBs has been shown to be related to its internal cholesterol level. That means MVBs containing high concentrations of cholesterol more readily escape degradation from lysosomes and are released by plasma membrane fusion.48 The mechanism of exosome biogenesis is extremely complex and involves biological processes such as cargo sorting, vesicle formation, vesicle translocation, and exosome release. The current view is that MVB and exosome formation and release are primarily related to mechanisms mediated by the endosomal sorting complex required for transport (ESCRT) and related proteins (eg VPS4, VTA1, ALIX).49,50 ESCRT is a complex protein machine consisting of four different protein complexes, including ESCRT-0, -I, -II, -III.51,52 Specifically, ubiquitinated (UB)-cargo proteins are recognized by ESCRT-0, which has a ubiquitin structural domain (UBD), and are sorted into the lipid structural domain, thereby initiating an ESCRT-dependent mechanism. Ubiquitinated structural domains are also present in ESCRT-I and ESCRT-II, which can work together with ESCRT-0 and produce a high affinity for ubiquitinated cargo at the site of ILV formation sorting domains.49,53 ESCRT-I then recruits ESCRT -III via ESCRT-II or Alix, and finally, ESCRT is mechanically dissociated by the action of AAA-ATPase VPS4.54 However, the depletion of ESCRT components only reduces exosome secretion and does not completely block its secretion, so it is speculated that non-ESCRT-dependent mechanisms.55 Often, exosomal membranes are enriched in tetraspanins, which are known to be markers of exosomes. These proteins have been found to be associated with exosome release and the independent-ESCRT pathway. For example, an Epstein Barr virus-associated oncoprotein (latent membrane protein 1) was found to bind to the tetraspanin CD63, can be sorted into exosomes, avoid lysosomal degradation, and promote exosome release.56 Another study found that in HEK 293T cells, tetraspanins CD9 and CD82 promote β-linked protein secretion via a ceramide-dependent exosome pathway.57 Notably, sphingomyelin ceramide has been shown to play a role in exosome biogenesis and to inhibit neutral sphingomyelinase, a key enzyme that facilitates the breakdown of sphingomyelin to phosphorylcholine and ceramide, reducing exosome secretion.58 In addition, some small GTPases of the Rab-related protein (Rabs) family have been shown to play an important role in intracellular vesicle transport, affecting exosome secretion.59 Rab35 regulates the docking of MVB to the plasma membrane in oligodendrocytes, and affects the secretion of exosomes.60 Ostrowski et al used RNA interference screening to find that Rab27a and Rab27b mediate MVB-plasma membrane docking, while Rab27a knockdown inhibits MVB-plasma membrane docking, and two effector proteins of Rab27, Slp4 and Slac2b, were also identified, which are associated with exosomal secretion of Rab27a and Rab27b.61 Furthermore, genomic DNA (gDNA) is mainly found in the nucleus, while exosomes occur in the cytoplasm, and the mechanism of how DNA is loaded remains unclear. A recent study found that nuclear contents, including gDNA, are loaded into exosomes with the assistance of the tetraspanin CD63 after the collapse of cytoplasmic structures encased by the nuclear membrane (micronucleus).62
Uptake
Exosomes serve as the primary mediator of intercellular communication, and multiple mechanisms were identified to mediate this biological process, such as endocytosis, direct plasma membrane fusion and intercellular receptor pathways (Figure 2).63 Of these, endocytosis is the most extensively studied and includes mechanisms of endocytosis mediated by the clathrin, caveolin, macropinocytosis, phagocytosis, and lipid rafts.64 Most endocytosed exosomes target lysosomes to be degraded to release cargo, or they can escape from lysosomes and form endosomal cycles.65 In some cases, exosomes activate cellular signaling pathways through specific binding to target cell receptor proteins and do not involve endocytosis. Previous studies have demonstrated that neural stem cell-derived exosomes activate the Stat1 signaling pathway in target cells via IFN-γ/Ifngr1.66 Another possible uptake mechanism is the direct fusion of the exosome membrane with the target cell membrane, releasing the cargo directly into the cytoplasm. Melanoma cells were observed to take up exosomes via membrane fusion by fluorescent lipid decolorization technique, and the acidic pH environment may facilitate this mechanism.67 Exosomes from different cells preferentially interact with specific cell types. For instance, exosomes derived from oligodendrocyte precursor cells are endocytosed by microglia but not by astrocytes, while exosomes from cortical neurons are not taken up by glial cells and tend to bind to other neuronal cells.68,69 The mechanism of this specific uptake is not well unknown and may depend on the cell type or the specific recognition of various lipid and adhesion proteins of the exosome membrane with the target cell surface, such as tetraspanins, integrins, ECM proteins and proteoglycans, which determine the localization and entrance of the exosome.64,70
Isolation Techniques of Exosomes
Ultracentrifugation is currently the most commonly used technique for exosome isolation. The principle is that different extracellular components in the sample can be separated successively according to density, size and shape at appropriate centrifugal forces, two main methods included, differential speed and gradient density. Firstly, dead cells, cell debris and large extracellular vesicles are removed after a series of successive low to medium centrifugation, and then exosomes are further separated by centrifugation at 100,000 x g.71 All components with overlapping density, size and mass including exosomes, microvesicles, proteins, can be precipitated in the centrifuge tube.72 Therefore, the composition obtained at the end of differential ultracentrifugation contains proteins or other non-exosomal components, which affects the purity and is extremely unfavorable for further studies of exosomes. While adding the sample to the top of a density gradient medium (iodixanol or sucrose) followed by prolonged centrifugation, extracellular components, such as exosomes, apoptotic vesicles, and protein aggregates, can be easily separated into components with different buoyant densities. For example, protein aggregations are mainly concentrated at the bottom of the centrifuge tube, while exosomes are retained in the media layer between 1.1–1.2 g/mL.73 Therefore, density gradient differential centrifugation is often combined with differential ultracentrifugation to improve the purity of exosome isolation.
Ultrafiltration is a method of isolating exosomes using nanomembranes based on the size of the exosomes. The classical design is non-exosomal components, large vesicles including apoptotic vesicles, are filtered out by a 200 nm membrane, while the remaining 20–200 nm diameter vesicles are left behind and smaller particles (proteins) are further filtered through a 20 nm microfilter, finally, leaving the exosomes.74 Compared to ultracentrifugation, ultrafiltration is less time consuming and simple to operate. However, some disadvantages such as membrane pore clogging have also been reported, leading to low capture efficiency or membrane damage.75 The shear stress caused by the membrane pores to exosomes is also an issue that has to be considered.74 In addition, some NPs that are similar in size to exosomes cannot be filtered out and can affect their purity.75
The exosome membrane contains a large number of tetraspanins considered to be markers of exosomes (eg CD9, CD63, CD81), as well as the presence of MHC I and II molecules and heat shock proteins. Immunoaffinity capture techniques have been developed to isolate exosomes by exploiting the immunological interaction between these proteins (antigens) and their antibodies. A recent study successfully captured exosomes from the plasma of COVID-19 patients using the immunoaffinity of CD81 antibody with the exosomal surface antigen protein CD81.76 In another study, researchers used magnetic beads coated with MHC II antibodies to isolate exosomes from cell culture supernatants.77 Typically, the purity of exosomes isolated by immunoaffinity techniques is higher compared to other exosome isolation techniques.78 However, this highly specific isolation method may result in low yields and cannot be used for high-throughput isolation of exosomes.
Polymer precipitation methods usually use the interaction between highly hydrophilic polymers and water molecules surrounding the exosomes to form a hydrophobic microenvironment that reduces the solubility of the exosomes and isolates them under centrifugal conditions.79 Polyethylene glycol (PEG) is a widely accepted non-toxic polymer that is commonly used in polymer precipitation methods.80 The polymer precipitation method is relatively simple to perform and has a high yield. Although it is possible to precipitate exosomes, this may lead to high contamination rates and low purity of the isolated exosomes.81,82
Size exclusion chromatography (SEC) is also a technique for isolating exosomes based on molecular size. Specifically, as the sample passes through a stationary phase consisting of porous resin particles, molecules smaller than the pores of the stationary phase enter the pores preferentially resulting in longer distances and relatively slower speeds, and larger molecules that cannot enter the pores are forced to surround the porous particles and elute from the column. Commonly used materials such as dextran polymers, agarose and polyacrylamide.83 Hong et al successfully isolated high-purity exosomes of approximately 50–200 nm in size from acute myeloid leukemia plasma using a small-size exclusion chromatography column with agarose 2B.84 Although this method does not affect the structure and activity of exosomes and is low-cost, the disadvantage is time-consuming and difficult to be used for high-throughput isolation.85 In addition, the size of exosomes may overlap with some contaminants, which is a common drawback of size-based exosome isolation methods. To improve this problem, it can be solved by combining different separation methods. Previous studies have shown that combining ultrafiltration and SEC to separate exosomes significantly improves exosome purity compared to SEC or ultrafiltration alone, and does not affect their functionality.86
Microfluidic technology enables the integration of multiple assays, sensors and other components on the same chip to manipulate small fluid volumes in microchannels of tens to hundreds of microns in size, exploiting the biological or physical properties of exosomes for isolation and detection, demonstrating high efficiency, rapidity and low sample requirements.87 One commonly used microfluidic technique is based on immunoaffinity. For instance, researchers recently developed a microfluidic device made of polydimethylsiloxane (PDMS) and functionalized using a CD63 antibody that specifically binds to the CD63 antigen on the surface of exosomes to isolate them. The source of ExoChip-immobilized exosomes was confirmed using immunoelectron microscopy and protein blotting.88 Another more common technique is the acoustic nanofilter, in which a matrix containing exosomes and other extracellular components is injected into a chamber exposed to ultrasound, using acoustic waves to isolate extracellular vesicles according to their size and density, larger particles are subjected to stronger radiation forces and therefore migrate more rapidly toward the pressure node, and the ultrasound can be adjusted to separate particles of the desired size.89 Viscoelastic microfluidic separation techniques developed in recent years have also been shown to isolate exosomes with high efficiency and purity by exploiting the different elastic lift forces of particles of different sizes through viscoelastic media such as polyoxyethylene.90 In conclusion, a variety of microfluidic-based isolation techniques for high-throughput exosome acquisition have been developed as a result of advances in microfluidics.
