Back to Journals » Nature and Science of Sleep » Volume 14
Current Insights into Optimal Lighting for Promoting Sleep and Circadian Health: Brighter Days and the Importance of Sunlight in the Built Environment
Authors Fernandez FX
Received 23 October 2021
Accepted for publication 14 December 2021
Published 6 January 2022 Volume 2022:14 Pages 25—39
DOI https://doi.org/10.2147/NSS.S251712
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Fabian-Xosé Fernandez
Department of Psychology, University of Arizona, Tucson, AZ, USA
Correspondence: Fabian-Xosé Fernandez
Department of Psychology, University of Arizona, Tucson, Az, USA
Email [email protected]
Abstract: This perspective considers the possibility that daytime’s intrusion into night made possible by electric lighting may not be as pernicious to sleep and circadian health as the encroachment of nighttime into day wrought by 20th century architectural practices that have left many people estranged from sunlight.
Keywords: human-centric lighting, sleep, circadian entrainment, photohistory, sunlight, daylight
Introduction
The alternating availability and predictable ratio of sunlight to darkness emerging from the Earth’s rotation on its axis and revolution around the sun drives climate weather patterns, daily changes in temperature and humidity, as well as cycles of photosynthesis and chemical energy transfer across global food webs.1 Given the predictive power of this primordial signal (which lies at the root of all periodic change in the environment), the solar light/dark schedule became imprinted within the genome of the earliest organisms, who evolved molecular feedback loops matching the approximate timing of the Earth’s rotation calibrated by a centralized pacemaker network situated in immediate contact with photoreceptive organs.2 Sunlight is fundamental to our sense of time,3,4 and it is perhaps no surprise that the proliferation of light sources independent of the sun—starting with the domestication of fire—has increasingly challenged the circadian organization and robustness of the sleep-wake cycle.5 While the introduction of electric lighting in the late 19th century is often charged with being at the forefront of this disruption,6 anecdotal evidence suggests that illumination from flame-based sources could also delay bedtimes and perturb sleep, so much so that candlelight avoidance at night was recommended as a sleep hygiene technique in the year 1800.7 Contemporary research reveals that some individuals in the present day continue to exhibit circadian sensitivity to light levels approximating candlelight (< 4 lux, melanopic illuminance).8,9 An individual’s photic history may play a role in creating this heightened sensitivity.9,10
In the current manuscript, we provide a perspective on human-centric lighting, starting with the premise that such considerations are only made possible by the retinal divergence of the image-forming visual system and the non-visual system associated with circadian timekeeping, melatonin suppression, and arousal. If these two entities could not be biologically distinguished, then it is unlikely that most societies would be willing to trade the medical/emergency-care, security, work/business, and leisure opportunities afforded by nighttime illumination for improvements in sleep and circadian function (eg, current use trends for self-luminous devices would suggest as much11). Next, we summarize the available data suggesting that domestic and community light at night (LAN) has detrimental effects on sleep timing, duration, and quality. LAN directly modulates sleep but, in doing so, also influences many physical and mental health outcomes, some of which are outlined in communiqués from the World Health Organization, American Medical Association, and the United States National Toxicology Program.12 Upon reviewing the new standards that are being developed for reporting light exposure and integrating luminaires within the built environment (light-emitting diodes [LEDs] with controls permitting dynamic/customizable lighting regimes), we deconstruct the notion of “bright days, dark nights,” a simple principle that many in industry and academia use to describe optimal lighting conditions for sleep and circadian entrainment.13,14 In particular, we focus on work suggesting that the zeitgeber strength of the light prevailing throughout the day protects a person from the non-visual effects of ectopic light exposure that night, thereby improving sleep. In so far as there is a primacy lurking in this rule of thumb—that maximizing light availability in the photoperiod might be more conducive to maintaining sleep-wake rhythms than minimizing contact with evening light—it reorients discussion away from LAN towards a discussion about which practices might best convey a daytime signal during waking hours. Zeitgeber strength can be enhanced by dynamic LED regimes or increased exposure to daylight, though daylight is arguably the preferred choice because of its added health benefits, including essential vitamin D synthesis. If the most germane “human-centric lighting” is natural sunlight, a final point thus emerges with regard to solar rights (arguably a subset of universal human rights) and the role of daylight in architecture. We end our perspective by discussing the history of solar rights extending back to antiquity and the evolution of building design in urban centers, raising the possibility that postwar conveniences permitting taller and deeper construction (eg, electricity and air conditioning) are just as responsible for modern sleep disruption as universal access to electric lighting.
The Non-Image Forming System in Brief
English dictionaries such as Merriam-Webster and Oxford define the “eye” as the image-forming organ of sight, with other entries suggesting concepts related to “seeing,” “looking,” and “attending.” Definitions for “retina” also converge on the notion that it is a sensory membrane that transduces light signals in the service of vision. In all these narrative variations, without exception, the eye and retina are described as accoutrements of the visual system. The everyday vernacular use of the words belies their equally central role in non-image forming (NIF) pathways, even though such knowledge has been available for almost two decades. The NIF system is anchored by a small subpopulation of photosensitive retinal ganglion cells (pRGCs), so-called because they express melanopsin, a photopigment that absorbs short-wavelength light at maximum sensitivity around 490 nm after pre-receptoral filtering through the cornea, lens, and ocular media.15–20 pRGCs comprise only about 1% of the total retinal ganglion cell population in humans (which numbers upwards of 1.07 million cells)20 but send direct connections to an array of sites in the brain to regulate diverse functions.21–24 The most prominent of the connections is a monosynaptic pathway running along the retinohypothalamic tract to the brain’s circadian pacemaker, the suprachiasmatic nucleus (SCN), which enables entrainment to light-dark cycles.25–28
NIF processes mediated by pRGCs, including entrainment29–31 but also light suppression of pineal melatonin synthesis32–36 and light-mediated arousal,36–38 exhibit short-wavelength sensitivity consistent with the action spectrum for melanopsin. Blind humans and animals with mutations causing degeneration of classic photoreceptors retain many NIF functions.29,39–42 While these observations and others support the idea of there being an absolute functional dichotomy between the visual and NIF systems, it is important to note that rods and cones send inputs to pRGCs and influence their firing rates.16,43–47 What’s more, no single spectral sensitivity function can account for even simple NIF processes such as pupillary constriction (where time-gated contributions are likely such that rods and cones initiate responses that are later sustained by pRGCs).48,49 The interplay between rods, cones, and pRGCs in refining other NIF responses, with cones for example helping to specify phase-shifting responses to the kinds of rapidly changing light information experienced at dawn and dusk,50–52 underscores the strategic interconnectedness of the visual and NIF systems when complex real-world stimuli are considered. Nuances aside, NIF processes do evince a surprising degree of separation from classic visual processes and can likely be targeted with dynamic lighting approaches to improve sleep/circadian health whilst maintaining adequate illumination for work-related tasks. Part of the current focus of lighting practitioners, in fact, is in designing dynamic lighting standards for the workplace.
LAN’s Disruptive Effects on Sleep and Health
Exposure to community and household LAN affects dimensions of sleep health involving basic architecture, sleep timing and duration, as well as subjective quality. In the case of community LAN, the absolute effects of exposure—or lack of exposure—to nighttime illumination have been studied in the context of traditional societies devoid of electrification,53–56 people living in homes not wired for electricity,57 and individuals moving temporarily away from an urban setting to a campground.58 Under all these scenarios, people tend to sleep more relative to those with exposure to LAN and will retire to bed sooner (though results can be variable for sleep duration).54–56,59,60
The epidemiological effects of LAN have also been studied with respect to the intensity of upwardly directed outdoor illumination quantified from satellite imagery provided by the US Defense Meteorological Satellite Program (Operational Linescan System),61–63 the Visible and Infrared Imaging Radiometer Suite Day/Night Band (in the 500–900 nm spectral range),64,65 the International Space Station,66 and aerospace companies such as ImageSat International.67 Measurements suggest that 80% of the global population and upwards of 99% of the population in Europe and the United States are exposed to ground-level LAN via street, commercial, and neighborhood lighting.68 This accumulated exposure results in 23% of the world’s land mass being covered by indirect skyglow.68 Projections estimate that the radiance and extent of LAN will grow 2% annually from current numbers.69 LAN is omnipresent in the lives of many people, but there is geographic variation in its intensity and corresponding changes in the probability that individuals will experience issues with sleep or mental and physical health conditions connected to sleep. For example, when crosschecked with at-home sleep surveys done by telephone, greater outdoor LAN is associated with delayed and shortened sleep schedules, increased daytime sleepiness, and more dissatisfaction with sleep quality.70 In US adolescents (13–18 years of age), nationally representative surveys analyzed in the context of satellite imagery data suggest that higher outdoor levels of LAN are associated with half-hour later weeknight bedtimes along with increased odds of having a mood or anxiety disorder in the past year.71 Similar trends in the reporting of depression symptoms and suicide ideation have been noted among South Koreans living in districts with brighter outdoor LAN.72 Beyond mental health, several studies have also linked outdoor LAN exposure to the incidence of chronic disease,73 particularly cancer.12,74–76 The association may result from light-induced melatonin suppression, downstream changes in the immune system, and facilitation of tumor growth.77 An important asterisk for many of the semi-ecological population studies of outdoor LAN is the potential for residual confounding by variables other than nighttime illumination that distinguish large urban centers from suburban and rural areas (such variables are difficult to control simultaneously). Further investigations in humans and animal models will be necessary to distinguish the exact strength of LAN’s health associations.78–81
Inside or outside the influence of community LAN, people freely choose to use electric lighting in their homes at night for daily chores, self-care, work, and leisure. When simulating domestic overhead illumination (eg, from kitchens, bathroom vanities, or bedside lamps) or typical use of self-luminous devices, controlled experiments indicate that real-world intensities of household LAN can suppress melatonin secretion in the hours before habitual bedtime (ie, after 20.00), delay sleep timing, shorten sleep duration, and reduce next-morning alertness.82–88 These observations suggest that human-centric lighting practices require buy-in from individuals at a very personal level to be completely effective in optimizing sleep and circadian function. In other words: human-centric lighting is as much about personal choice as it is about technology and architecture.
Health Organizations Follow the Data and Voice New Concerns
The World Health Organization was one of the first agencies to suggest long-term shiftwork is a carcinogen. Monographs to this effect were released in 2007,89 with updates maintaining this outlook in 2019.90 In each statement, the impacts of LAN on cancer risk and tumor development were emphasized, singling out the possible role of immunodeficiency resulting from chronic melatonin suppression.91,92 The American Medical Association (AMA) followed up with their own position statement in 2012, in which they suggested that further research was warranted on environmental exposure to LAN and risk of cancer and other chronic diseases.93 In the Executive Summary of the AMA report, the carcinogenic effects associated with melatonin suppression were highlighted along with LAN’s possible role in exacerbating diseases such as obesity, diabetes, and depression.93 While these associations are plausibly causal, longitudinal exposure assessments of sleep in epidemiological research would help to fortify them into stronger policy statements.94
Unique to the 2012 AMA report was an additional commentary suggesting that children and adolescents might be particularly vulnerable to LAN’s disruption of sleep. This warning was prescient, as subsequent research has confirmed that preschool-age children, older children, and adolescents demonstrate enhanced melatonin suppression responses to evening light exposure in the hours preceding scheduled bedtime.95–99 Responses are not only two-fold more robust in children versus adults,98 but are also longer lived; in one study, most of the children participating in the experimental protocol did not see their levels of melatonin recover to 50% of baseline in the hour after the light exposure had ended.99 The arrest of melatonin secretion in children may result from increased retinal illumination stemming from the higher transparency of their lenses and larger steady-state pupil sizes.100–102 Adding to the pediatric issues surrounding LAN is that the lens transparency difference across age is most prominent at shorter wavelengths,102 the range of the visible spectrum at which the emissions peak for self-luminous devices .103 Nearly all individuals in the United States (ie, 90%) use self-luminous devices in the hour before bedtime and may not turn digital media off upon initiating sleep.11,104 For good sleep hygiene, the National Sleep Foundation recommends at least 30 minutes without technology before bedtime. However, this guideline is not customized for younger individuals who may require more time after their last light exposure to fully recover melatonin secretion. The concern is borne out in a meta-analysis of studies involving over 125,000 children, which found that evening use of self-luminous devices was associated with significantly worse sleep outcomes.105 Any future recommendations for limiting LAN exposure (and assuring some standardized level of daytime light exposure), as well as future dynamic lighting standards, would benefit from age-specific metrics analogous to those used by the National Sleep Foundation106 to benchmark sleep duration across the lifespan.
The Protective Role of Daytime Light Against LAN
LAN has dominated much of the discussion regarding human-centric lighting and is formalized as a medical concern by the World Health Organization and American Medical Association. While the “bright days” part of the circadian ledger has received some attention,14 there is no current consensus as to which—brighter days or darker nights—is more influential for shaping good sleep and circadian outcomes. Data suggest that the brighter days component might carry weight, however. First, in-lab manipulations of photohistory that vary the brightness of the indoor lighting a participant is exposed to over the subjective day (broad-spectrum fluorescent, 1 lux vs 90 lux) show that housing under dim light enhances subsequent arousal responses to a nighttime light stimulus.107 Rises in alertness in a 1-lux history condition versus a 90-lux history condition are observed whether measured through subjective rating (the Karolinska Drowsiness Test), objective neuropsychological assessment (auditory psychomotor vigilance task), or physiologically, by quantifying power density in the delta/theta band of the waking electroencephalograph.107 Accompanying the changes in arousal are other enhanced NIF functions combined with poorer sleep.108,109 Several independent groups have demonstrated that pre-exposure to inadequate daytime light leads to greater melatonin suppression upon viewing a test-light at night,10,110,111 as well as greater circadian phase-shifting responses.10,112 When measured across a divergent series of real-world or in-lab conditions, sparser patterns of ambient illumination (with lower zeitgeber strength) during the day also associate with later sleep initiation, less sleep pressure/slow-wave-sleep buildup under states of rest or sleep deprivation, more nighttime awakenings, worse perceptions of sleep quality, less circadian-robust sleep cycles, and increases in reported insomnia symptoms.113–126 These results are consistent with seasonally oriented studies of participants stationed in Antarctica that quantified (1) more light-induced melatonin suppression, (2) increased pupillary constriction, and (3) delayed phasing of the sleep-wake cycle in winter versus summer.127,128
Converging lines of evidence suggest that sensitivity to LAN increases when there is a lack of preceding daytime light, raising the possibility—in turn—that the health vulnerabilities associated with LAN can be counteracted or neutralized by adequate exposure to sunlight or electric indoor lighting. This premise has the potential to make a meaningful clinical impact once there is a data-driven consensus as to how much light a person requires throughout the day (intensity and duration of exposure) to inoculate themselves against LAN’s non-visual physiological effects. Though such data are generally lacking, one dose–response study conducted by Kozaki and colleagues did maintain individuals in-lab under varying intensities of white fluorescent light (4523K) for 3 hours in the morning (09.00–12.00) before testing light-induced melatonin responses overnight (01.00–03.00).111 The design of this study was somewhat unconventional (eg, with the LAN stimulus being introduced 18 hours after the morning intervention), but in principle, the results indicate that melatonin suppression associated with LAN can be prevented with bright early-day illumination. Future investigation will be necessary to clarify how full-day profiles of light exposure counteract nocturnal NIF responses and the relative importance of these effects in optimizing melanopic EDI in the morning versus afternoon. Yet the way is clear towards establishing an evidence-based standard for the daily allowance of light. One must answer the question: is there a daytime level of melanopic equivalent daylight illuminance that will completely minimize all NIF responses to LAN at LAN exposure levels anticipated to prevail domestically in most homes? The answer to this question remains unknown.
Evolution of Lighting Objectives
Up to the late 2000s, visual performance and comfort were the two cornerstones of lighting industry standards and design practices within the built environment. The emphasis is epitomized by the photopic luminous efficiency function V(λ) (grounded in human visual perception), which is the spectral weighting function commercially used to provide quantitative descriptions of light emissions between 380 and 780 nm.129–131 While architectural lighting is often still communicated in terms of illuminance (lux) and lumens per watt, a newer standard was devised by Lucas and colleagues132 and initially disseminated as a technical note by the Commission Internationale de l’Eclairage in 2015 before being formalized by international stakeholders in 2018 (CIE S 026/E:2018).133 It recommends defining exposure based on light’s activation of each of the five photoreceptor types contributing to NIF responses (S cone, M cone, L cone, rhodopsin, and the melanopsin-encoded photoreception of pRGCs). The necessity of this new standard was clarified within a few years after the discovery of pRGCs and, since then, has grown more urgent as the deleterious effects of LAN exposure have come into scientific view.