Recently, advances in nanomaterials technology are expected to improve the efficiency of exosome capture and isolation. For example, researchers developed an Fe3O4@TiO2-CD63 aptamer composite nanomaterial for the rapid isolation of exosomes, double capture was achieved by relying on the high affinity of TiO2 to phosphate groups on the lipid bilayer of exosomes and the specific binding of CD63 aptamer to exosome membrane proteins, and the results showed that 92.6% of exosomes were captured <10 min.91 Zhang et al developed a nano-interfaced microfluidic platform for exosome isolation using nanostructured graphene oxide/polydopamine coated membranes on the channel and microcolumn surfaces to increase the surface area and antibody immobilization density, greatly improving the efficiency of exosome immunocapture and effectively inhibiting the adsorption of nonspecific exosomes, while the reactive sites on the polydopamine coating allow easy covalent coupling of protein G to immobilize captured antibodies in a targeted manner, further improving purity.92
The Role of Exosomes in Disease
Exosomes act as communication mediators between cells, and a large number of studies have shown that they are involved in the pathological processes of numerous diseases. The tumor microenvironment (TME) is mainly composed of tumor cells, stromal cells and stromal cell molecules. A growing number of research have shown that exosome biogenesis and cargo delivery serve an essential role in the regulation of the TME and are involved in biological processes such as tumor cell growth, tumor metastasis, and drug resistance. For example, compared with normal colonic epithelial cell-derived exosomes, colorectal cancer cell-derived exosomes promote cancer cell proliferation and inhibit apoptosis, participating in tumor progression.93 Paracrine exosomes from breast cancer cells inhibit T-cell activation through the programmed death 1 (PD-1)/PD ligand pathway, allowing cancer cells to evade surveillance from the immune system and metastasize to other tumor cells that do not express PDL1, facilitating metastasis between tumor cells.94 Ma et al found that exosomes derived from non-small cell lung cancer cells contained miR-3157-3p delivered to vascular endothelial cells, targeted downregulation of TIMP/KLF2 levels in endothelial cells, increased VEGF/MMP expression, promoted angiogenesis, increased vascular permeability, thereby promoting tumor progression.95 In addition, tumor cell-derived exosomes can promote tumor metastasis and drug resistance.96–99 Furthermore, the researchers found that injections of exosome secretion inhibitors significantly reduced overall exosome secretion and inhibited tumor growth and tumor cell metastasis, possibly owing to the predominance of tumor cell numbers in TME.93,100 It also suggests that exosomes are not only involved in tumor progression, and inhibiting the secretion of exosomes from tumor cells may be beneficial to intervene in tumor progression. Within the central nervous system (CNS), exosomes carry and release a variety of molecules related to neuronal function and neurotransmission in the brain, and have been shown to facilitate neuron-glia communication,101 synaptic plasticity,102 and neuronal development.103 On the other hand, exosomes are also involved in disease progression. Studies have shown that brain-derived exosomes in Alzheimer’s disease (AD) contain increased amounts of toxic amyloid (Aβ) and found that AD brain-derived exosomes can mediate the transmission of Aß.104 These findings indicated that exosomes may mediate the pathological progression of AD. Another study showed that microglia secrete tau proteins via exosomes to facilitate neuronal transmission, while inhibition of exosome synthesis dramatically decreased tau protein transmission in vitro and in vivo.105 Moreover, Aβ and hyperphosphorylated tau are considered to be the central mechanisms in the pathogenesis of AD. Similarly, it is unclear whether the number of exosomes is up- or down-regulated in neurological diseases. Interestingly, a study in a transgenic animal model of AD showed that the use of a neutral sphingomyelinase inhibitor reduced the level of exosome release and decreased the Aβ load as well as the number of plaques.106 In osteoarthritis (OA), exosomes derived from synovial cells and chondrocytes interact to cause cellular phenotypic changes that lead to cartilage degradation, inflammatory cytokine release, and promote OA pathological progression.107,108 A recent study compared osteoblast-derived exosomes from the sclerotic and non-sclerotic regions of OA subchondral bone and found that osteoblast-derived exosomes from the sclerotic region significantly inhibited chondrocyte extracellular matrix synthesis, and further RNA sequencing revealed that miR-210-5p mediated communication between osteoblasts and chondrocytes.109 Hence, the progression of OA is facilitated by communication between the subchondral microenvironment, synovium and articular cartilage via exosomes. This provides a new mechanism for the pathogenesis of OA. Current studies have shown that exosomes mediate the pathological progression of related diseases in almost all parts of the body, such as the cardiovascular, digestive and respiratory systems.110–112 In recent years, exosomes have been recognized to play an important role in the interaction between intestinal flora and host cells, mediating the formation of intestinal microbial structures, regulating food metabolism, intestinal epithelial barrier integrity and intestinal immunity, and together maintaining the homeostasis of the intestinal microenvironment. (Figure 3).17,36 For example, Liu et al found that EVs of Fusobacterium nucleatum induced macrophage shift to a pro-inflammatory phenotype and oxidative stress and mediated intestinal epithelial cell death by targeting RIPK1 and disrupted the intestinal barrier.113 In another study, probiotic (Clostridium butyricum)-derived EVs were found to protect intestinal barrier function, improve gut microbiota homeostasis in ulcerative colitis, and contribute to the relief of colitis.114 And in inflammatory bowel disease (IBD) and colorectal cancer, a potential association was found between intestinal miRNA expression profiles and intestinal microbiota abundance.115,116 To further visualize the communication between EVs from gut microbial and the host, researchers used the Cre-LoxP system to report in vivo transfer of bacterial-derived EVs to recipient cells in a mouse model, and colonization of the intestines of these mice with E. coli Cre resulted in Cre-recombinase-induced expression of fluorescent reporter genes in intestinal epithelial cells, even well beyond the intestine, and bacterial-derived Cre induced a wide range of host tissues with expanded marker gene expression, including heart, liver, kidney, spleen, and brain, indicating that intestinal flora-derived EVs can act as a biological shuttle system to transfer functional biomolecules laterally between bacterial and mammalian host cells.117
The Role of Exosomes in the Diagnosis and Treatment of Diseases
Since exosomes carry a large number of active substances from donor cells and can be detected in almost all body fluids, exosomes show great potential as biomarkers for disease diagnosis. For instance, Han et al developed a novel technique of microfluidic combined with surface-enhanced Raman spectroscopy to analyze protein biomarker levels on plasma exosomes from osteosarcoma patients and healthy donors, with significantly higher levels of CD63, vimentin and epithelial cell adhesion molecules on plasma exosomes from osteosarcoma patients compared to healthy controls, which were used in a rapid diagnostic classification model for osteosarcoma with 100% sensitivity, 90% specificity and 95% accuracy, respectively.118 Tetraspanin 1 (TSPAN1) was reported to be significantly elevated in plasma exosomes of patients with hepatocellular carcinoma, and it has been shown to be superior to carcinoembryonic antigen (CEA) as a novel biomarker for the diagnosis of hepatocellular carcinoma.119 Protein levels of P-S396-tau, P-T181-tau and Aβ1-42 in neurogenic blood exosomes were found to be higher than controls in a 10-year clinical study, which can be used to predict the progression of AD, showing potential as a diagnostic marker for AD.120 MicroRNA is the most abundant RNA in exosomes and is not easily degraded by RNA enzymes, while lncRNA, although low in abundance, is easily extracted and highly stable in the external environment.121–123 Therefore, microRNAs and lncRNAs have been widely used as biomarkers for disease diagnosis in recent years. For instance, Karimi et al found that serum exosomes miR-23a, mir-301a were highly enriched in patients with colorectal cancer, and characteristic curve analysis revealed that these exosomes were able to distinguish patients with colorectal cancer from non-colon cancer patients.124 A recent study showed that miR-17-5p, miR-92a-3p levels were significantly elevated in serum exosomes of rectal cancer patients with liver metastases, which were closely associated with liver metastasis and could be used as a predictor of metastasis.125 Recently, lncRNA (SAP30L-AS1, SChLAP1) in circulating exosomes has been reported to be used for the diagnosis of prostate disease. In benign prostatic hyperplasia (BPH), SAP30L-AS1 levels were upregulated, and SAP30L-AS1 levels were significantly higher in prostate cancer than in normal and BPH, ROC curves indicated that SAP30L-AS1 and SChLAP1 had sufficient diagnostic value to distinguish prostate cancer from controls.126 In addition, SAP30L-AS1 expression levels were associated with PSA values and tumor invasion.126 Compared to the traditional use of circulating DNA (ctDNA) alone, the combination of exosomal RNA/DNA and ctDNA increases the total number of mutant copies available for sampling, increasing the sensitivity and specificity of the detection.127,128 A recent meta-analysis analyzed the diagnostic value of ctDNA, circulating tumor cells and exosomes for pancreatic cancer, with exosomes exhibiting the highest sensitivity and specificity.129 Detection of changes in microbial-derived exosomes in blood, feces and urine provides insight into the composition of the gut microbiota and allows timely detection of imbalances. Recently, these microbial-derived EVs have been used as new markers for the diagnosis of gastrointestinal and extraintestinal diseases. For example, using macrogenomic analysis of microbiome and microbial-derived EV in stool samples, researchers found that analysis of gut microbial-derived EV better distinguished patients with IBD from healthy controls than did analysis of the stool microbiome.130 The microbial signature of EVs in urine can also serve as a potential biomarker for colorectal cancer diagnosis.131 Based on macroscopic genomic analysis of serum microbial EVs, researchers developed a diagnostic model for atopic dermatitis.132 Together with the advantages of relatively minimally invasive exosome acquisition and the abundance of exosome sources, exosomes are increasingly used in liquid biopsies, or in combination with other adjunctive tests, to further improve diagnostic accuracy and sensitivity. Some examples of exosomes as biomarkers for disease diagnosis are summarized in Table 1.
 |
Table 1 Partial Demonstration of Exosomes as Diagnostic Markers for Diseases |
Natural exosomes exert therapeutic effects by delivering biologically active substances to target cells and have been heavily investigated as cell-free therapies in recent years. These studies covered various fields such as tumor vaccine development, inflammatory disease treatment, and regenerative medicine. For example, exosomes from dendritic cells (DCs) carrying MHCI/II peptide complexes activate the differentiation of CD8+ T cells into cytotoxic lymphocytes (CTL) and have been shown to have anti-immune tumor effects.142 Studies have shown that this anti-immune tumor effect is positively correlated with the high maturation of DCs.143 A previous study showed that CD8+ T cells-derived exosomes target tumor mesenchymal cells via miR-298-5p and disrupt tumor stroma to inhibit tumor invasion and metastasis.144 NK cell-derived exosomes showed antitumor effects, and the number of exosomes and antitumor effects were increased in hypoxia-treated NK cells. It was further found that increased expression of functional proteins (specific FasL, perforin and granzyme B-as) was found in hypoxia-treated NK cell-derived exosomes, which may mediate the anti-tumor mechanism of NK-exosomes.145 In recent years, the polarized phenotype of macrophages has been found to be closely associated with inflammation and tumor progression. Studies have shown that M1 macrophage-exosomes exhibit antitumor properties,146,147 while M2 macrophage-exosomes promote tumor progression.148,149 Hence, exosomes derived from immune cells are potential candidates for tumor immune vaccines. Mesenchymal stem cells (MSCs) are known to be one of the abundant sources of exosomes, while their secreted exosomes show immunomodulatory functions and tissue protection properties. For instance, in a mouse model of atherosclerosis, BMSC-derived exosomes induced polarization of macrophages from pro-inflammatory phenotype M1 to anti-inflammatory phenotype M2 via miR-let7, which attenuated disease progression.150 Similarly, MSC-derived exosomes have been shown to induce synovial macrophage polarization from M1 to M2, thereby suppressing inflammation and slowing the progression of OA.151 In addition, MSC-derived exosomes can inhibit immune cells such as B-cell proliferation,152 T-cell differentiation and proliferation,153 and DC cell maturation,154 exerting immunosuppressive capacity. Hence, MSC-derived exosomes possess great potential for the treatment of inflammatory diseases. An increasing number of studies have shown that MSC-derived exosomes carry microRNAs that promote chondrocyte migration, attenuate chondrocyte extracellular matrix degradation, and demonstrate chondroprotective properties, such as miR-136-5p and miR-125a-5p.155,156 Additionally, MSC-derived exosomes deliver specific mRNAs and growth factors that promote proliferation and anti-apoptosis of renal tubular epithelial cells, thereby promoting recovery of kidney function.157 In a mouse model of acute myocardial infarction, MSC-derived exosome miR-22 exhibits cardioprotective effects by reducing myocardial apoptosis and fibrosis through downregulation of methyl CpG-binding protein 2 (Mecp2).158 These studies suggest that MSC-derived exosomes represent a promising approach to regenerative medicine. Within the CNS, the MSC-derived exosome miR-542-3p attenuates ischemia-induced cerebral infarct size and suppresses inflammatory responses in a mouse model by inhibiting Toll-like receptor 4 (TLR4).159 Another study showed that BMSCs reduce inflammation while improving cognitive function by transferring exosomal miR-146a to astrocytes in a mouse model of AD, suggesting that exosomes can penetrate the blood-brain barrier (BBB) and represent an effective vehicle for treating CNS diseases.160 In fact, exosome-based cell-free therapy has been considered a promising approach in recent years and is now in clinical trials. Selected clinical trials of exosomes for the treatment of diseases were showed in Table 2.