The human experience of light can potentially be balanced with respect to both the visual and NIF systems and tailored to fit the needs of each across the day or through the seasons. This delicate precision would be enabled by the development of light-emitting diodes (LEDs), semiconductor devices that generate narrowband light when passed by an electric current.13 LED chips made of a mix of gallium nitride (GaN) and indium nitride (InN) or manufactured from aluminium, indium, gallium and phosphorus (AlInGap) are highly tunable with regard to an emission’s wavelength and intensity and—when assembled in array with the proper controls—can produce different patterns of light exposure with microsecond resolution.134 The government, commercial, and domestic adoption of stationary LED systems (ie, with a fixed predefined light spectrum) represents one of the fastest technology transitions in history.135,136 LEDs are 40% more efficient than conventional fluorescent lighting,136 which leads to corresponding reductions in greenhouse gases; a 2014 report from the Department of Energy estimates that US LED installations prevented 7.1 million metric tons of CO2 emission.137 Concerns about energy efficiency and climate change have fueled a widespread conversion to stationary LED lighting, but health concerns about the non-visual effects of light are poised to add another stage of evolution in the form of dynamic lighting. With LED luminaires, room lighting does not have to remain static but can be adjusted in real time to accommodate the performance of the NIF system. At its most basic, dynamic lighting ensures that a waxing daytime signal is conveyed to building occupants in the late morning and early afternoon, while a waning daytime signal is reinforced in the late afternoon. At a time consistent with local dusk, evening illumination provoking little NIF response can be made available to those on a day-active schedule or reprogrammed to start another progression of sunrise and sundown for those arriving at the night shift.
The international standards that will provide a framework for devising dynamic light regimes in public spaces are still under discussion and require different conversations about how electric light exposure should be scheduled in high-or-low-latitude areas with wider swings in daylight hours between summer and winter (ie, does one track the solar cycle in high winter or set an artificial schedule between 07.00 and 19.00, for instance?). Irrespective of the finer details, it is likely that daytime light exposure will be formulated around a lighting system’s ability to meet a minimum zeitgeber strength measured in the vertical plane by melanopic equivalent daylight illuminance (EDI, in units of lux; for reference, see the Well Building StandardTM recommendations for circadian lighting design along with the CIE 2019 position statement).138 In everyday situations, this would mean that polychromatic stimuli regulated by automatic dimmers are blended between: a brighter intensity of blue-enriched spectrum of the highest acceptable color temperature and a dimmer intensity of blue-depleted spectrum of the lowest acceptable color temperature. Further consideration is necessary to determine what the exact light dosing should be at peak times of midday exposure for the typical population and what accommodations, if any, are required for children or subgroups with environmental sensitivities.139 As already mentioned, there is no general “Recommended Dietary Allowance” for light analogous to the daily nutrient intake recommendations maintained by the US Institute of Medicine or health authorities in other countries.
Whichever light-dosing standards are established in the future, it is likely they can be better met with natural sunlight. Even with advances in dynamic lighting, lighting practitioners under most circumstances will be integrating luminaires within the drop ceilings of commercial buildings and offices, bundling them with materials connected to the heating, ventilation, and air conditioning (HVAC) systems. While cost-effective, this approach results in light emissions that are angled downward toward desks and floors to optimize occupant vision of the surrounding environment in the horizontal plane. It is not efficient for providing direct illumination into the occupants’ eyes, which is necessary for registering the light’s zeitgeber strength. Workarounds to redirect vertical light include changing the wall reflectance and using ceiling-suspended “pendent” luminaires that distribute illumination more evenly. These workarounds, however, belie the fact that electric lighting solutions alone are ill-equipped to provide the light levels necessary for circadian entrainment (and protection against LAN effects) at standard rates of energy consumption. LED arrays can theoretically provide an adequate daytime signal, but challenges remain with regard to the cost-effectiveness of this approach.
Given what we are learning about the importance of daytime light exposure in the built environment, these indoor lighting efforts may not be sufficient. As such, are the health risks associated with inadequate light exposure sufficiently concerning that they should be granted deeper consideration in present-day building construction and renovation? Should all public architectural plans start first and foremost with design centered around windows or other “daylighting” methods that enhance the availability of sunlight and should this design-first principle be codified into law? The answers to these questions need to be informed by what we currently know about the health effects of daylight140 and by historical insights related to legal protections for one’s “human right to the sun.” We touch on these issues below.
Daylight: Sleep, Performance, and Long-Term Health
Academic and industry studies have quantified the beneficial role of daylight exposure on worker productivity and school performance.119,141–143 A more circumscribed literature has evaluated its effects with respect to sleep and psychological wellbeing. Relative to participants working in daylight-rich offices (ie, upwards of 10-fold more equivalent melanopic lux), those working in windowless environments or behind traditional blinds exhibit (1) poorer overall sleep quality measured by the Pittsburgh Sleep Quality Index’s global score and sleep disturbances component as well as (2) ~30–45-minute shorter sleep duration measured via actigraphy.144 Sleep differences track worse scores on decision-making tests and on dimensions of wellbeing itemized in the Short Form Health Survey, including indices related to vitality and perception of physical ability/limitations.143,144 Larger cross-sectional and longitudinal analyses of over 400,000 individuals from the UK Biobank offer a demonstration of the daylight → sleep connection at population scale. Cross-sectionally, the number of daytime hours spent by Biobank participants outdoors was associated with less sleep inertia, fewer insomnia symptoms, and advanced sleep-wake phases.145 These associations remained significant after adjusting for demographic, lifestyle, and employment factors. Auto-Regressive Cross-Lagged models examining the longitudinal relationship between daylight exposure and sleep-related outcomes suggested moreover that the number of hours spent outdoors could statistically predict the likelihood of experiencing sleep inertia and insomnia.145
Findings at this stage suggest that sleep/circadian health problems are seeded in people when they are not exposed to the adequate amounts of daylight, thereby potentially increasing risk for other medical issues (genetic predispositions may leave some individuals particularly vulnerable to these exposure deficits146,147). In discussions concerning the relative advantages of daylight versus indoor electric lighting in meeting exposure needs, one could emphasize that the more circadian-optimal spectral/intensity qualities of sunlight are difficult to simulate with overhead luminaires. Sunlight above the atmosphere and at the Earth’s surface has a broad continuous spectral power distribution with a prominent shoulder over the range of the spectrum to which melanopsin-bearing pRGCs are most sensitive. Intensity-wise, outdoor levels of sunlight extend beyond 100,000 lux at midday and remain above 3000 lux when it rains. Outdoor illuminance is still at 1000 lux at the start of civil twilight, which is two-fold brighter than the set point throughout the day for most office lighting.148
Beyond these attributes though, sunlight has a few unique properties that electric lighting does not. Due to its ultraviolet-B content, sunlight can direct the synthesis of vitamin D through the skin,149 which is essential for calcium absorption by bones and strengthening them against conditions like osteoporosis and rickets.150 Sequestered under electric lighting, around one billion people globally are now deficient in vitamin D.151 As a result, the bone disease, rickets, is seeing a resurgence almost a century after it was thought to have been eliminated.152 A rise in shortsightedness (myopia) has also taken hold in many East Asian countries with dense urban environments such as China, Singapore and Japan, afflicting 70–80% of the pediatric and young-adult populations there.153–156 Being outdoors for 2–3 hours daily through the first several years of life can prevent the condition157 and may be the only realistic prescription considering that the biochemical pathways stimulated by daylight to control eye growth are not understood.158,159 Placed in as complete of a health context as possible, it becomes obvious that daylight is rooted in our biology. Our external organs assume its daily presence, and a person’s physical and cognitive potential may depend on it. The right to daylight exposure has been codified under various legal protections since antiquity in observance of this (long-assumed) importance. This has changed in the electrified world, where some safeguards remain, some do not, and many go unheeded in urban development.
Daylighting: Law
For much of recorded history, starting in ancient Greece, issues surrounding solar rights drove building codes, design architecture, and top-down approaches to urban planning that positioned structures along street layouts collectively optimizing daylight exposure (a complete review can be found in160). As early as the 6th century A.D., the Justinian code also provided for the enforcement of solar rights through court decrees, prescriptive easements (that enabled a citizen to assert property claims against an owner’s land), and government allocations. Day-to-day in Rome/Byzantine, this meant that occupants were protected from illegal shadows or compensated when incident daylight on their property was blocked by an adjacent dwelling. The tradition of solar rights that started in Greece and Rome materialized in English Common Law (ie, derived from custom and judicial precedent) in the form of the 1663 “Ancient Lights” rule and was then codified into the United Kingdom statutory law by the Prescription Act of 1832.161 The doctrine asserts that the windows an owner uses to access daylight cannot be obstructed by another party if those windows have been in use for 20 years or more. Similar legal approaches have been taken in Japan, with Japanese courts shepherding a present-day permit system that recognizes a right to light not only for interiors via windows but also for the entire lot including the house and garden.162 The binding thread for most solar law is that sunlight travels a path across multiple parcels in any urban zone; everyone along that path should enjoy equal access to the illumination that is produced. Japanese courts have often cited health concerns as the justification for enforcing solar protections.
Current daylighting standards are in limited evidence in most local jurisdictions beyond the United Kingdom, Japan, and Germany, which recently became the first country in the European Union to adopt EN 17037 (a new building standard that specifies design practices for achieving adequate daylight exposure indoors).163 Even across the metropolises of the United States, municipal legislation can only really be found in lower Manhattan’s 1916 solar zoning legislation, which ensures the availability of sunlight at street-level by prescribing minimum street widths, building setbacks (eg, a wedding-cake-shaped typology), and structural height limitations.164 The American legal system has yet to recognize the solar rights of property owners or office workers and, like most of the rest of the world, lacks a coherent legal framework by which to do so. The international vacuum has undoubtedly contributed to daylight’s absence from: the World Health Organization’s Declaration on Occupational Health for All and “Healthy Cities” concept, the United Nations Sustainable Development Goals, as well as the United Nations Universal Declaration of Human Rights. In some tangible capacity, solar rights must become a part of global discussions on human wellbeing if they have any chance of being codified into building mandates wherein employers and community members are charged with the (properly placed) social responsibility of ensuring adequate daylight exposure.
Daylighting: Architecture
In an engineering context, daylighting refers to practices that admit “beam sunlight, diffuse skylight, and reflected light from the exterior into a building” (Illuminating Engineering Society, 2013). Historically, inside or outside of mandates, developers have typically made efforts to direct daylight into enclosed spaces, starting with classic Roman architects that designed public bath houses around solar heating.165 These efforts were deprioritized after the late 19th century invention of the fluorescent lamp followed by that of air conditioning in 1902. With these tools, architects were no longer reliant on windows to regulate indoor lighting and temperature, and thus could design tall structures (eg, skyscrapers) maximizing building occupancy with deep floor plans and lower floor-to-ceiling heights.166 Financial incentives for skyscraper construction became increasingly relevant in the early 20th century as the price of land surged in major American cities, such as New York City, Chicago, and Philadelphia. Soon, in a shift that accelerated around the world in the 1960s, fluorescent indoor lighting and HVAC systems offered the promise of comfortable indoor environments that enabled people to live and build anywhere they wanted irrespective of the surrounding climate. During these decades of urban development, little distinction was made between daylight and fluorescent lighting as both could be engineered with visual performance in mind. It took scientists until approximately the 1990s to form a consensus that endogenous circadian rhythms existed, were entrained by light–dark exposure, and helped to organize the sleep-wake cycle. The lag between the invention of electric lighting, on one hand—and our scientific understanding of circadian rhythmicity and sleep, on the other—has resulted in the problem we face today: People spend most of their time indoors at workstations far removed from daylight and suffer unacknowledged health problems associated with this exposure deficit.
An expert discussion of what architectural steps should be taken to enhance the availability of daylight within the built environment is outside the purview of this commentary. Yet, a few advancements are worth noting that illustrate the variety of ways the problem can be approached. The first concerns advanced systems based on anidolic lighting (non-image optics) that use reflection, refraction, and diffraction methods to channel the delivery of daylight deeper into buildings. Here, light ducts or windows can be adapted with reflective louvers and prismatic film tiles to enhance daylight penetration. With a second approach, electrochromic (EC) glazing, the transparency of the window glass can also be changed by application of a low-voltage electric current. These transparency changes are adjustable so that glare is minimized while optimizing the amount of biologically active short-wavelength light reaching the occupants. Anidolic lighting is best deployed when vertically integrated with the design features of the building façade. Fenestration schemes that incorporate atria, internal gardens, skylights, and room-individualized balconies are significantly reinforced when anidolic optical components are used to help distribute light throughout the space. Notably, current metrics that evaluate daylight performance under a given fenestration design (eg, Spatial Daylight Autonomy, Useful Daylight Illuminance, and Daylight Factor)167 require conversion to melanopic variants to be most informative for human-centric lighting objectives.
One More Thing: A Thought About Seasonality
A consideration that sometimes gets lost in lighting discussions is the relative importance of seasonality. Metropolitan areas in Sweden, Norway, and Finland might receive only 6 hours of daylight in high winter, about half the daylength observed in tropical countries near the equator. Due to conditions such as seasonal affective disorder, it seems intuitive that the built environment should compensate for this lack of winter daylight exposure. However, how does one execute this strategy? Should one respect seasonal distinctions and make light available in the spectrum consistent with the latitude of the locale and time-of-year only for those 6 hours—perhaps at two-fold the normal radiance? Or should a protracted spectral-appropriate sequence be made available for 12 hours? What aspects of human physiology would benefit or suffer should one or the other regime be implemented? We have the wherewithal to engineer the light–dark cycle at will, and the knowledge to intuit a fairly accurate daily pattern of healthy lighting. However, what happens when we have the power to abolish seasons? One could argue that lack of seasonality is already endemic to the modern world; the argument is not especially satisfying, though, when seasonal trends in depression and other conditions are still measurable at population-scale. Advances in LEDs and daylighting will bring this question and others increasingly into focus.
Conclusion
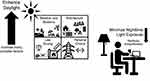
In the current perspective, we have attempted to synthesize a number of considerations that factor into the concept of healthy circadian lighting beyond the elegant neurophysiological data that originally highlighted the importance of pRGCs to the NIF system and the cells’ mechanistic role in translating light’s effects on the sleep and circadian systems (for more involved discussion of these topics, the reader is referred to Blume and colleagues, 2019168). From this synthesis, we suggest that sunlight is the most effective zeitgeber for entraining the sleep-wake cycle and a potential lever arm for preventing the unintended non-visual physiological effects of LAN exposure. Introducing more daylight into the built environment, however, is not without future challenges and involves a complex set of factors that sometimes go unrecognized and are difficult to negotiate in the short term (Figure 1).
Foremost among these challenges is that few legal statutes recognize the importance of sunlight to human wellbeing and even fewer that furnish protections guaranteeing access to it. For healthy circadian lighting to become a fundamental part of daily living, there must be an acknowledgement by international agencies and national governments that inadequate exposure to daylight is pernicious to human health, akin to the environmental laws that already acknowledge the dangers of smog to breathable air or the dangers of pesticides and heavy metals in drinking water. Regulatory bodies such as the US Environmental Protection Agency look to protect air quality, public water distribution systems, and agriculture by evolving standards that recognize the various ways these vital resources can be corrupted. The next step for human health would be for these agencies to develop and enforce analogous requirements for daylight exposure in offices, commercial buildings, and domestic homes that could be integrated with new architectural codes and design objectives. This evolution in environmental protection might take time to unfold but can be guided by empirical data that clearly establish how much daytime light exposure is necessary to offset responses to LAN (ie, the factor that the WHO and AMA have associated with negative health outcomes, including cancer). Surprisingly, such data do not exist despite previous academic attempts to describe daytime light recommendations for indoor environments. Acquisition of these data is a sensible future direction for circadian lighting research.