 |
Table 2 Selected Clinical Trials of Exosomes for the Treatment of Diseases |
Cargo Loading Engineering for Exosome
Previously, we stated that natural exosomes are considered as nanocarriers for the treatment of diseases by delivering active substances to target cells. The personalized design of exosome cargo loading can improve cargo utilization efficiency and reduce side effects and is a classic way to use exosomes as a nano-delivery platform. Including direct exosome loading and indirect loading based on parental cells (Figure 4A and B). For example, the antioxidant (curcumin) was loaded into the exosome by direct co-incubation with the exosome, increasing the stability of the drug in circulation and maintaining its biological activity compared to free curcumin, enhancing the anti-inflammatory properties of curcumin (Figure 4C).166 In another study, exosomes from MSC were co-incubated with doxorubicin (Dox) and the prepared Exo-Dox exhibited higher cellular uptake efficiency, lower cytotoxicity and stronger antitumor effects in osteosarcoma MG63 cells compared to free Dox (Figure 4D).167 In addition, co-incubation of paclitaxel (PTX) with MSCs showed that the secreted vesicles were successfully loaded with PTX and exhibited strong anti-proliferative activity.168 Due to the ability of siRNA to bind to the target mRNA, resulting in the degradation of the target mRNA by cellular nucleases and blocking the expression of the target gene. In recent years, the targeted delivery of siRNA has become a new direction in gene therapy research. In 2011, researchers first loaded siRNA by electroporation into neuron-targeted peptide (GVG)-modified dendritic cell (DC)-derived exosomes, which were successfully delivered to the brain, resulting in neuronal and glial cell-specific gene knockdown.169 Yang et al directly transfected VEGF-siRNA to exosomes derived from brain endothelial cells that were delivered to the zebrafish brain and inhibited VEGF expression to exert anti-tumor effects.170 Also, siRNA can be transfected into parental cells for loading. For example, researchers demonstrated that transfection of embryonic kidney 293T cells with HGF-siRNA, isolated exosomes significantly reduced the expression of HGF and VEGF.171 In addition, Chen et al transfected therapeutic miRNA-150-5p into MSCs by plasmid and isolated exosomes targeted to rheumatoid arthritis synovial tissue significantly inhibited fibroblast expression of MMP14 and VEGF and alleviated joint inflammation in mice.172 (Figure 4E). Transfection is the most commonly used technique for loading miRNAs, while studies of miRNAs in EVs relevant to disease diagnosis and therapy have gained significant popularity in recent years. As erythrocytes are anucleate cells, loading RNA does not pose a risk of gene transfer. Therefore, the researchers developed a simple and efficient method for erythrocyte-based exosome delivery, including loading short RNAs such as antisense oligonucleotides (ASOs) and gRNAs and long RNAs (Cas9mRNA) by electroporation for targeted delivery.173 Recently, Yang et al reported a cellular nano-perforation method that allows the passage of transient electrical pulses through nanochannels to shuttle DNA plasmids from buffers into donor cells, producing 50-fold more exosomes and 103-fold more mRNA transcripts than electroporation and other exosome production strategies.174 More importantly, this new approach is not limited to specific cells and addresses the challenges of loading large amounts of mRNA. Exosome-targeted delivery of DNA can be used for transgenic therapy and can also be loaded by different methods. For instance, plasmid encoding peroxidase (pDNA) transfected macrophages and isolated exosomes successfully delivered to the CNS for the treatment of Parkinson’s disease (PD) mice with sustained levels of oxidase expression in the brain (>1 month) and significantly reduced levels of inflammation (>3-fold).175 Another study reported loading of exogenous linear DNA into exosomes by electroporation, with loading efficiency and volume depending on DNA size, but no functional gene delivery was observed.176 Some specific protein molecules can be loaded by applying co-incubation methods. For example, dopamine can be loaded into exosomes by co-incubation with blood exosomes for the systemic treatment of PD, showing a more than 15-fold increase in brain distribution of dopamine compared to free dopamine, with lower systemic toxicity.177 However, large molecular proteins or peptides are limited for exosome-targeted delivery due to their poor stability and large molecular weight. Current studies mainly focused on indirect loading with the genetic engineering of parent cells. Recently, researchers designed a self-assembled structure capture, known as an enveloped protein nanocage (EPN) for EV delivery of proteins, where the EPN is transfected through the vesicular stomatitis virus G protein (VSV-G) into donor cells and overexpressed in released EVs, and each EPN can be actively loaded with up to 60 therapeutic protein molecules.178 Ilahibaks et al developed a novel technique (TOP-EVs) for loading proteins via EVs integrating VSV-G and rapamycin-induced heterodimeric T82L mutant FRB (DmrC) fused to a target protein with FKBP12 (DmrA), different target proteins such as GFP, Cre and CRISPR/Cas9 ribonucleoprotein complexes, were efficiently loaded and functionally delivered to different cell types in vitro, and it was also demonstrated that the technique can mediate the functional transfer of proteins in vivo.29 The other loading methods, such as extrusion, freeze-thaw cycles,179 saponin treatment180 and ultrasonic treatment181 are also commonly used for cargo loading.
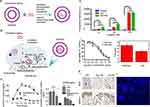 |
Figure 4 Strategies and applications of exosome loading cargo. (A) Direct loading cargo strategies. (B) Indirect cargo loading strategy via parental cells. (C) Exosomes loaded with curcumin via direct co-incubation for targeted anti-inflammatory therapy.166 Reprinted from Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle delivery system: enhanced anti-inflammatory activity of curcumin when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. http://creativecommons.org/licenses/by/4.0/. (i) Plasma circulation times of exosome-loaded curcumin and free curcumin, **P < 0.01. (ii) TNF-α and IL-6 levels in supernatants after PBS, exosomes, free curcumin and curcumin-loaded exosomes treatment of LPS-stimulated RAW 264.7 cells, *P < 0.05, **P < 0.01. (D) Exosomes from MSC loaded with Dox by direct co-incubation for osteosarcoma treatment.167 Reprinted from Wei H, Chen J, Wang S, et al. A Nanodrug Consisting of Doxorubicin and Exosome Derived from Mesenchymal Stem Cells for Osteosarcoma Treatment In Vitro. Int J Nanomedicine. 2019; 14:8603–8610. http://creativecommons.org/licenses/by-nc/3.0/. (i) Fluorescence intensity of exosome-loaded Dox and free Dox co-incubated with osteosarcoma cells for different times, ****P < 0.0001. (ii) Cell viability of osteosarcoma cells treated with the same concentration of free Dox and exosome-loaded Dox for 24 hours and the half-maximal inhibitory concentration (IC50) of free Dox and exosome-loaded Dox. (E) Treatment of CIA mice by transfection of MSC loaded to exosomes with miRNA-150-5p.172 Reprinted with permission from Chen Z, Wang H, Xia Y, Yan F, Lu Y. Therapeutic Potential of Mesenchymal Cell-Derived miRNA-150-5p-Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J Immunol. 2018;201(8):2472–2482.www.jimmunol.org. Copyright © 2018 by The American Association of Immunologists, Inc. (i) Immunohistochemistry showed the expression levels of VEGF and MMP14 in the synovium after transfection of exosomes with miRNA-150-5p to treat CIA mice. (ii) The DiO-labeled MSC exosomes in the joint cavity of CIA model as shown by immunofluorescence. |
Different methods affect cargo loading capacity, which is related to the properties of the cargo being loaded, such as hydrophilicity, hydrophobicity and molecular weight. A previous study comparing the loading of hydrophobic molecules paclitaxel (PTX) onto exosomes by incubation and ultrasound methods showed higher ultrasound loading rates due to the easier diffusion of PTX molecules onto lipid bilayers by ultrasound.182 Another study evaluated different techniques for loading catalase into exosomes, incubation, freeze-thaw cycling, sonication or extrusion for Parkinson’s disease drug delivery, and showed that sonication loading efficiency loading was the most efficient.179 Ultrasonication and extrusion caused diffusion of peroxidase on the lipid bilayer, resulting in high loading efficiency of exosome carriers, while saponin selectively removed cholesterol from exosome membranes and formed pores on the lipid bilayer of exosome membranes, thus promoting binding of peroxidase and improving loading efficiency.179 In addition, loading methods can affect the stability of exosomes, such as sonication, electroporation, or permeants can disrupt the integrity of EV membranes and reduce stability.179 Electroporation produces siRNA aggregation effects during the loading process and can affect the actual loading rate. To circumvent this phenomenon, the researchers suggested adding ethylenediaminetetraacetic acid to the electroporation buffer.183
Exosome Membrane Modification Engineering
Direct Exosome Membrane Surface Modification
However, depending on exosome-loaded drug delivery alone is not the primary factor in increasing drug accumulation in the lesioned region, and is more dependent on targeted modifications of exosomes. Since some specific structures on the exosome membrane surface mediate the targeting function and homing properties of exosomes, appropriate membrane surface modifications can further improve the delivery efficiency, including direct modification of the exosome membrane surface and indirectly modified exosome-derived parental cells (Figure 5A and B).
 |
Figure 5 Strategies and applications of exosome membrane modification. (A) Direct modification of exosome membrane strategies. (B) Indirect modification of exosome membrane strategies by genetic engineering. (C) Targeting peptide c (RGDyK)-modified exosomes loaded with miR-210 for the treatment of cerebral ischemia models.184 Reprinted from Zhang H, Wu J, Wu J, et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology. 2019;17(1):29. http://creativecommons.org/licenses/by/4.0/. (i) Fluorescence intensity of GRD-modified exosomes and unmodified exosomes loaded with miR-210 in a brain ischemia-reperfusion model in the lesioned (intralesional) and matched non-lesioned (contralateral) areas 6 hours after intravenous injection, *P < 0.05, **P < 0.01. (ii) VEGF expression in the lesion area at 7 and 14 days after reperfusion and administration of RGD-exo:NC or RGD-exo:miR-210, *P < 0.05, **P < 0.01. (D) FPC-modified exosome for RA-targeted therapy.25 Reprinted from Yan F, Zhong Z, Wang Y, et al. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J Nanobiotechnology. 2020;18(1):115. http://creativecommons.org/licenses/by/4.0/. (i) In vivo imaging of plasma and organs 24 hours after intravenous injection of Dex-loaded liposomes, Dex-loaded exosomes, and Dex-loaded FPC-modified exosomes. (ii) Serum levels of inflammatory cytokines (TNF-α, IL-1β and IL-10) in normal and treated CIA mice, *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.05. (iii) Serum levels of ALT and AST in normal and treated CIA mice. (E). IMTP-Exosomes for Myocardial Ischemia Targeted Therapy.185 Reprinted from Wang X, Chen Y, Zhao Z, et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J Am Heart Assoc. 2018;7(15):e008737. http://creativecommons.org/licenses/by/4.0/. (i) Fluorescence imaging of DiR-labeled blank-Exos and IMTP-Exos in a mouse model of myocardial ischemia after 72h of intravenous injection. (ii) Expression levels of inflammatory factors (TNF-α, IL-1β and IL-6) after IMTP-Exos and blank-Exos treatment, ****p < 0.001. |
Using exogenous molecules such as proteins and peptides to modify the surface of exosomes by various chemical and mechanical methods can enhance their active targeting ability and improve their efficacy. For instance, exosome membranes were coupled to neuropilin-1 targeting peptide (RGERPPR, RGE) by click chemistry to design therapeutically functional glioma-targeting exosomes, and these engineered exosomes were found to cross the BBB, further enhancing targeting ability.186 The targeting peptide c (RGDyK) of the vascular endothelial cell integrin αvβ3 receptor was coupled to the surface of exosomes loaded with miR-210 by click chemistry.184 In vivo experiments showed that target-modified exosomes increased the ability to target areas of cerebral ischemia and increased miR-210 and VEGF levels in lesioned areas (Figure 5C).184 Click chemistry is a method for covalent modification of exosome membrane surfaces. In addition, some non-covalent modifications have been applied to surface modification. Researchers modified exosome membranes with cationized pullulan via electrostatic interactions, and the cationic branched-chain starch specifically targeted hepatocyte asialoglycoprotein receptor (AGPR). In a mouse model of liver injury, the engineered exosomes accumulated more in liver tissue compared to unmodified exosomes and are expected to be a targeted drug delivery system for liver disease.187 Since target cell-derived exosome membranes express target cell-specific receptors on their surface, the exosome membranes can be engineered with targeted fractions through ligand/receptor binding method. For example, A33-positive exosomes were isolated from A33 overexpressing tumor cells, and A33 antibody-modified superparamagnetic iron oxide NPs (US) bound to the exosome membrane via ligand/receptor interactions were designed as a drug delivery system for the treatment of colon cancer, loaded with Adriamycin, with significantly enhanced targeting ability and reduced cardiotoxicity.188 Similarly, exosomes derived from reticulocytes bind to superparamagnetic magnetite colloidal nanocrystal clusters via surface transferrin receptors, significantly enhancing the targeting ability and anti-cancer capacity of exosomes.189 The dual lipid structure of exosomes provides hydrophobic properties allowing some hydrophobic molecules to be incorporated within the exosome membrane. For example, exosomal membrane can be loaded with both hydrophobic small molecules and modified by hydrophobic insertion. Targeted ligand-modified PEG phospholipids can insert into exosomal lipid membrane and is a commonly used technique in the development of targeted drug delivery systems in recent years.190 Folic acid (FA)-modified PEG-cholesterol (FPC) can specifically target FA receptors expressed on the surface of synovial macrophages by inserting into the membrane surface of macrophage-derived exosomes. Loaded dexamethasone (DEX) for intra-articular delivery is precisely targeted to inflamed synovial tissue with longer retention time and enhanced therapeutic efficacy, no significant hepatic toxicity was observed, and superiority over synthetic liposomes (Figure 5D).25 Similarly, tumor cell-derived exosomes based on PEG-FA modification for targeted drug delivery in breast cancer significantly improved targeting ability and tumor killing effect by up to 15%.191 Moreover, PEG enhances the stability and circulation time of drug delivery systems.192 In fact, PEG coating technique is widely utilized for membrane modification of NPs to evade the clearance of MPS.193