Another research priority is to determine whether the needs of the visual and NIF systems can be balanced with dynamic indoor lighting alone. LEDs can help achieve this balance across the day but might be cost-prohibitive in windowless rooms. In such cases, light therapy from tabletop devices directed at eye-level could supplement overhead LED lighting to ensure a daytime signal. However, experiments to test these interventions in real-world settings are still rare, and an open question remains as to whether electric lighting can truly create environments that reinforce the circadian system while meeting all the performance objectives of vision. In the event that an adequate daytime signal cannot be easily accommodated by electric lighting solutions that optimize visual perception, it creates more of an impetus to develop legal sun rights that can be codified into newer building standards.
Finally, the influence of non-photic zeitgebers in setting the threshold of daytime light exposure that will offset LAN responses is not known (to our knowledge, it is not even an active area of research). It is possible that proper meal timing and exercise during the day will lower this threshold, while mistiming of the two zeitgebers might raise it. A common limitation in circadian lighting research is the general lack of consideration of non-photic zeitgebers. The aforementioned studies—evaluating photic and non-photic zeitgebers side-by-side in an attempt to determine how one influences the functionality of the other—would thus represent a valuable intersection with significant implications for public health.
Healthy circadian lighting ultimately depends on personal choice. Where available, a person can choose to prioritize outdoor activities that will guarantee exposure to sunlight. They can also choose to limit the illumination they are exposed to at night and opt to align their eating and exercise schedules so that they are best positioned with the light–dark cycle. That said, efforts to ensure public access to daylight are not mutually exclusive of these personal choices. The more tangible daylighting efforts become, the more they enter public consciousness, and the more likely they will factor into the health-related decision-making of the average person. Efforts to institutionalize the importance of daylight can thus produce halo effects, with success resulting in more people willing to commit to choices that will strengthen their sleep and circadian health. Let us not lose sight of this process.
Funding
Financial support for the author was provided by the Velux Stiftung (Project No. 1360).
Disclosure
The author reports no other conflicts of interest in this work.
References
1. Hafker NS, Tessmar-Raible K. Rhythms of behavior: are the times changin’? Curr Opin Neurobiol. 2020;60:55–66. doi:10.1016/j.conb.2019.10.005
2. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–179. doi:10.1038/nrg.2016.150
3. Roenneberg T, Foster RG. Twilight times: light and the circadian system. Photochem Photobiol. 1997;66(5):549–561. doi:10.1111/j.1751-1097.1997.tb03188.x
4. Kreitzman L, Foster R. Rhythms of Life: The Biological Clocks That Control the Daily Lives of Every Living Thing. Profile Books; 2011.
5. Ludtke LE. Sleep, disruption and the ‘nightmare of total illumination’ in late nineteenth and early twentieth-century dystopian fiction. Interface Focus. 2020;10(3):20190130. doi:10.1098/rsfs.2019.0130
6. Lockley S, Foster R. Sleep: A Very Short Introduction. Oxford University Press; 2012.
7. Willich AFM. Lectures on Diet and Regimen. Longman, Hurst, Rees&Orme; 1800
8. Phillips AJK, Vidafar P, Burns AC, et al. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc Natl Acad Sci U S A. 2019;116(24):12019–12024. doi:10.1073/pnas.1901824116
9. Cain SW, McGlashan EM, Vidafar P, et al. Evening home lighting adversely impacts the circadian system and sleep. Sci Rep. 2020;10(1):19110. doi:10.1038/s41598-020-75622-4
10. Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589(Pt 5):1095–1102. doi:10.1113/jphysiol.2010.201194
11. Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, Czeisler CA. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9(12):1291–1299. doi:10.5664/jcsm.3272
12. Lunn RM, Blask DE, Coogan AN, et al. Health consequences of electric lighting practices in the modern world: a report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ. 2017;607–608:1073–1084. doi:10.1016/j.scitotenv.2017.07.056
13. Soler R, Voss E. Biologically Relevant Lighting: an Industry Perspective. Front Neurosci. 2021;15:637221. doi:10.3389/fnins.2021.637221
14. Vetter C, Phillips AJK, Silva A, Lockley SW, Glickman G. Light Me up? Why, When, and How Much Light We Need. J Biol Rhythms. 2019;34(6):573–575. doi:10.1177/0748730419892111
15. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi:10.1126/science.1067262
16. Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749–754. doi:10.1038/nature03387
17. Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (lambda max approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci. 2013;280(1759):20122987. doi:10.1098/rspb.2012.2987
18. Do MTH. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: biophysics to Behavior. Neuron. 2019;104(2):205–226. doi:10.1016/j.neuron.2019.07.016
19. Spitschan M. Melanopsin contributions to non-visual and visual function. Curr Opin Behav Sci. 2019;30:67–72. doi:10.1016/j.cobeha.2019.06.004
20. Mure LS. Intrinsically Photosensitive Retinal Ganglion Cells of the Human Retina. Front Neurol. 2021;12:636330. doi:10.3389/fneur.2021.636330
21. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. doi:10.1126/science.1069609
22. Li JY, Schmidt TM. Divergent projection patterns of M1 ipRGC subtypes. J Comp Neurol. 2018;526(13):2010–2018. doi:10.1002/cne.24469
23. Rupp AC, Ren M, Altimus CM, et al. Distinct ipRGC subpopulations mediate light’s acute and circadian effects on body temperature and sleep. Elife. 2019:8. doi:10.7554/eLife.44358
24. Aranda ML, Schmidt TM. Diversity of intrinsically photosensitive retinal ganglion cells: circuits and functions. Cell Mol Life Sci. 2021;78(3):889–907. doi:10.1007/s00018-020-03641-5
25. Kim KY, Rios LC, Le H, et al. Synaptic Specializations of Melanopsin-Retinal Ganglion Cells in Multiple Brain Regions Revealed by Genetic Label for Light and Electron Microscopy. Cell Rep. 2019;29(3):628–644 e6. doi:10.1016/j.celrep.2019.09.006
26. Stinchcombe AR, Hu C, Walch OJ, Faught SD, Wong KY, Forger DB. M1-Type, but Not M4-Type, Melanopsin Ganglion Cells Are Physiologically Tuned to the Central Circadian Clock. Front Neurosci. 2021;15:652996. doi:10.3389/fnins.2021.652996
27. Mouland JW, Martial FP, Lucas RJ, Brown TM. Modulations in irradiance directed at melanopsin, but not cone photoreceptors, reliably alter electrophysiological activity in the suprachiasmatic nucleus and circadian behaviour in mice. J Pineal Res. 2021;70(4):e12735. doi:10.1111/jpi.12735
28. Dannerfjord AA, Brown LA, Foster RG, Peirson SN. Light Input to the Mammalian Circadian Clock. Methods Mol Biol. 2021;2130:233–247. doi:10.1007/978-1-0716-0381-9_18
29. Zaidi FH, Hull JT, Peirson SN, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17(24):2122–2128. doi:10.1016/j.cub.2007.11.034
30. Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2(31):31ra33. doi:10.1126/scitranslmed.3000741
31. Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001;18(5):801–808. doi:10.1081/cbi-100107515
32. Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi:10.1523/JNEUROSCI.21-16-06405.2001
33. Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535(Pt 1):261–267. doi:10.1111/j.1469-7793.2001.t01-1-00261.x
34. Brainard GC, Hanifin JP, Warfield B, et al. Short-wavelength enrichment of polychromatic light enhances human melatonin suppression potency. J Pineal Res. 2015;58(3):352–361. doi:10.1111/jpi.12221
35. Prayag AS, Najjar RP, Gronfier C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J Pineal Res. 2019;66(4):e12562. doi:10.1111/jpi.12562
36. Spitschan M, Lazar R, Yetik E, Cajochen C. No evidence for an S cone contribution to acute neuroendocrine and alerting responses to light. Curr Biol. 2019;29(24):R1297–R1298. doi:10.1016/j.cub.2019.11.031
37. Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37(2):271–281. doi:10.5665/sleep.3396
38. Nowozin C, Wahnschaffe A, Rodenbeck A, et al. Applying Melanopic Lux to Measure Biological Light Effects on Melatonin Suppression and Subjective Sleepiness. Curr Alzheimer Res. 2017;14(10):1042–1052. doi:10.2174/1567205014666170523094526
39. Czeisler CA, Shanahan TL, Klerman EB, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332(1):6–11. doi:10.1056/NEJM199501053320102
40. Lucas RJ, Foster RG. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology. 1999;140(4):1520–1524. doi:10.1210/endo.140.4.6672
41. Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):505–507. doi:10.1126/science.284.5413.505
42. Freedman MS, Lucas RJ, Soni B, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284(5413):502–504. doi:10.1126/science.284.5413.502
43. Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi:10.1038/nature01761
44. Guler AD, Altimus CM, Ecker JL, Hattar S. Multiple photoreceptors contribute to nonimage-forming visual functions predominantly through melanopsin-containing retinal ganglion cells. Cold Spring Harb Symp Quant Biol. 2007;72:509–515. doi:10.1101/sqb.2007.72.074
45. Guler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi:10.1038/nature06829
46. Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582(Pt 1):279–296. doi:10.1113/jphysiol.2007.133751
47. Lee SK, Sonoda T, Schmidt TM. M1 Intrinsically Photosensitive Retinal Ganglion Cells Integrate Rod and Melanopsin Inputs to Signal in Low Light. Cell Rep. 2019;29(11):3349–3355e2. doi:10.1016/j.celrep.2019.11.024
48. Gooley JJ, Ho Mien I, St Hilaire MA, et al. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. 2012;32(41):3219–3253. doi:10.3390/ijerph17093219
49. Joyce DS, Feigl B, Cao D, Zele AJ. Temporal characteristics of melanopsin inputs to the human pupil light reflex. Vision Res. 2015;107:58–66. doi:10.1016/j.visres.2014.12.001
50. Walmsley L, Hanna L, Mouland J, et al. Colour as a signal for entraining the mammalian circadian clock. PLoS Biol. 2015;13(4):e1002127. doi:10.1371/journal.pbio.1002127
51. van Diepen HC, Schoonderwoerd RA, Ramkisoensing A, Janse JAM, Hattar S, Meijer JH. Distinct contribution of cone photoreceptor subtypes to the mammalian biological clock. Proc Natl Acad Sci U S A. 2021;118(22):e2024500118. doi:10.1073/pnas.2024500118
52. Wong KY, Fernandez FX. Circadian Responses to Light-Flash Exposure: conceptualization and New Data Guiding Future Directions. Front Neurol. 2021;12:627550. doi:10.3389/fneur.2021.627550
53. Yetish G, Kaplan H, Gurven M, et al. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015;25(21):2862–2868. doi:10.1016/j.cub.2015.09.046
54. Pilz LK, Levandovski R, Oliveira MAB, Hidalgo MP, Roenneberg T. Sleep and light exposure across different levels of urbanisation in Brazilian communities. Sci Rep. 2018;8(1):11389. doi:10.1038/s41598-018-29494-4
55. Moreno CR, Vasconcelos S, Marqueze EC, et al. Sleep patterns in Amazon rubber tappers with and without electric light at home. Sci Rep. 2015;5:14074. doi:10.1038/srep14074
56. Samson DR, Manus MB, Krystal AD, Fakir E, Yu JJ, Nunn CL. Segmented sleep in a nonelectric, small-scale agricultural society in Madagascar. Am J Hum Biol. 2017;29(4). doi:10.1002/ajhb.22979
57. Peixoto CA, Da Silva AG, Carskadon MA, Louzada FM. Adolescents living in homes without electric lighting have earlier sleep times. Behav Sleep Med. 2009;7(2):73–80. doi:10.1080/15402000902762311
58. Wright KP
59. de la Iglesia HO, Fernandez-Duque E, Golombek DA, et al. Access to Electric Light Is Associated with Shorter Sleep Duration in a Traditionally Hunter-Gatherer Community. J Biol Rhythms. 2015;30(4):342–350. doi:10.1177/0748730415590702
60. Beale AD, Pedrazzoli M, Goncalves B, et al. Comparison between an African town and a neighbouring village shows delayed, but not decreased, sleep during the early stages of urbanisation. Sci Rep. 2017;7(1):5697. doi:10.1038/s41598-017-05712-3
61. Kloog I, Haim A, Stevens RG, Barchana M, Portnov BA. Light at night co-distributes with incident breast but not lung cancer in the female population of Israel. Chronobiol Int. 2008;25(1):65–81. doi:10.1080/07420520801921572
62. Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. Limiting the impact of light pollution on human health, environment and stellar visibility. J Environ Manage. 2011;92(10):2714–2722. doi:10.1016/j.jenvman.2011.06.029
63. de Miguel AS, Zamorano J, Castaño JG, Pascual S. Evolution of the energy consumed by street lighting in Spain estimated with DMSP-OLS data. J Quant Spectrosc Radiat Transf. 2013;139:109–117. doi:10.1016/j.jqsrt.2013.11.017
64. Elvidge CD, Baugh KE, Zhizhin M, Hsu F-C. Why VIIRS data are superior to DMSP for mapping nighttime lights. Proc Asia Pacific Adv Network. 2013;35:62–69. doi:10.7125/APAN.35.7
65. Lane KJ, Stokes EC, Seto KC, Thanikachalam S, Thanikachalam M, Bell ML. Associations between Greenness, Impervious Surface Area, and Nighttime Lights on Biomarkers of Vascular Aging in Chennai, India. Environ Health Perspect. 2017;125(8):087003. doi:10.1289/EHP541
66. de Miguel AS, Castaño JG, Zamorano J, et al. Atlas of astronaut photos of earth at night. Astron Geophys. 2014;55:436. doi:10.1093/astrogeo/atu165
67. Katz Y, Levin N. Quantifying urban light pollution—a comparison between field measurements and EROS-B imagery. Remote Sens Environ. 2016;177:65–77. doi:10.1016/j.rse.2016.02.017
68. Falchi F, Cinzano P, Duriscoe D, et al. The new world atlas of artificial night sky brightness. Sci Adv. 2016;2(6):e1600377. doi:10.1126/sciadv.1600377
69. Kyba CCM, Kuester T, Sanchez de Miguel A, et al. Artificially lit surface of Earth at night increasing in radiance and extent. Sci Adv. 2017;3(11):e1701528. doi:10.1126/sciadv.1701528
70. Ohayon MM, Milesi C. Artificial Outdoor Nighttime Lights Associate with Altered Sleep Behavior in the American General Population. Sleep. 2016;39(6):1311–1320. doi:10.5665/sleep.5860
71. Paksarian D, Rudolph KE, Stapp EK, et al. Association of Outdoor Artificial Light at Night With Mental Disorders and Sleep Patterns Among US Adolescents. JAMA Psychiatry. 2020;77(12):1266–1275. doi:10.1001/jamapsychiatry.2020.1935
72. Min JY, Min KB. Outdoor light at night and the prevalence of depressive symptoms and suicidal behaviors: a cross-sectional study in a nationally representative sample of Korean adults. J Affect Disord. 2018;227:199–205. doi:10.1016/j.jad.2017.10.039