Indirect Modification of Exosome-Derived Parental Cells
Genetic modification of exosome-derived cells is another important method of exosome surface functionalization. By transfecting target cell receptor genes into parental cells, these receptor proteins are fused to exosomal transmembrane proteins during exosome biogenesis to form the targeting portion expressed on the exosome surface. Wang et al used the ischemic myocardial targeting peptide CSTSMLKAC (IMTP) plasmid to transfect MSC with fused exosomal membrane protein (Lamp2b), and the isolated exosomes expressed Lamp2b-IMTP complex on the surface and found that IMTP-exosomes were more efficiently taken up by injured cardiomyocytes than unmodified exosomes, accumulated more in the ischemic region of the heart, and showed an enhanced therapeutic effect on acute myocardial infarction.185 (Figure 5E) In another study, researchers genetically engineered DCs to produce exosomes of Lamp2b fused to the neuron-targeting peptide RVG, loaded with exogenous siRNA via electroporation, and effectively delivered to the plasmatic brain through the BBB for targeted therapy of AD.169 Similarly, anti-epidermal growth factor receptor (EGFR), a tumor cell receptor, was expressed on the surface of EVs by transfection of EV-producing cells encoding EGFR antibodies and fused to glycosylphosphatidylinositol (GPI) anchor signaling peptides, significantly enhancing exosome-tumor cell binding and depending on the density of EGFR.194 Using tetraspanins expressed on the surface of exosomes, Liang et al designed Apo-A1-CD63 complexes on the surface of exosomes and loaded miR-26a by electroporation to target the scavenger receptor class B (SR-B1) receptor on the surface of hepatocellular carcinoma cells and successfully delivered anti-tumor RNA.195 Tian et al transfected RGD-4C targeting peptide fused to Lactadherin C1C2 structural domain on EV membrane by plasmid transfection significantly improved the ability of EV to target brain ischemic regions and significantly decreased the level of inflammation compared to unmodified EV.196 In addition, the interaction of CD47 with the immunosuppressive receptor SIRPα on the surface of macrophages can stimulate the immune escape system.197,198 Since macrophages are primarily responsible for the clearance of circulating exosomes, the CD47-SIRPα signaling axis has been used to engineer exosomes to escape macrophage phagocytosis.199 By transfecting CD47 into donor cells, CD47 overexpressed exosomes effectively escaped phagocytosis from MPS, prolonged the circulation time of exosomes, and increased the distribution in tumor tissues.200 Meanwhile, CD47 can target CD47 receptors on the surface of tumor cells and enhance the anti-tumor effect.200
Hybrid Engineering of Exosomes and NPs
Advances in nanotechnology greatly contributed to the development of engineered exosomes. Incorporating the advantages of liposomes and exosomes, hybrid exosomes based on membrane fusion have emerged as a novel nanodrug delivery system. For instance, lipid charge affects the uptake of target cells, and depending on the target cell properties, personalized liposomes can be selected to hybridize with exosomes to improve the uptake rate.201,202 A previous study using freeze-thaw cycling techniques to hybridize exosomes and lipids found that neutral and anionic lipids did not affect cellular uptake of hybrid exosomes, while hybridizing cationic lipids to exosomes decreased cellular uptake efficiency.203 In contrast, the cellular uptake of hybrid exosomes with PEG-DOPS was significantly increased nearly 2-fold compared to unmodified exosomes.203 In addition, the pH, optical and thermal sensitive liposomes provide controlled release properties to the drug delivery system, further improving drug delivery efficiency and reducing drug side effects. For instance, recently developed exosomes from macrophages were hybridized with PH-sensitive liposomes and loaded with Adriamycin. Under acidic conditions, the drug was released, exhibiting enhanced toxicity to tumor cells, suggesting that this hybrid exosome is a potential drug delivery system for the acidic tumor environment.204 On the other hand, membrane fusion-based approaches may disrupt the integrity of membrane proteins and increase the size of exosomes, affecting the stability and targeting ability of exosomes.205 From the perspective of synthetic NPs, exosome membranes can be used for membrane coating technology of synthetic nanoparticles, which is beneficial to reduce their immunogenicity.
Apart from organic liposomes, inorganic nanomaterials such as metal NPs, metal oxides and quantum dots (QDs) display excellent physical properties such as plasmonic, magnetic and fluorescent properties. For example, the magnetic navigation properties of iron oxide NPs loaded with MSC-derived exosomes can significantly improve targeting properties and increase exosome accumulation in disease regions, such as cerebral ischemia (>5-fold) and myocardial ischemia (>2-fold), compared to unmodified exosomes.206 In addition to loading therapeutic agents for targeted drug delivery, these magnetic NPs can also generate heat when disturbed by external alternating magnetic fields, which can promote the ablation of targeted tumor cells through magnetothermal therapy, further enhancing the efficacy of the treatment.207 Also, iron oxide NPs loaded in exosomes are used for bioimaging and are suitable contrast agents for MRI.208 Au NPs are widely used for CT imaging due to their high imaging resolution and safety.209 In addition, exosomes loaded with Au NPs respond to near-infrared radiation and selectively induce target cell death through photothermal effects.210 Cao et al loaded vanadium carbide quantum dots (V2C QD) into RGD-targeting peptide-modified breast cancer cell-derived exosomes for tumor-targeting therapy by electroporation, and also using photothermal therapy to kill tumor cells.211 Therefore, based on the many properties of inorganic NPs, the hybridization strategy of exosomes and inorganic NPs can design multifunctional nanocarriers with both bioimaging, targeted therapy, and intelligent control. Hybridization with inorganic NPs can be achieved by covalent binding of chemically modified junctions to exosomal surface proteins or by hydrophobic insertion into the lipid bilayer of exosomes.212,213 Inorganic NPs can also be loaded directly into exosomes by various physical methods such as electroporation, extrusion, sonication, and freeze-thawing.214 A recent study found that including electroporation, diffusion passive loading, thermal shock, ultrasound and saponin-assisted loading of gold NPs (GNs), the loading rate was <15% and some loading methods affected the morphology and integrity of exosomes. However, the loading rate of exosomes secreted via the exosome biogenesis pathway was >50% by co-incubation of cells with polyethylene glycolized HGNs (PEG-GNs).214 More importantly, loading inorganic NPs via the biogenesis pathway can increase exosome yield and enrich more functional molecules, which is beneficial to further improve the therapeutic effect.206
Bioactive Scaffolds for Delivery of Exosomes
Due to the rapid clearance effect of exosomes, the delivery of exosomes by bioactive materials can further improve the retention of exosomes in the disease area and achieve long-term release, on the other hand, these bioactive materials can also enhance additional repair effects. Bioactive scaffolds are now widely used for therapeutic agent or nanocarrier delivery, particularly in tissue repair and regenerative medicine. The ideal scaffold material should have good biocompatibility, maintain exosome activity and structural integrity, achieve long-term exosome release, and facilitate the migration of target cells to the scaffold to take up exosomes to exert therapeutic effects.215 Decellularized extracellular matrix (ECM) has good biocompatibility, promotes cell recruitment and differentiation, and facilitates exosome homing by delivering exosomes as a biological scaffold.216 Jiang et al prepared a chondrocyte-derived ECM scaffold loaded with umbilical cord MSC-derived exosomes for cartilage defect repair in rabbit knee, and showed that the cartilage repair effect of the ECM+exosome combination was superior to the exosome or ECM scaffold groups alone.217 Hydrogels are the most commonly used biological scaffolds for exosome delivery and include both natural and synthetic hydrogels. Such as polysaccharides (chitosan, hyaluronic acid and alginate) and proteins (fibronectin, silk cardiac protein, collagen and gelatin), can be used in the preparation of natural hydrogels.218 Natural hydrogels have good biocompatibility, biodegradability and proper cellular interactions. However, their disadvantages are also evident with lack of adequate mechanical properties and low levels of cell adhesion.219 Therefore, an increasing number of studies tend to design synthetic or composite hydrogels with bionic properties for exosome delivery. For instance, Zhou et al used Pluronic F-127 thermosensitive hydrogel loaded with MSC-exosomes for the treatment of skin wounds and achieved controlled release based on thermal sensitivity, with exosome release still detectable up to 96 h.220 The retention time was 2-fold longer compared to the unapplied hydrogel group, achieving a lower dose frequency of administration to promote wound healing.220 In addition, the addition of some nanomaterials, such as nano-hydroxyapatite (nHAP), nano-silicate, polymer micelles, nanoclay and liposomes can overcome the local defects of hydrogels.31 Lu et al prepared GelMA-HAMA / nHAP nanocomposite hydrogels loaded with exosomes derived from human urine stem cells, showing excellent controlled release properties and appropriate mechanical properties to promote the repair of cranial defects in a rat model.221 Wu et al developed a gelatin methacrylate (Gelma)/nanoclay hydrogel (Gel-nano) with stronger mechanical properties, better biocompatibility, and longer exosome release time compared to the Gelma group, possibly due to the unique physical and biological properties of the nanomaterials.222 This new system showed excellent therapeutic efficacy when used to load MSC-derived exosomes for cartilage injury, mediated via miR-23a-3p.222 3D printing technology allows better tuning of composite scaffolds in terms of shape, size, and porosity for in vivo transplantation. In a recent study, MSC-derived exosomes were composite with collagen/chitosan by 3D printing technology with good mechanical properties and biocompatibility, and 3D-CC-BMExos scaffold with porous network structure and high porosity promoted cell adhesion with significantly higher cumulative exosome release than CC-BMExos scaffold for the treatment of post-traumatic brain injury to improve cognitive function and recovery of sensorimotor function.223 Yue et al prepared nanocomposite scaffolds composed of ECM, gelatin, QCs and nano-hydroxyapatite with high pore structure, proper degradability, good biocompatibility and mechanical properties by 3D printing for loading MSC-derived exosomes showed significantly enhanced osteogenic differentiation and vascular regeneration in in vitro and in vivo trials.32 Nanofiber reinforcement is another important method for hydrogel modification with excellent porosity, mechanical properties, good biodegradability, controlled release design potential, and similar to ECM, nanofibers have regenerative ability to promote cell growth, adhesion and proliferation.224 For example, nanofiber-reinforced alginate hydrogels prepared by electrostatic spinning technology successfully mimic the ECM structure, enhance the mechanical strength, deliver PRP to the nucleus pulposus, achieve the protection and controlled release of PRP in nucleus pulposus fibers, with long-term replenishment of bioactive molecules.225 Zhou et al developed a matrix metalloproteinase-2 (MMP2)-sensitive controlled-release self-assembling peptide (KMP2) nanofiber hydrogel that was used for local delivery of MSC-EVs for tissue repair.226 More importantly, natural or synthetic polymers can now be prepared into nanofibers by different techniques such as electrospinning, phase separation, and self-assembly.227 Hence, nanocomposite hydrogels and nanofiber hydrogels, may be promising strategies for future delivery of exosomes. We summarized the potential delivery strategies for hydrogel-loaded exosomes in Figure 6.
 |
Figure 6 Summary of hydrogel-loaded exosome delivery strategies. |
Exosome Bioimaging
The combination of some biofluorescent materials or NPs with imaging capabilities with exosomes is beneficial for tracking exosomes in vivo, which is used to determine the biodistribution and pharmacokinetics of exosomes. For instance, Gaussian luciferase (Gluc)228 and Renilla luciferase (Rluc)229 can be loaded into exosomes by transfection to produce bioluminescence effects after the addition of enzyme substrates, and then bioluminescence imaging is used to show the distribution of exosomes in different organs of the body. Radioisotopes, such as 99mTc, 131I, and 111In-oxine for labeling exosomes, and single photon emission computed tomography (SPECT) or positron emission tomography (PET) tracking are the most commonly used non-invasive techniques for radiolabeled exosome imaging.230–232 The unique imaging properties of nanomaterials are ideal imaging agents for exosome bioimaging. For example, inorganic NPs (superparamagnetic iron oxide) have been loaded into exosomes by researchers via electroporation or co-incubation, offering excellent imaging depth using magnetic resonance imaging (MRI).233,234
Plant-Derived Exosomes
In recent years, plant-derived exosomes have gained popularity owing to their wide range of sources and equally low immunogenicity. Another advantage is that plants do not carry zoonotic or human pathogens compared to mammals. Numerous studies have demonstrated that plant-derived exosomes are favorable candidates for development as nanocarriers into delivery platforms. For example, ginger-derived exosome-like NPs, conjugated with the targeting ligand FA, successfully delivered Adriamycin to colon cancer cells. While improving the efficacy, the ginger-derived nanocarriers showed more efficient drug loading efficiency compared to commercially available liposomes.235 In another study, grapefruit-derived nanocarriers were modified with FA ligands for enhanced targeting and rapid delivery of miR17 across the BBB via intranasal administration to target folic acid receptor-positive GL-26 brain tumor cells and inhibit tumor growth.236 Apart from being engineered exosomes for targeted delivery systems, plant-derived exosomes carry natural components that have demonstrated anti-tumor, anti-inflammatory and tissue regenerative properties. Exosome-like particles from Artemisia annua inhibit tumor growth and enhance anti-tumor immunity by remodeling the tumor microenvironment and reprogramming tumor-associated macrophages in a mouse model of lung cancer.237 Plant-derived mitochondrial DNA (mtDNA) was also found to be internalized into macrophages via vesicles and promote the transformation of tumor macrophages to an anti-tumor phenotype, revealing for the first time that mtDNA is exchanged between plants and mammals.237 Other studies demonstrated that exosome-like NPs derived from ginger rhizomes and shiitake mushrooms inhibited NLRP3 inflammasome activation and reduced the release of inflammatory factors after being taken up by macrophages.238,239 A recent study found that orally administered ginger-derived exosomes can be taken up by intestinal macrophages and epithelial cells, reduce the release of pro-inflammatory cytokines, and promote the proliferation of epithelial cells, while exhibiting anti-inflammatory and protective properties of intestinal tissues.240 Due to the advantage of edibility, plant-derived exosomes can be selectively absorbed by the gut microbiota and contain microRNAs that alter microbiome composition and host physiology, remodeling the intestinal flora and exert therapeutic effects.241 Recently, researchers isolated tea flowers-derived exosomes that accumulate at breast tumor and lung metastasis sites after intravenous or oral administration (intravenous > oral) and inhibit breast cancer growth and metastasis, with comparable therapeutic efficacy between oral and intravenous administration, attributed to the fact that treatment by the oral route substantially increases the community abundance and diversity of the gastrointestinal microbiota.242 In addition, plant exosomes inherently carry active plant derivatives. For example, ginger-derived exosomes enriched in 6-gingerol and 6-gingerenol, which have anti-inflammatory properties.238 Broccoli-derived exosomes contain high levels of carotenoids, which are recognized as antioxidants or anticancer agents.243
Challenges, Opportunities, and Future Perspectives
Despite significant progress in the field of exosome research in the past, the biogenesis of exosomes is still not clearly explained, especially the cargo sorting mechanism of RNA and DNA, the factors and mechanisms affecting exosome secretion. These basic studies on exosomes are essential for our comprehensive understanding of exosomes and for further research on their applications. Current isolation techniques for exosomes do not allow for both high-throughput and high-purity screening, further optimization of isolation protocols may contribute to overcoming these shortcomings and accelerating exosome research for both basic and clinical applications. Notably, nanocomposites combined with nanomaterial-modified microfluidic channels are expected to achieve high capture rates and high-throughput screening of exosomes, which may be a new direction for the future.
Natural exosomes possess anti-tumor, immunomodulatory and tissue repair abilities. We summarized the relevant preclinical and clinical studies on exosome therapy for these diseases, and exosome therapy is a promising cell-free therapy. Exosomes are the main communication signal for intercellular communication mechanisms, involved in the development of almost all diseases in the human body, and provide new insights into the pathological mechanisms of some diseases, such as the role of exosomes in the intestinal microenvironment, and synovial microenvironment. Also, exosomes show great potential for disease diagnosis and promising clinical applications as rapid and minimally invasive liquid biopsies. Exosomes can be used as drug delivery carriers to assist in the more accurate delivery of clinical drugs to targeted tissues, improving both drug efficacy and reducing systemic toxicities. We summarized and analyzed the current loading techniques of different cargoes and the advantages and disadvantages of different loading techniques, and suggested that the appropriate loading technique should be considered according to the chemical nature and molecular weight of the cargo. In addition, factors affecting exosome stability such as exosome membrane integrity and zeta potential should also be considered. We summarized various loading schemes for drugs, proteins, and nucleic acids, however, large-scale loading and loading of large molecule therapeutic proteins and nucleic acids remain challenging. Therefore, we focused on new techniques, such as cellular nanoporation for large-scale loading of large nucleic acids, envelope protein nanocage (EPN) capture and TOP-EVs technology for efficient loading of large molecule proteins, which are expected to solve the current challenges but still need to be validated by extensive in vivo animal experiments. Excitingly, several exosome-loaded therapeutic agents are currently in clinical trials such as plant-derived exosomes loaded with curcumin for intestinal cancer (NCT01294072), MSC-exosome loaded siRNA for metastatic pancreatic adenocarcinoma (NCT03608631), and tumor-derived vesicles loaded with cisplatin for malignant pleural or ascites fluid (NCT01854866). It is believed that exosomes will be used more often as drug delivery platforms for clinical targeted therapies in the future. Engineering of exosome membrane modifications facilitates enhanced active targeting ability and evasion of MPS clearance, further improving delivery efficiency. Hybridization with NPs can further enhance targeting capabilities by exploiting the unique properties of these NPs, including inorganic NPs such as liposomes, organic NPs such as peroxisomes, metal NPs, and QDs, for controlled release, in vivo bioimaging, and adjuvant therapy. Several multifunctional delivery platforms have been developed focusing on exosome membrane modification, therapeutic agent loading and controlled release, and adjuvant therapies such as thermotherapy and photothermal therapy (Figure 7). For instance, by loading both doxorubicin and Ag 2S QDs into the exosome, controlled release of the drugs and QDs can be achieved using external infrared light, and photothermal therapy can also be used to kill tumor cells, further enhancing the therapeutic efficacy.244 In another study, the anticancer drug (Adriamycin) was designed to bind to milk-derived exosomal membranes via PH-sensitive imine bonds, using the acidic microenvironment inside the tumor to break the imine bonds, and the drug was rapidly released while effectively killing tumor cells.245 Click chemically modified M1 macrophage-derived exosomes are coupled to aCD47 and aSIRPα (antibodies) via pH-sensitive bonds, where aCD47 targets tumor cells and the acidic tumor microenvironment cleaves the pH-sensitive bonds to release aSIRPα and aCD47, blocking the CD47-SIRPα axis between tumor cells and macrophages to exert phagocytosis. Meanwhile, natural M1 exosomes can effectively reprogram macrophages from pre-tumor M2 to anti-tumor M1.246 These multifunctional nano-delivery platforms developed based on exosomes, which may be emerging therapeutic approaches for targeted therapies, are currently limited to in vivo experimental studies in animals and more preclinical studies should be conducted to accumulate more data and to prepare for relevant clinical studies. Bioactive scaffold-loaded exosome delivery can achieve increased retention and long-term release of exosomes at disease sites, which are now widely used in tissue repair and regenerative medicine and have shown good therapeutic effects, such as hydrogel-loaded exosomes. In recent years, advances in nanomaterials technology have facilitated the development of nanocomposite biomimetic scaffolds and nanohydrogel scaffolds that integrate the positive properties of natural and synthetic materials and may be a new direction for future research on bio-scaffold-loaded exosomes, especially combined with 3D printing technology. Due to the complex physiological microenvironment in the human body, the biocompatibility, degradability, and mechanical properties of these scaffold materials require extensive testing to validate before clinical translation. In addition, plant derived exosomes are considered ideal delivery platforms for gut diseases and diseases related to gut microbial dysbiosis owing to their oral availability, wide source and natural carriage of anticancer and anti-inflammatory components, and should be considered in the future.
Conclusions
Exosomes, being natural nanocarriers, possess significant clinical translational and applicative potential in the diagnosis and treatment of diseases. In the foreseeable future, exosomes, as natural nanocarriers, would emerge as a novel therapeutic alternative in the fields of oncology, immunology, and regenerative medicine.