73. Rybnikova NA, Haim A, Portnov BA. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int J Obes. 2016;40(5):815–823. doi:10.1038/ijo.2015.255
74. James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM, Laden F. Outdoor Light at Night and Breast Cancer Incidence in the Nurses’ Health Study II. Environ Health Perspect. 2017;125(8):087010. doi:10.1289/EHP935
75. Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin. 2014;64(3):207–218. doi:10.3322/caac.21218
76. Stevens RG, Rea MS. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control. 2001;12(3):279–287. doi:10.1023/a:1011237000609
77. Blask DE, Brainard GC, Dauchy RT, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65(23):11174–11184. doi:10.1158/0008-5472.CAN-05-1945
78. Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ. Dim light at night provokes depression-like behaviors and reduces CA1 dendritic spine density in female hamsters. Psychoneuroendocrinology. 2011;36(7):1062–1069. doi:10.1016/j.psyneuen.2011.01.004
79. Bedrosian TA, Fonken LK, Nelson RJ. Endocrine Effects of Circadian Disruption. Annu Rev Physiol. 2016;78:109–131. doi:10.1146/annurev-physiol-021115-105102
80. Russart KLG, Nelson RJ. Light at night as an environmental endocrine disruptor. Physiol Behav. 2018;190:82–89. doi:10.1016/j.physbeh.2017.08.029
81. Fonken LK, Bedrosian TA, Zhang N, Weil ZM, DeVries AC, Nelson RJ. Dim light at night impairs recovery from global cerebral ischemia. Exp Neurol. 2019;317:100–109. doi:10.1016/j.expneurol.2019.02.008
82. Cajochen C, Frey S, Anders D, et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol. 2011;110(5):1432–1438. doi:10.1152/japplphysiol.00165.2011
83. Gooley JJ, Chamberlain K, Smith KA, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96(3):E463–72. doi:10.1210/jc.2010-2098
84. Chellappa SL, Steiner R, Oelhafen P, et al. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013;22(5):573–580. doi:10.1111/jsr.12050
85. Cho JR, Joo EY, Koo DL, Hong SB. Let there be no light: the effect of bedside light on sleep quality and background electroencephalographic rhythms. Sleep Med. 2013;14(12):1422–1425. doi:10.1016/j.sleep.2013.09.007
86. Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112(4):1232–1237. doi:10.1073/pnas.1418490112
87. Cho CH, Lee HJ, Yoon HK, et al. Exposure to dim artificial light at night increases REM sleep and awakenings in humans. Chronobiol Int. 2016;33(1):117–123. doi:10.3109/07420528.2015.1108980
88. Hysing M, Pallesen S, Stormark KM, Jakobsen R, Lundervold AJ, Sivertsen B. Sleep and use of electronic devices in adolescence: results from a large population-based study. BMJ Open. 2015;5(1):e006748. doi:10.1136/bmjopen-2014-006748
89. Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–1066. doi:10.1016/S1470-2045(07)70373-X
90. Erren TC, Morfeld P, Gross JV, Wild U, Lewis P. IARC 2019: ”Night shift work” is probably carcinogenic: what about disturbed chronobiology in all walks of life? J Occup Med Toxicol. 2019;14:29. doi:10.1186/s12995-019-0249-6
91. Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25(4):187–192. doi:10.1016/j.it.2004.02.001
92. Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10(5):643–653. doi:10.1096/fasebj.10.5.8621064
93. Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Adverse health effects of nighttime lighting: comments on American Medical Association policy statement. Am J Prev Med. 2013;45(3):343–346. doi:10.1016/j.amepre.2013.04.011
94. Erren TC, Lewis P. Towards standard assessments of sleep as an exposure: an initiative for an important research area. Sleep Med. 2021;88:187–188. doi:10.1016/j.sleep.2021.10.001
95. Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased Sensitivity of the Circadian System to Light in Early/Mid-Puberty. J Clin Endocrinol Metab. 2015;100(11):4067–4073. doi:10.1210/jc.2015-2775
96. Lee SI, Matsumori K, Nishimura K, et al. Melatonin suppression and sleepiness in children exposed to blue-enriched white LED lighting at night. Physiol Rep. 2018;6(24):e13942. doi:10.14814/phy2.13942
97. Nagare R, Plitnick B, Figueiro MG. Effect of exposure duration and light spectra on nighttime melatonin suppression in adolescents and adults. Light Res Technol. 2019;51(4):530–543. doi:10.1177/1477153518763003
98. Higuchi S, Nagafuchi Y, Lee SI, Harada T. Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab. 2014;99(9):3298–3303. doi:10.1210/jc.2014-1629
99. Akacem LD, Wright KP
100. Weale RA. Human lenticular fluorescence and transmissivity, and their effects on vision. Exp Eye Res. 1985;41(4):457–473. doi:10.1016/s0014-4835(85)80004-x
101. Yang Y, Thompson K, Burns SA. Pupil location under mesopic, photopic, and pharmacologically dilated conditions. Invest Ophthalmol Vis Sci. 2002;43(7):2508–2512.
102. Charman WN. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthalmic Physiol Opt. 2003;23(2):181–187. doi:10.1046/j.1475-1313.2003.00105.x
103. Gringras P, Middleton B, Skene DJ, Revell VL. Bigger, Brighter, Bluer-Better? Current Light-Emitting Devices - Adverse Sleep Properties and Preventative Strategies. Front Public Health. 2015;3:233. doi:10.3389/fpubh.2015.00233
104. Exelmans L, Van den Bulck J. Technology and Sleep: how Electronic Media Exposure Has Impacted Core Concepts of Sleep Medicine. Behav Sleep Med. 2015;13(6):439–441. doi:10.1080/15402002.2015.1083025
105. Carter B, Rees P, Hale L, Bhattacharjee D, Paradkar MS. Association Between Portable Screen-Based Media Device Access or Use and Sleep Outcomes: a Systematic Review and Meta-analysis. JAMA Pediatr. 2016;170(12):1202–1208. doi:10.1001/jamapediatrics.2016.2341
106. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. doi:10.1016/j.sleh.2014.12.010
107. Chang AM, Scheer FA, Czeisler CA, Aeschbach D. Direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans depend on prior light history. Sleep. 2013;36(8):1239–1246. doi:10.5665/sleep.2894
108. Rahman SA, St Hilaire MA, Lockley SW. The effects of spectral tuning of evening ambient light on melatonin suppression, alertness and sleep. Physiol Behav. 2017;177:221–229. doi:10.1016/j.physbeh.2017.05.002
109. Chellappa SL, Ly JQ, Meyer C, et al. Photic memory for executive brain responses. Proc Natl Acad Sci U S A. 2014;111(16):6087–6091. doi:10.1073/pnas.1320005111
110. Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33(4):198–203. doi:10.1034/j.1600-079x.2002.01885.x
111. Kozaki T, Kubokawa A, Taketomi R, Hatae K. Effects of day-time exposure to different light intensities on light-induced melatonin suppression at night. J Physiol Anthropol. 2015;34:27. doi:10.1186/s40101-015-0067-1
112. Zeitzer JM, Friedman L, Yesavage JA. Effectiveness of evening phototherapy for insomnia is reduced by bright daytime light exposure. Sleep Med. 2011;12(8):805–807. doi:10.1016/j.sleep.2011.02.005
113. Carrier J, Dumont M. Sleep propensity and sleep architecture after bright light exposure at three different times of day. J Sleep Res. 1995;4(4):202–211. doi:10.1111/j.1365-2869.1995.tb00171.x
114. Ancoli-Israel S, Gehrman P, Martin JL, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1(1):22–36. doi:10.1207/S15402010BSM0101_4
115. Viola AU, James LM, Schlangen LJ, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34(4):297–306. doi:10.5271/sjweh.1268
116. Figueiro MG, Rea MS. Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students. Neuro Endocrinol Lett. 2010;31(1):92–96.