Acknowledgment
KT and WZ would like to acknowledge the support from the National Natural Science Foundation of China (No.81601901, 82002908) and the Natural Science Foundation of Liaoning, China (No.2019-MS-079). KT would like to acknowledge the support from the Climbing Program of Key Disciplinary Construction Dalian, China (No.2022DF012).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17(4):251–266. doi:10.1038/s41571-019-0308-z
2. Dangkoub F, Sankian M, Tafaghodi M, Jaafari MR, Badiee A. The impact of nanocarriers in the induction of antigen-specific immunotolerance in autoimmune diseases. J Control Release. 2021;339:274–283. doi:10.1016/j.jconrel.2021.09.037
3. Alyautdin R, Khalin I, Nafeeza MI, Haron MH, Kuznetsov D. Nanoscale drug delivery systems and the blood-brain barrier. Int J Nanomed. 2014;9:795–811. doi:10.2147/IJN.S52236
4. Mao L, Wu W, Wang M, et al. Targeted treatment for osteoarthritis: drugs and delivery system. Drug Deliv. 2021;28(1):1861–1876. doi:10.1080/10717544.2021.1971798
5. Crommelin DJA, van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release. 2020;318:256–263. doi:10.1016/j.jconrel.2019.12.023
6. Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118(14):6844–6892. doi:10.1021/acs.chemrev.8b00199
7. Yamamoto K, Imaoka T, Tanabe M, Kambe T. New horizon of nanoparticle and cluster catalysis with dendrimers. Chem Rev. 2020;120(2):1397–1437. doi:10.1021/acs.chemrev.9b00188
8. Wang TT, Xia YY, Gao JQ, Xu DH, Han M. Recent progress in the design and medical application of in situ self-assembled polypeptide materials. Pharmaceutics. 2021;13(5):753. doi:10.3390/pharmaceutics13050753
9. Song S, Qin Y, He Y, et al. Functional nanoprobes for ultrasensitive detection of biomolecules. Chem Soc Rev. 2010;39(11):4234–4243. doi:10.1039/c000682n
10. Long Z, Wu Y-P, Gao H-Y, et al. Functionalization of halloysite nanotubes via grafting of dendrimer for efficient intracellular delivery of siRNA. Bioconjug Chem. 2018;29(8):2606–2618. doi:10.1021/acs.bioconjchem.8b00321
11. Pulskamp K, Diabaté S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168(1):58–74. doi:10.1016/j.toxlet.2006.11.001
12. Akçan R, Aydogan HC, Yildirim M, Taştekin B, Sağlam N. Nanotoxicity: a challenge for future medicine. Turk J Med Sci. 2020;50(4):1180–1196. doi:10.3906/sag-1912-209
13. Sun D, Zhuang X, Zhang S, et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65(3):342–347. doi:10.1016/j.addr.2012.07.002
14. Luan X, Sansanaphongpricha K, Myers I, et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–763. doi:10.1038/aps.2017.12
15. Ailuno G, Baldassari S, Lai F, Florio T, Caviglioli G. Exosomes and extracellular vesicles as emerging theranostic platforms in cancer research. Cells. 2020;9(12):2569. doi:10.3390/cells9122569
16. He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi:10.7150/thno.21945
17. Díaz-Garrido N, Badia J, Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10(13):e12161. doi:10.1002/jev2.12161
18. Li YJ, Wu JY, Liu J, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnol. 2021;19(1):242. doi:10.1186/s12951-021-00986-2
19. Qambrani A, Rehman FU, Tanziela T, et al. Biocompatible exosomes nanodrug cargo for cancer cell bioimaging and drug delivery. Biomed Mater. 2021;16(2):025026. doi:10.1088/1748-605X/abaaa2
20. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi:10.7150/thno.52570
21. Patel S, Schmidt KF, Farhoud M, et al. In vivo tracking of [(89)Zr]Zr-labeled engineered extracellular vesicles by PET reveals organ-specific biodistribution based upon the route of administration. Nucl Med Biol. 2022;112–113:20–30. doi:10.1016/j.nucmedbio.2022.06.004
22. Gupta D, Liang X, Pavlova S, et al. Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J Extracell Vesicles. 2020;9(1):1800222. doi:10.1080/20013078.2020.1800222
23. Wiklander OP, Nordin JZ, O’Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi:10.3402/jev.v4.26316
24. Matsumoto A, Takahashi Y, Nishikawa M, et al. Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected B16BL6-derived exosomes by macrophages. J Pharm Sci. 2017;106(1):168–175. doi:10.1016/j.xphs.2016.07.022
25. Yan F, Zhong Z, Wang Y, et al. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J Nanobiotechnol. 2020;18(1):115. doi:10.1186/s12951-020-00675-6
26. Cheng L, Zhang X, Tang J, Lv Q, Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275:120964. doi:10.1016/j.biomaterials.2021.120964
27. Hade MD, Suire CN, Suo Z. An effective peptide-based platform for efficient exosomal loading and cellular delivery of a microRNA. ACS Appl Mater Interfaces. 2023;15(3):3851–3866. doi:10.1021/acsami.2c20728
28. Hao R, Yu Z, Du J, et al. A high-throughput nanofluidic device for exosome nanoporation to develop cargo delivery vehicles. Small. 2021;17(35):e2102150. doi:10.1002/smll.202102150
29. Ilahibaks NF, Ardisasmita AI, Xie S, et al. TOP-EVs: technology of protein delivery through extracellular vesicles is a versatile platform for intracellular protein delivery. J Control Release. 2023;355:579–592. doi:10.1016/j.jconrel.2023.02.003
30. Akbari A, Jabbari N, Sharifi R, et al. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 2020;249:117447. doi:10.1016/j.lfs.2020.117447
31. Niemczyk-Soczynska B, Zaszczyńska A, Zabielski K, Sajkiewicz SP. Electrospun and composite materials for bone/cartilage and neural tissue engineering. Materials. 2021;14(22):6899. doi:10.3390/ma14226899
32. Kang Y, Xu J, Meng L, et al. 3D bioprinting of dECM/Gel/QCS/nHAp hybrid scaffolds laden with mesenchymal stem cell-derived exosomes to improve angiogenesis and osteogenesis. Biofabrication. 2023;15(2):024103. doi:10.1088/1758-5090/acb6b8
33. Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. doi:10.1038/s41392-020-00261-0
34. Fan W-J, Liu D, Pan L-Y, et al. Exosomes in osteoarthritis: updated insights on pathogenesis, diagnosis, and treatment. Front Cell Dev Biol. 2022;10:949690. doi:10.3389/fcell.2022.949690
35. Gharavi AT, Hanjani NA, Movahed E, Doroudian M. The role of macrophage subtypes and exosomes in immunomodulation. Cell Mol Biol Lett. 2022;27(1):83. doi:10.1186/s11658-022-00384-y
36. Shen Q, Huang Z, Yao J, Jin Y. Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J Adv Res. 2022;37:221–233. doi:10.1016/j.jare.2021.07.002
37. Li D-F, Yang M-F, Xu J, et al. Extracellular vesicles: the next generation theranostic nanomedicine for inflammatory bowel disease. Int J Nanomed. 2022;17:3893–3911. doi:10.2147/IJN.S370784
38. Cai Y, Zhang L, Zhang Y, Lu R. Plant-derived exosomes as a drug-delivery approach for the treatment of inflammatory bowel disease and colitis-associated cancer. Pharmaceutics. 2022;14(4):822. doi:10.3390/pharmaceutics14040822
39. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi:10.3390/cells8070727
40. Ridder K, Keller S, Dams M, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12(6):e1001874. doi:10.1371/journal.pbio.1001874
41. Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2019;60(1):9–18. doi:10.1194/jlr.R084343
42. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–948. doi:10.1016/j.bbagen.2012.03.017
43. Mashouri L, Yousefi H, Aref AR, et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. doi:10.1186/s12943-019-0991-5
44. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9(1):19. doi:10.1186/s13578-019-0282-2
45. Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi:10.1038/nrm2755
46. Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL. Late endosomes derive from early endosomes by maturation. Cell. 1991;65(3):417–427. doi:10.1016/0092-8674(91)90459-C
47. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
48. Möbius W, Ohno-Iwashita Y, van Donselaar EG, et al. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J Histochem Cytochem. 2002;50(1):43–55. doi:10.1177/002215540205000105
49. Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002;3(2):271–282. doi:10.1016/S1534-5807(02)00220-4
50. Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–869. doi:10.1038/nature08849
51. Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013;5(9):a016766. doi:10.1101/cshperspect.a016766
52. Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34(19):2398–2407. doi:10.15252/embj.201592484
53. Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445–452. doi:10.1038/nature07961
54. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. doi:10.1146/annurev-cellbio-101512-122326
55. Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi:10.1242/jcs.128868
56. Verweij FJ, van Eijndhoven MA, Hopmans ES, et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J. 2011;30(11):2115–2129. doi:10.1038/emboj.2011.123
57. Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190(6):1079–1091. doi:10.1083/jcb.201002049
58. Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi:10.1126/science.1153124
59. Blanc L, Vidal M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases. 2018;9(1–2):95–106. doi:10.1080/21541248.2016.1264352
60. Hsu C, Morohashi Y, Yoshimura S, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi:10.1083/jcb.200911018
61. Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30; sup pp 11–13. doi:10.1038/ncb2000
62. Yokoi A, Villar-Prados A, Oliphint PA, et al. Mechanisms of nuclear content loading to exosomes. Sci Adv. 2019;5(11):eaax8849. doi:10.1126/sciadv.aax8849
63. S ELA, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi:10.1038/nrd3978
64. Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641.
65. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
66. Cossetti C, Iraci N, Mercer TR, et al. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56(2):193–204. doi:10.1016/j.molcel.2014.08.020
67. Parolini I, Federici C, Raggi C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi:10.1074/jbc.M109.041152
68. Fitzner D, Schnaars M, van Rossum D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124(Pt 3):447–458. doi:10.1242/jcs.074088
69. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3.24722
70. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
71. Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30(1):. doi:10.1002/0471143030.cb0322s30
72. Langevin SM, Kuhnell D, Orr-Asman MA, et al. Balancing yield, purity and practicality: a modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 2019;16(1):5–12. doi:10.1080/15476286.2018.1564465
73. Onódi Z, Pelyhe C, Terézia Nagy C, et al. Isolation of high-purity extracellular vesicles by the combination of iodixanol density gradient ultracentrifugation and bind-elute chromatography from blood plasma. Front Physiol. 2018;9:1479. doi:10.3389/fphys.2018.01479
74. Dehghani M, Lucas K, Flax J, McGrath J, Gaborski T. Tangential flow microfluidics for the capture and release of nanoparticles and extracellular vesicles on conventional and ultrathin membranes. Adv Mater Technol. 2019;4(11):1900539. doi:10.1002/admt.201900539
75. Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82(9):1024–1032. doi:10.1038/ki.2012.256
76. Ning B, Huang Z, Youngquist BM, et al. Liposome-mediated detection of SARS-CoV-2 RNA-positive extracellular vesicles in plasma. Nat Nanotechnol. 2021;16(9):1039–1044. doi:10.1038/s41565-021-00939-8
77. Clayton A, Court J, Navabi H, et al. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods. 2001;247(1–2):163–174. doi:10.1016/S0022-1759(00)00321-5
78. Nakai W, Yoshida T, Diez D, et al. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 2016;6(1):33935. doi:10.1038/srep33935
79. Soares Martins T, Catita J, Martins Rosa I, da Cruz e Silva OA, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One. 2018;13(6):e0198820. doi:10.1371/journal.pone.0198820
80. García-Romero N, Madurga R, Rackov G, et al. Polyethylene glycol improves current methods for circulating extracellular vesicle-derived DNA isolation. J Transl Med. 2019;17(1):75. doi:10.1186/s12967-019-1825-3
81. Sim S-L, He T, Tscheliessnig A, et al. Protein precipitation by polyethylene glycol: a generalized model based on hydrodynamic radius. J Biotechnol. 2012;157(2):315–319. doi:10.1016/j.jbiotec.2011.09.028
82. Kimura T, Ferran B, Tsukahara Y, et al. Production of adeno-associated virus vectors for in vitro and in vivo applications. Sci Rep. 2019;9(1):13601. doi:10.1038/s41598-019-49624-w
83. Ruysschaert T, Marque A, Duteyrat J-L, et al. Liposome retention in size exclusion chromatography. BMC Biotechnol. 2005;5(1):11. doi:10.1186/1472-6750-5-11
84. Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5:29289. doi:10.3402/jev.v5.29289
85. Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, et al. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci Rep. 2016;6(1):33641. doi:10.1038/srep33641
86. Shu S, Yang Y, Allen CL, et al. Purity and yield of melanoma exosomes are dependent on isolation method. J Extracell Vesicles. 2020;9(1):1692401. doi:10.1080/20013078.2019.1692401
87. Lin S, Yu Z, Chen D, et al. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small. 2020;16(9):e1903916. doi:10.1002/smll.201903916
88. Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14(11):1891–1900. doi:10.1039/C4LC00136B
89. Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9(3):2321–2327. doi:10.1021/nn506538f
90. Contreras-Naranjo JC, Wu HJ, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17(21):3558–3577. doi:10.1039/C7LC00592J
91. Zhang N, Sun N, Deng C. Rapid isolation and proteome analysis of urinary exosome based on double interactions of Fe(3)O(4)@TiO(2)-DNA aptamer. Talanta. 2021;221:121571. doi:10.1016/j.talanta.2020.121571
92. Zhang P, He M, Zeng Y. Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab Chip. 2016;16(16):3033–3042. doi:10.1039/C6LC00279J
93. Wang B, Wang Y, Yan Z, Sun Y, Su C. Colorectal cancer cell-derived exosomes promote proliferation and decrease apoptosis by activating the ERK pathway. Int J Clin Exp Pathol. 2019;12(7):2485–2495.