117. Kozaki T, Miura N, Takahashi M, Yasukouchi A. Effect of reduced illumination on insomnia in office workers. J Occup Health. 2012;54(4):331–335. doi:10.1539/joh.12-0049-fs
118. Najjar RP, Wolf L, Taillard J, et al. Chronic artificial blue-enriched white light is an effective countermeasure to delayed circadian phase and neurobehavioral decrements. PLoS One. 2014;9(7):e102827. doi:10.1371/journal.pone.0102827
119. Figueiro MG, Steverson B, Heerwagen J, et al. The impact of daytime light exposures on sleep and mood in office workers. Sleep Health. 2017;3(3):204–215. doi:10.1016/j.sleh.2017.03.005
120. Gimenez MC, Geerdinck LM, Versteylen M, et al. Patient room lighting influences on sleep, appraisal and mood in hospitalized people. J Sleep Res. 2017;26(2):236–246. doi:10.1111/jsr.12470
121. Wams EJ, Woelders T, Marring I, et al. Linking Light Exposure and Subsequent Sleep: a Field Polysomnography Study in Humans. Sleep. 2017;40(12). doi:10.1093/sleep/zsx165
122. Cajochen C, Reichert C, Maire M, et al. Evidence That Homeostatic Sleep Regulation Depends on Ambient Lighting Conditions during Wakefulness. Clocks Sleep. 2019;1(4):517–531. doi:10.3390/clockssleep1040040
123. Cajochen C, Freyburger M, Basishvili T, et al. Effect of daylight LED on visual comfort, melatonin, mood, waking performance and sleep. Lighting Res Technol. 2019;51:1044–1062. doi:10.1177/1477153519828419
124. Te Kulve M, Schlangen LJM, van Marken Lichtenbelt WD. Early evening light mitigates sleep compromising physiological and alerting responses to subsequent late evening light. Sci Rep. 2019;9(1):16064. doi:10.1038/s41598-019-52352-w
125. Juda M, Liu-Ambrose T, Feldman F, Suvagau C, Mistlberger RE. Light in the Senior Home: effects of Dynamic and Individual Light Exposure on Sleep, Cognition, and Well-Being. Clocks Sleep. 2020;2(4):557–576. doi:10.3390/clockssleep2040040
126. Stefani O, Freyburger M, Veitz S, et al. Changing color and intensity of LED lighting across the day impacts on circadian melatonin rhythms and sleep in healthy men. J Pineal Res. 2021;70(3):e12714. doi:10.1111/jpi.12714
127. Kawasaki A, Wisniewski S, Healey B, et al. Impact of long-term daylight deprivation on retinal light sensitivity, circadian rhythms and sleep during the Antarctic winter. Sci Rep. 2018;8(1):16185. doi:10.1038/s41598-018-33450-7
128. Owen J, Arendt J. Melatonin suppression in human subjects by bright and dim light in Antarctica: time and season-dependent effects. Neurosci Lett. 1992;137(2):181–184. doi:10.1016/0304-3940(92)90399-r
129. Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms. 2010;8(1):2. doi:10.1186/1740-3391-8-2
130. Schlangen LJM, Price LLA. The Lighting Environment, Its Metrology, and Non-visual Responses. Front Neurol. 2021;12:624861. doi:10.3389/fneur.2021.624861
131. Spitschan M, Mead J, Roos C, et al. luox: novel validated open-access and open-source web platform for calculating and sharing physiologically relevant quantities for light and lighting. Wellcome Open Res. 2021;6:69. doi:10.12688/wellcomeopenres.16595.2
132. Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37(1):1–9. doi:10.1016/j.tins.2013.10.004
133. Vetter C, Pattison PM, Houser K, et al. A Review of Human Physiological Responses to Light: implications for the Development of Integrative Lighting Solutions. LEUKOS;2021. 1–28. doi:10.1080/15502724.2021.1872383
134. Pattison PM, Tsao JY, Brainard GC, Bugbee B. LEDs for photons, physiology and food. Nature. 2018;563(7732):493–500. doi:10.1038/s41586-018-0706-x
135. Sachs G. The low carbon economy: technology in the driver’s seat. Goldman Sachs Equity Res. 2016.
136. Sachs G. The low carbon economy. Goldman Sachs Equity Res. 2015.
137. Bauer M, Glenn T, Monteith S, et al. The potential influence of LED lighting on mental illness. World J Biol Psychiatry. 2018;19(1):59–73. doi:10.1080/15622975.2017.1417639
138. Houser KW, Esposito T. Human-Centric Lighting: foundational Considerations and a Five-Step Design Process. Front Neurol. 2021;12:630553. doi:10.3389/fneur.2021.630553
139. Spitschan M, Garbazza C, Kohl S, Cajochen C. Sleep and circadian phenotype in people without cone-mediated vision: a case series of five CNGB3 and two CNGA3 patients. Brain Commun. 2021;3(3):fcab159. doi:10.1093/braincomms/fcab159
140. Munch M, Wirz-Justice A, Brown SA, et al. The Role of Daylight for Humans: gaps in Current Knowledge. Clocks Sleep. 2020;2(1):61–85. doi:10.3390/clockssleep2010008
141. Kuller R, Lindsten C. Health and behavior of children in classrooms with and without windows. J Environ Psychol. 1992;12:305–317. doi:10.1016/S0272-4944(05)80079-9
142. Grant LK, Kent BA, Mayer MD, Stickgold R, Lockley SW, Rahman SA. Daytime Exposure to Short Wavelength-Enriched Light Improves Cognitive Performance in Sleep-Restricted College-Aged Adults. Front Neurol. 2021;12:624217. doi:10.3389/fneur.2021.624217
143. Boubekri M, Lee J, MacNaughton P, et al. The Impact of Optimized Daylight and Views on the Sleep Duration and Cognitive Performance of Office Workers. Int J Environ Res Public Health. 2020;17(9). doi:10.3390/ijerph17093219
144. Boubekri M, Cheung IN, Reid KJ, Wang CH, Zee PC. Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. J Clin Sleep Med. 2014;10(6):603–611. doi:10.5664/jcsm.3780
145. Burns AC, Saxena R, Vetter C, Phillips AJK, Lane JM, Cain SW. Time spent in outdoor light is associated with mood, sleep, and circadian rhythm-related outcomes: a cross-sectional and longitudinal study in over 400,000 UK Biobank participants. J Affect Disord. 2021;295:347–352. doi:10.1016/j.jad.2021.08.056
146. Roecklein KA, Rohan KJ, Duncan WC, et al. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114(1–3):279–285. doi:10.1016/j.jad.2008.08.005
147. Roecklein KA, Wong PM, Franzen PL, et al. Melanopsin gene variations interact with season to predict sleep onset and chronotype. Chronobiol Int. 2012;29(8):1036–1047. doi:10.3109/07420528.2012.706766
148. Wirz-Justice A, Skene DJ, Munch M. The relevance of daylight for humans. Biochem Pharmacol. 2021;191:114304. doi:10.1016/j.bcp.2020.114304
149. Jukic AMZ, Hoofnagle AN, Lutsey PL. Measurement of Vitamin D for Epidemiologic and Clinical Research: shining Light on a Complex Decision. Am J Epidemiol. 2018;187(4):879–890. doi:10.1093/aje/kwx297
150. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi:10.1016/j.annepidem.2007.12.001
151. Naeem Z. Vitamin d deficiency- an ignored epidemic. Int J Health Sci. 2010;4(1):V–VI.
152. Lowdon J. Rickets: concerns over the worldwide increase. J Fam Health Care. 2011;21(2):25–29.
153. Holden BA, Fricke TR, Wilson DA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi:10.1016/j.ophtha.2016.01.006
154. Spillmann L. Stopping the rise of myopia in Asia. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):943–959. doi:10.1007/s00417-019-04555-0
155. Lam CS, Lam CH, Cheng SC, Chan LY. Prevalence of myopia among Hong Kong Chinese schoolchildren: changes over two decades. Ophthalmic Physiol Opt. 2012;32(1):17–24. doi:10.1111/j.1475-1313.2011.00886.x
156. Hobday R. Myopia and daylight in schools: a neglected aspect of public health? Perspect Public Health. 2016;136(1):50–55. doi:10.1177/1757913915576679
157. Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115(8):1279–1285. doi:10.1016/j.ophtha.2007.12.019
158. Lingham G, Mackey DA, Lucas R, Yazar S. How does spending time outdoors protect against myopia? A review. Br J Ophthalmol. 2020;104(5):593–599. doi:10.1136/bjophthalmol-2019-314675
159. Lagrèze WA, Schaeffel F. Preventing Myopia. Dtsch Arztebl Int. 2017;114:575–580. doi:10.3238/arztebl.2017.0575
160. Bronin SC. Solar Rights. Boston Univ Law Rev. 2009;89:1217–1265.
161. Reitze GL. A solar rights zoning guarantee: seeking new law in old concepts. Washington Univ Law Quarterly. 1976;1976(3):375–402.
162. Aronson BE. Review essay: environmental law in Japan. Harvard Envion Law Rev. 1983;7:135.
163. Šprah N, Košir M. Daylight provision requirements according to EN 17037 as a restriction for sustainable urban planning of residential developments. Sustainability. 2020;12:315. doi:10.3390/su12010315
164. Boubekri M. A overview of the current state of daylight legislation. J Human Environ Sys. 2004;7:57–63. doi:10.1618/jhes.7.57
165. Hobday RA. Sunlight therapy and solar architecture. Med Hist. 1997;41(4):455–472. doi:10.1017/s0025727300063043
166. Boubekri M, Shishegar N, Khama TR. Sustainability with Health in Mind: a Case for Daylighting. Int J Constructed Environ. 2016;8:1–13. doi:10.18848/2154-8587/CGP/v08i02/1-13
167. Lee J, Boubekri M, Liang F. Impact of building design parameters on daylighting metrics using an analysis, prediction, and optimization approach based on statistical learning technique. Sustainability. 2019;11:1474. doi:10.3390/su11051474
168. Blume C, Garbazza C, Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie. 2019;23(3):147–156. doi:10.1007/s11818-019-00215-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.