94. Yang Y, Li CW, Chan LC, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862–864. doi:10.1038/s41422-018-0060-4
95. Ma Z, Wei K, Yang F, et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Dis. 2021;12(9):840. doi:10.1038/s41419-021-04037-4
96. Liu Y, Gu Y, Han Y, et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30(2):243–256. doi:10.1016/j.ccell.2016.06.021
97. Sun H, Wang C, Hu B, et al. Exosomal S100A4 derived from highly metastatic hepatocellular carcinoma cells promotes metastasis by activating STAT3. Signal Transduct Target Ther. 2021;6(1):187. doi:10.1038/s41392-021-00579-3
98. Wei Y, Lai X, Yu S, et al. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res Treat. 2014;147(2):423–431. doi:10.1007/s10549-014-3037-0
99. Li Z, Meng X, Wu P, et al. Glioblastoma cell-derived lncRNA-containing exosomes induce microglia to produce complement C5, promoting chemotherapy resistance. Cancer Immunol Res. 2021;9(12):1383–1399. doi:10.1158/2326-6066.CIR-21-0258
100. Fabbri M, Paone A, Calore F, et al. MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–E2116. doi:10.1073/pnas.1209414109
101. Frühbeis C, Fröhlich D, Kuo WP, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604. doi:10.1371/journal.pbio.1001604
102. Koniusz S, Andrzejewska A, Muraca M, et al. Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci. 2016;10:109. doi:10.3389/fncel.2016.00109
103. Guitart K, Loers G, Buck F, et al. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia. 2016;64(6):896–910. doi:10.1002/glia.22963
104. Sardar Sinha M, Ansell-Schultz A, Civitelli L, et al. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136(1):41–56. doi:10.1007/s00401-018-1868-1
105. Asai H, Ikezu S, Tsunoda S, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18(11):1584–1593. doi:10.1038/nn.4132
106. Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging. 2014;35(8):1792–1800. doi:10.1016/j.neurobiolaging.2014.02.012
107. Kato T, Miyaki S, Ishitobi H, et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):R163. doi:10.1186/ar4679
108. Ni Z, Kuang L, Chen H, et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019;10(7):522. doi:10.1038/s41419-019-1739-2
109. Wu X, Crawford R, Xiao Y, Mao X, Prasadam I. Osteoarthritic subchondral bone release exosomes that promote cartilage degeneration. Cells. 2021;10(2):251. doi:10.3390/cells10020251
110. Henning RJ. Cardiovascular exosomes and MicroRNAs in cardiovascular physiology and pathophysiology. J Cardiovasc Transl Res. 2021;14(2):195–212. doi:10.1007/s12265-020-10040-5
111. Zarin B, Rafiee L, Daneshpajouhnejad P, Haghjooy Javanmard S. A review on the role of CAFs and CAF-derived exosomes in progression and metastasis of digestive system cancers. Tumour Biol. 2021;43(1):141–157. doi:10.3233/TUB-200075
112. Purghè B, Manfredi M, Ragnoli B, Baldanzi G, Malerba M. Exosomes in chronic respiratory diseases. Biomed Pharmacother. 2021;144:112270. doi:10.1016/j.biopha.2021.112270
113. Liu L, Liang L, Yang C, Zhou Y, Chen Y. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes. 2021;13(1):1–20. doi:10.1080/19490976.2021.1902718
114. Ma L, Shen Q, Lyu W, et al. Clostridium butyricum and its derived extracellular vesicles modulate gut homeostasis and ameliorate acute experimental colitis. Microbiol Spectr. 2022;10(4):e0136822. doi:10.1128/spectrum.01368-22
115. Yuan C, Burns MB, Subramanian S, Blekhman R, Sharpton T. Interaction between host MicroRNAs and the gut microbiota in colorectal cancer. mSystems. 2018;3(3). doi:10.1128/mSystems.00205-17
116. Ji Y, Li X, Zhu Y, et al. Faecal microRNA as a biomarker of the activity and prognosis of inflammatory bowel diseases. Biochem Biophys Res Commun. 2018;503(4):2443–2450. doi:10.1016/j.bbrc.2018.06.174
117. Bittel M, Reichert P, Sarfati I, et al. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo. J Extracell Vesicles. 2021;10(12):e12159. doi:10.1002/jev2.12159
118. Han Z, Peng X, Yang Y, et al. Integrated microfluidic-SERS for exosome biomarker profiling and osteosarcoma diagnosis. Biosens Bioelectron. 2022;217:114709. doi:10.1016/j.bios.2022.114709
119. Lee CH, Im EJ, Moon PG, Baek MC. Discovery of a diagnostic biomarker for colon cancer through proteomic profiling of small extracellular vesicles. BMC Cancer. 2018;18(1):1058. doi:10.1186/s12885-018-4952-y
120. Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 2015;11(6):600–607.e601. doi:10.1016/j.jalz.2014.06.008
121. Taylor DD, Gercel-Taylor C. Retracted: microRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21. doi:10.1016/j.ygyno.2008.04.033
122. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi:10.1073/pnas.0804549105
123. Xie L, Zhang Q, Mao J, Zhang J, Li L. The roles of lncRNA in myocardial infarction: molecular mechanisms, diagnosis biomarkers, and therapeutic perspectives. Front Cell Dev Biol. 2021;9:680713. doi:10.3389/fcell.2021.680713
124. Karimi N, Ali Hosseinpour Feizi M, Safaralizadeh R, et al. Serum overexpression of miR-301a and miR-23a in patients with colorectal cancer. J Chin Med Assoc. 2019;82(3):215–220. doi:10.1097/JCMA.0000000000000031
125. Fu F, Jiang W, Zhou L, Chen Z. Circulating exosomal miR-17-5p and miR-92a-3p predict pathologic stage and grade of colorectal cancer. Transl Oncol. 2018;11(2):221–232. doi:10.1016/j.tranon.2017.12.012
126. Wang YH, Ji J, Wang BC, et al. Tumor-derived exosomal long noncoding RNAs as promising diagnostic biomarkers for prostate cancer. Cell Physiol Biochem. 2018;46(2):532–545. doi:10.1159/000488620
127. Krug AK, Enderle D, Karlovich C, et al. Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma. Ann Oncol. 2018;29(10):2143. doi:10.1093/annonc/mdy261
128. Castellanos-Rizaldos E, Grimm DG, Tadigotla V, et al. Exosome-based detection of EGFR T790M in plasma from non-small cell lung cancer patients. Clin Cancer Res. 2018;24(12):2944–2950. doi:10.1158/1078-0432.CCR-17-3369
129. Zhu Y, Zhang H, Chen N, et al. Diagnostic value of various liquid biopsy methods for pancreatic cancer: a systematic review and meta-analysis. Medicine. 2020;99(3):e18581. doi:10.1097/MD.0000000000018581
130. Heo M, Park YS, Yoon H, et al. Potential of gut microbe-derived extracellular vesicles to differentiate inflammatory bowel disease patients from healthy controls. Gut Liver. 2023;17(1):108–118. doi:10.5009/gnl220081
131. Yoon H, Kim NE, Park J, et al. Analysis of the gut microbiome using extracellular vesicles in the urine of patients with colorectal cancer. Korean J Intern Med. 2023;38(1):27–38. doi:10.3904/kjim.2022.112
132. Yang J, McDowell A, Seo H, et al. Diagnostic models for atopic dermatitis based on serum microbial extracellular vesicle metagenomic analysis: a Pilot Study. Allergy Asthma Immunol Res. 2020;12(5):792–805. doi:10.4168/aair.2020.12.5.792
133. Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–182. doi:10.1038/nature14581
134. Sohn W, Kim J, Kang SH, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47(9):e184. doi:10.1038/emm.2015.68
135. Qiu X, Zhu H, Liu S, et al. Expression and prognostic value of microRNA-26a and microRNA-148a in gastric cancer. J Gastroenterol Hepatol. 2017;32(4):819–827. doi:10.1111/jgh.13533
136. Wei C, Chen X, Ji J, et al. Urinary exosomal prostate-specific antigen is a noninvasive biomarker to detect prostate cancer: not only old wine in new bottles. Int J Cancer. 2023;152(8):1719–1727. doi:10.1002/ijc.34388
137. Kim JE, Eom JS, Kim WY, et al. Diagnostic value of microRNAs derived from exosomes in bronchoalveolar lavage fluid of early-stage lung adenocarcinoma: a pilot study. Thorac Cancer. 2018;9(8):911–915. doi:10.1111/1759-7714.12756
138. Shen X, Xue Y, Cong H, et al. Circulating lncRNA DANCR as a potential auxillary biomarker for the diagnosis and prognostic prediction of colorectal cancer. Biosci Rep. 2020;40(3). doi:10.1042/BSR20191481
139. Liu CG, Song J, Zhang YQ, Wang PC. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol Med Rep. 2014;10(5):2395–2400. doi:10.3892/mmr.2014.2484
140. Skriner K, Adolph K, Jungblut PR, Burmester GR. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006;54(12):3809–3814. doi:10.1002/art.22276
141. Jiang H, Toscano JF, Song SS, et al. Author correction: differential expression of circulating exosomal microRNAs in refractory intracranial atherosclerosis associated with antiangiogenesis. Sci Rep. 2021;11(1):15266. doi:10.1038/s41598-021-94233-1
142. Hao S, Bai O, Li F, et al. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120(1):90–102. doi:10.1111/j.1365-2567.2006.02483.x
143. Chen Z, Dehm S, Bonham K, et al. DNA array and biological characterization of the impact of the maturation status of mouse dendritic cells on their phenotype and antitumor vaccination efficacy. Cell Immunol. 2001;214(1):60–71. doi:10.1006/cimm.2001.1883
144. Seo N, Shirakura Y, Tahara Y, et al. Activated CD8(+) T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. 2018;9(1):435. doi:10.1038/s41467-018-02865-1
145. Jiang Y, Jiang H, Wang K, et al. Hypoxia enhances the production and antitumor effect of exosomes derived from natural killer cells. Ann Transl Med. 2021;9(6):473. doi:10.21037/atm-21-347
146. Wang X, Huang R, Lu Z, et al. Exosomes from M1-polarized macrophages promote apoptosis in lung adenocarcinoma via the miR-181a-5p/ETS1/STK16 axis. Cancer Sci. 2022;113(3):986–1001. doi:10.1111/cas.15268
147. Zhao Y, Zheng Y, Zhu Y, et al. Docetaxel-loaded M1 macrophage-derived exosomes for a safe and efficient chemoimmunotherapy of breast cancer. J Nanobiotechnol. 2022;20(1):359. doi:10.1186/s12951-022-01526-2
148. Yang Y, Guo Z, Chen W, et al. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2. Mol Ther. 2021;29(3):1226–1238. doi:10.1016/j.ymthe.2020.11.024
149. Lan J, Sun L, Xu F, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79(1):146–158. doi:10.1158/0008-5472.CAN-18-0014
150. Li J, Xue H, Li T, et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE(-/-) mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 2019;510(4):565–572. doi:10.1016/j.bbrc.2019.02.005
151. Zhang J, Rong Y, Luo C, Cui W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging. 2020;12(24):25138–25152. doi:10.18632/aging.104110
152. Khare D, Or R, Resnick I, et al. Mesenchymal stromal cell-derived exosomes affect mRNA expression and function of B-lymphocytes. Front Immunol. 2018;9:3053. doi:10.3389/fimmu.2018.03053
153. Hu W, Song X, Yu H, Sun J, Zhao Y. Released exosomes contribute to the immune modulation of cord blood-derived stem cells. Front Immunol. 2020;11:165. doi:10.3389/fimmu.2020.00165
154. Reis M, Mavin E, Nicholson L, et al. Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front Immunol. 2018;9:2538. doi:10.3389/fimmu.2018.02538
155. Xia Q, Wang Q, Lin F, Wang J. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered. 2021;12(2):11225–11238. doi:10.1080/21655979.2021.1995580
156. Chen X, Shi Y, Xue P, et al. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res Ther. 2020;22(1):256. doi:10.1186/s13075-020-02325-6
157. Bruno S, Tapparo M, Collino F, et al. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A. 2017;23(21–22):1262–1273. doi:10.1089/ten.tea.2017.0069
158. Feng Y, Huang W, Wani M, Yu X, Ashraf M, Qin G. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9(2):e88685. doi:10.1371/journal.pone.0088685
159. Cai G, Cai G, Zhou H, et al. Retracted article: mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Res Ther. 2021;12(1):2. doi:10.1186/s13287-020-02030-w
160. Nakano M, Kubota K, Kobayashi E, et al. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10(1):10772. doi:10.1038/s41598-020-67460-1
161. Sengupta V, Sengupta S, Lazo A, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi:10.1089/scd.2020.0080
162. Dai S, Wei D, Wu Z, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16(4):782–790. doi:10.1038/mt.2008.1
163. Escudier B, Dorval T, Chaput N, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3(1):10. doi:10.1186/1479-5876-3-10
164. Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5(4):e1071008. doi:10.1080/2162402X.2015.1071008
165. Nassar W, El-Ansary M, Sabry D, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20(1):21. doi:10.1186/s40824-016-0068-0
166. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
167. Wei H, Chen J, Wang S, et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int J Nanomed. 2019;14:8603–8610. doi:10.2147/IJN.S218988
168. Pascucci L, Coccè V, Bonomi A, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi:10.1016/j.jconrel.2014.07.042
169. Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi:10.1038/nbt.1807
170. Yang T, Fogarty B, LaForge B, et al. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J. 2017;19(2):475–486. doi:10.1208/s12248-016-0015-y
171. Zhang H, Wang Y, Bai M, et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018;109(3):629–641. doi:10.1111/cas.13488
172. Chen Z, Wang H, Xia Y, Yan F, Lu Y. Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. 2018;201(8):2472–2482. doi:10.4049/jimmunol.1800304
173. Usman WM, Pham TC, Kwok YY, et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun. 2018;9(1):2359. doi:10.1038/s41467-018-04791-8
174. Yang Z, Shi J, Xie J, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4(1):69–83. doi:10.1038/s41551-019-0485-1
175. Haney MJ, Zhao Y, Harrison EB, et al. Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PLoS One. 2013;8(4):e61852. doi:10.1371/journal.pone.0061852
176. Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm. 2015;12(10):3650–3657. doi:10.1021/acs.molpharmaceut.5b00364
177. Qu M, Lin Q, Huang L, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–166. doi:10.1016/j.jconrel.2018.08.035
178. Ovchinnikova LA, Terekhov SS, Ziganshin RH, et al. Reprogramming extracellular vesicles for protein therapeutics delivery. Pharmaceutics. 2021;13(6):768. doi:10.3390/pharmaceutics13060768
179. Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi:10.1016/j.jconrel.2015.03.033
180. Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. doi:10.1016/j.jconrel.2014.11.029
181. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–664. doi:10.1016/j.nano.2015.10.012
182. Salarpour S, Forootanfar H, Pournamdari M, et al. Paclitaxel incorporated exosomes derived from glioblastoma cells: comparative study of two loading techniques. Daru. 2019;27(2):533–539. doi:10.1007/s40199-019-00280-5
183. Kooijmans SAA, Stremersch S, Braeckmans K, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release. 2013;172(1):229–238. doi:10.1016/j.jconrel.2013.08.014
184. Zhang H, Wu J, Wu J, et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnol. 2019;17(1):29. doi:10.1186/s12951-019-0461-7
185. Wang X, Chen Y, Zhao Z, et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc. 2018;7(15):e008737. doi:10.1161/JAHA.118.008737
186. Jia G, Han Y, An Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi:10.1016/j.biomaterials.2018.06.029
187. Tamura R, Uemoto S, Tabata Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017;57:274–284. doi:10.1016/j.actbio.2017.05.013
188. Li Y, Gao Y, Gong C, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine. 2018;14(7):1973–1985. doi:10.1016/j.nano.2018.05.020
189. Qi H, Liu C, Long L, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10(3):3323–3333. doi:10.1021/acsnano.5b06939
190. Yamamoto T, Teramura Y, Itagaki T, Arima Y, Iwata H. Interaction of poly(ethylene glycol)-conjugated phospholipids with supported lipid membranes and their influence on protein adsorption. Sci Technol Adv Mater. 2016;17(1):677–684. doi:10.1080/14686996.2016.1240006
191. Zhu L, Dong D, Yu Z-L, et al. Folate-engineered microvesicles for enhanced target and synergistic therapy toward breast cancer. ACS Appl Mater Interfaces. 2017;9(6):5100–5108. doi:10.1021/acsami.6b14633
192. Kooijmans SAA, Fliervoet LAL, van der Meel R, et al. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Control Release. 2016;224:77–85. doi:10.1016/j.jconrel.2016.01.009
193. Lu J, Gao X, Wang S, et al. Advanced strategies to evade the mononuclear phagocyte system clearance of nanomaterials. Exploration. 2023;3(1):20220045. doi:10.1002/EXP.20220045
194. Kooijmans SA, Aleza CG, Roffler SR, et al. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5(1):31053. doi:10.3402/jev.v5.31053
195. Liang G, Kan S, Zhu Y, et al. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomed. 2018;13:585–599. doi:10.2147/IJN.S154458
196. Tian T, Cao L, He C, et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics. 2021;11(13):6507–6521. doi:10.7150/thno.56367
197. Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19(2):72–80. doi:10.1016/j.tcb.2008.12.001
198. Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem Sci. 2012;37(8):325–332. doi:10.1016/j.tibs.2012.05.002
199. Parada N, Romero-Trujillo A, Georges N, Alcayaga-Miranda F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J Adv Res. 2021;31:61–74. doi:10.1016/j.jare.2021.01.001
200. Du J, Wan Z, Wang C, et al. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics. 2021;11(17):8185–8196. doi:10.7150/thno.59121
201. Ramasubramanian L, Kumar P, Wang A. Engineering extracellular vesicles as nanotherapeutics for regenerative medicine. Biomolecules. 2019;10(1). doi:10.1021/acsanm.0c01553
202. Villata S, Canta M, Cauda V. EVs and bioengineering: from cellular products to engineered nanomachines. Int J Mol Sci. 2020;21(17). doi:10.3390/ijms21176048
203. Sato YT, Umezaki K, Sawada S, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6(1):21933. doi:10.1038/srep21933
204. Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–494. doi:10.1016/j.actbio.2019.05.054
205. Mentkowski KI, Snitzer JD, Rusnak S, Lang JK. Therapeutic potential of engineered extracellular vesicles. AAPS J. 2018;20(3):50. doi:10.1208/s12248-018-0211-z
206. Lee JR, Park BW, Kim J, et al. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv. 2020;6(18):eaaz0952. doi:10.1126/sciadv.aaz0952
207. Altanerova U, Babincova M, Babinec P, et al. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int J Nanomed. 2017;12:7923–7936. doi:10.2147/IJN.S145096
208. Bull E, Madani SY, Sheth R, et al. Stem cell tracking using iron oxide nanoparticles. Int J Nanomed. 2014;9:1641–1653. doi:10.2147/IJN.S48979
209. Perets N, Betzer O, Shapira R, et al. Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 2019;19(6):3422–3431. doi:10.1021/acs.nanolett.8b04148
210. Sancho-Albero M, Navascués N, Mendoza G, et al. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J Nanobiotechnol. 2019;17(1):16. doi:10.1186/s12951-018-0437-z
211. Cao Y, Wu T, Zhang K, et al. Engineered exosome-mediated near-infrared-II region V(2)C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano. 2019;13(2):1499–1510. doi:10.1021/acsnano.8b07224
212. Zhang M, Vojtech L, Ye Z, Hladik F, Nance E. Quantum dot labeling and visualization of extracellular vesicles. ACS Appl Nano Mater. 2020;3(7):7211–7222.
213. Di H, Zeng E, Zhang P, et al. General approach to engineering extracellular vesicles for biomedical analysis. Anal Chem. 2019;91(20):12752–12759. doi:10.1021/acs.analchem.9b02268
214. Sancho-Albero M, Encabo-Berzosa MDM, Beltrán-Visiedo M, et al. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale. 2019;11(40):18825–18836. doi:10.1039/C9NR06183E
215. Poongodi R, Chen YL, Yang TH, et al. Bio-scaffolds as cell or exosome carriers for nerve injury repair. Int J Mol Sci. 2021;22(24):13347. doi:10.3390/ijms222413347
216. Rowland CR, Glass KA, Ettyreddy AR, et al. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials. 2018;177:161–175. doi:10.1016/j.biomaterials.2018.04.049
217. Jiang S, Tian G, Yang Z, et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact Mater. 2021;6(9):2711–2728. doi:10.1016/j.bioactmat.2021.01.031
218. Tsou YH, Khoneisser J, Huang PC, Xu X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact Mater. 2016;1(1):39–55. doi:10.1016/j.bioactmat.2016.05.001
219. Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;18:100522. doi:10.1016/j.mtbio.2022.100522
220. Zhou Y, Zhang XL, Lu ST, et al. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res Ther. 2022;13(1):407. doi:10.1186/s13287-022-02980-3
221. Lu W, Zeng M, Liu W, et al. Human urine-derived stem cell exosomes delivered via injectable GelMA templated hydrogel accelerate bone regeneration. Mater Today Bio. 2023;19:100569. doi:10.1016/j.mtbio.2023.100569
222. Hu H, Dong L, Bu Z, et al. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J Extracell Vesicles. 2020;9(1):1778883. doi:10.1080/20013078.2020.1778883
223. Liu X, Zhang J, Cheng X, et al. Integrated printed BDNF-stimulated HUCMSCs-derived exosomes/collagen/chitosan biological scaffolds with 3D printing technology promoted the remodelling of neural networks after traumatic brain injury. Regen Biomater. 2023;10:rbac085. doi:10.1093/rb/rbac085
224. Thakur S, Anjum MM, Jaiswal S, et al. Novel synergistic approach: tazarotene-calcipotriol-loaded-PVA/PVP-nanofibers incorporated in hydrogel film for management and treatment of psoriasis. Mol Pharm. 2023;20(2):997–1014. doi:10.1021/acs.molpharmaceut.2c00713
225. Li M, Wu Y, Li H, et al. Nanofiber reinforced alginate hydrogel for leak-proof delivery and higher stress loading in nucleus pulposus. Carbohydr Polym. 2023;299:120193. doi:10.1016/j.carbpol.2022.120193
226. Zhou Y, Liu S, Zhao M, et al. Injectable extracellular vesicle-released self-assembling peptide nanofiber hydrogel as an enhanced cell-free therapy for tissue regeneration. J Control Release. 2019;316:93–104. doi:10.1016/j.jconrel.2019.11.003
227. Smith LA, Liu X, Ma PX. Tissue engineering with nano-fibrous scaffolds. Soft Matter. 2008;4(11):2144–2149. doi:10.1039/b807088c
228. Takahashi Y, Nishikawa M, Shinotsuka H, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165(2):77–84. doi:10.1016/j.jbiotec.2013.03.013
229. Gangadaran P, Li XJ, Lee HW, et al. A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice. Oncotarget. 2017;8(66):109894–109914. doi:10.18632/oncotarget.22493
230. Faruqu FN, Wang JT, Xu L, et al. Membrane radiolabelling of exosomes for comparative biodistribution analysis in immunocompetent and immunodeficient mice - a novel and universal approach. Theranostics. 2019;9(6):1666–1682. doi:10.7150/thno.27891
231. Rashid MH, Borin TF, Ara R, et al. Differential in vivo biodistribution of (131)I-labeled exosomes from diverse cellular origins and its implication for theranostic application. Nanomedicine. 2019;21:102072. doi:10.1016/j.nano.2019.102072
232. Hwang DW, Choi H, Jang SC, et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using (99m)Tc-HMPAO. Sci Rep. 2015;5:15636. doi:10.1038/srep15636
233. Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn Reson Med. 2015;74(1):266–271. doi:10.1002/mrm.25376
234. Busato A, Bonafede R, Bontempi P, et al. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes. Int J Nanomed. 2016;11:2481–2490. doi:10.2147/IJN.S104152
235. Zhang M, Xiao B, Wang H, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24(10):1783–1796. doi:10.1038/mt.2016.159
236. Zhuang X, Teng Y, Samykutty A, et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol Ther. 2016;24(1):96–105. doi:10.1038/mt.2015.188
237. Liu J, Xiang J, Jin C, et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnology. 2023;21(1):78. doi:10.1186/s12951-023-01835-0
238. Chen X, Zhou Y, Yu J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol Pharm. 2019;16(6):2690–2699. doi:10.1021/acs.molpharmaceut.9b00246
239. Liu B, Lu Y, Chen X, et al. Protective role of shiitake mushroom-derived exosome-like nanoparticles in D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Nutrients. 2020;12(2):477.
240. Zhang M, Viennois E, Prasad M, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi:10.1016/j.biomaterials.2016.06.018
241. Teng Y, Ren Y, Sayed M, et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–652.e638. doi:10.1016/j.chom.2018.10.001
242. Chen Q, Li Q, Liang Y, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12(2):907–923. doi:10.1016/j.apsb.2021.08.016
243. Yepes-Molina L, Carvajal M. Nanoencapsulation of sulforaphane in broccoli membrane vesicles and their in vitro antiproliferative activity. Pharm Biol. 2021;59(1):1490–1504. doi:10.1080/13880209.2021.1992450
244. Xu Z, Huang H, Xiong X, et al. A near-infrared light-responsive extracellular vesicle as a “Trojan horse” for tumor deep penetration and imaging-guided therapy. Biomaterials. 2021;269:120647. doi:10.1016/j.biomaterials.2020.120647
245. Zhang Q, Xiao Q, Yin H, et al. Milk-exosome based pH/light sensitive drug system to enhance anticancer activity against oral squamous cell carcinoma. RSC Adv. 2020;10(47):28314–28323. doi:10.1039/D0RA05630H
246. Nie W, Wu G, Zhang J, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl. 2020;59(5):2018–2022. doi:10.1002/anie.201912524
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.