Back to Journals » Clinical Interventions in Aging » Volume 18
Current and Emerging Therapies for Atopic Dermatitis in the Elderly
Authors Teng Y , Zhong H, Yang X, Tao X, Fan Y
Received 1 July 2023
Accepted for publication 25 September 2023
Published 2 October 2023 Volume 2023:18 Pages 1641—1652
DOI https://doi.org/10.2147/CIA.S426044
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nandu Goswami
Yan Teng,1,* Huiting Zhong,2,* Xianhong Yang,1 Xiaohua Tao,1,* Yibin Fan1,*
1Center for Plastic & Reconstructive Surgery, Department of Dermatology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital of Hangzhou Medical College, Hangzhou, 310014, People’s Republic of China; 2Department of Dermatology, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yibin Fan; Xiaohua Tao, Center for plastic & Reconstructive Surgery, Department of Dermatology, Zhejiang provincial People’s Hospital, Affiliated People’s Hospital of Hangzhou Medical College, Tel +86-18806538451 ; +86-13505811700, Email [email protected]; [email protected]
Abstract: Atopic dermatitis (AD) in the elderly has recently emerged as a distinct subgroup of AD, garnering widespread concern due to its increasing global incidence rate. Epidermal barrier dysfunction, inflammatory response, and chronic pruritus interact with each other, contributing to the pathogenesis and pathophysiology of AD in the elderly. Although fundamental medications are essential for managing AD in the elderly, older adults often struggle with regular usage of moisturizing emollients, topical medications, and avoidance of environmental triggers, leading to recurrent or even exacerbated disease progression. Therefore, a systematic medication approach is necessary to control pruritus and skin lesions. Traditional systemic treatments may not adequately meet the treatment needs of moderate and severe AD in the elderly and may even pose certain safety risks. Biologics and Janus kinase (JAK) inhibitors, exhibiting excellent clinical efficacy, have made significant breakthroughs in AD treatment. Existing evidence suggests that dupilumab, a human monoclonal IgG4 antibody, has been confirmed as an effective and safe first-line systematic treatment for moderate to severe AD in the elderly, with no notable differences between adults and the elderly. However, the limited inclusion of elderly patients in related clinical studies hinders the generalizability of these findings. As older patients face a higher risk of adverse events with JAK inhibitors, JAK inhibitors are recommended when no other suitable treatment options are available. Obtaining population-specific data is crucial for making evidence-based treatment choices when managing AD in older adults with JAK inhibitors.
Keywords: atopic dermatitis in the elderly, treatments, biologics, JAK inhibitors
Introduction
Atopic dermatitis (AD) is a chronic, relapsing pruritic cutaneous disease characterized by type-2 inflammation and skin barrier dysfunction resulting from environmental stimuli and genetic factors.1 With societal development and an aging population, AD in the elderly has emerged as a distinct clinical subtype of AD, capturing widespread attention. Unlike the other three types of AD (infantile, childhood, and adult), older adults often experience concurrent pruritic skin diseases with similar clinical manifestations, such as seborrheic dermatitis, geriatric scabies, prurigo, bullous pemphigoid, along with underlying systemic conditions including hypertension, cardiovascular and cerebrovascular diseases, and diabetes. As a result, the diagnosis and treatment of AD in the elderly are more complex than the other types. The treatment of AD in the elderly follows a stepwise approach, which includes basic and systemic treatment, with an emphasis on safety. Compliance with basic treatment is generally poor among elderly patients with AD, and the significance of systemic treatments should be highlighted. Traditional systemic therapies, including systemic corticosteroids and immunosuppressants, have reasonable efficacy but are not well-tolerated in the elderly population. Biologics, particularly dupilumab, have been confirmed as effective and well-tolerated systemic treatments for moderate and severe AD in the elderly. Janus kinase 1 (JAK1) inhibitors, with their advantages of rapid onset and convenient administration, may offer a better treatment option for elderly patients with AD. However, their impact on safety limits their use in the elderly population. This article reviews the epidemiology, clinical characteristics, diagnosis, and current and emerging therapies for AD in the elderly, providing a new perspective on the diagnosis and treatment of AD in this age group.
The Prevalence of AD in the Elderly is Increasing
AD is classified into four stages based on age: infancy (birth–2 years), childhood (>2–12 years), adolescence and adulthood (>12–60 years), and older adult (>60 years).2 The global prevalence of AD has been rising over the past three decades. In recent years, with the population aging, the prevalence of AD among individuals aged >60 years has been notably high, displaying distinct clinical characteristics compared to AD in children and adults.3 Currently, there is limited epidemiological data on AD in the elderly, both domestically and internationally. In industrialized countries, the prevalence of AD in the elderly ranges from 1%–3%.4 In Poland, the incidence rate of AD among the elderly (aged ≥60 years) is 1.86%.5 In Japan, the incidence rate of AD (age ≥60 years) is approximately 2.6%.6 Germany reports an incidence rate of AD (age ≥50 years) at 4.3%.7 An analysis of data from The Health Improvement Network, a database of electronic health records from UK general practitioners, examined the prevalence of AD in different age groups (totaling 9,154,936 cases). The analysis demonstrated a gradual increase in the incidence rate of AD among the elderly over time, possibly attributed to increased senile xerosis and trans-epidermal water loss.8 Furthermore, while the prevalence of AD in adults is higher among women than among men, the opposite has been observed in the elderly.9 This difference may be related to the influence of sex hormones in the elderly.8,10
The Pathogenesis of AD in the Elderly Involves the Interaction of Epidermal Barrier Dysfunction, Inflammatory Response, and Chronic Pruritus
AD is a complex and multifactorial skin disorder mainly mediated by type II helper T cells (Th2) to environmental antigens and allergens. In older patients, the aging skin is commonly related to declined physical barrier function, resulting in exacerbation of AD. Aging skin becomes thinner, more translucent, and undergoes elastosis, making it more susceptible to physical damage. Furthermore, older individuals have a diminished ability to restore the skin barrier after irritation.11 The immune system undergoes dysregulation with aging and is characterized by chronic inflammation, often referred to as “inflamm-ageing”.12,13 Inflamm-ageing is believed to result from remodeling of both the innate and adaptive immune systems, resulting to the production of chronic inflammatory cytokines.14 Pruritus, or itching, is a common and distressing symptom experienced by a significant number of elderly patients. In the United States and other regions, approximately a quarter of patients over 65 years old visiting outpatient clinics report pruritus. In the elderly, pruritus is frequently secondary to xerosis (dry skin), often associated with other dermatologic conditions, like AD Figure 1.11
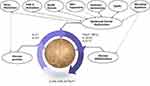 |
Figure 1 The pathogenesis of AD in the elderly that involves epidermal barrier dysfunction, chronic inflammation, and chronic pruritus. |
In general, there are two types of AD that have been identified, including IgE anaphylaxis (exogenous) and non-IgE anaphylaxis (intrinsic), where the former refers to elevated serum total IgE (>200 or 400 IU/L, according to facility criteria) and allergen-specific IgE levels are both elevated; The other group refers to normal serum total IgE levels and elevated allergen-specific IgE levels.15–17 Similar to other age groups, elderly patients with AD experience these two types when the diagnosis of AD is made based on standardized diagnostic criteria.17,18 Additionally, similar to infantile AD, elderly AD patients may also experience an unspecified allergic type with normal level of serum total IgE and elevated level of allergen-specific IgEs. This indeterminate allergic type represents an intermediate, state between the intrinsic and exogenous types. Unlike infantile AD, these three clinical phenotypes in AD patients often exhibit a discontinuous disease state, possibly indicating different underlying mechanisms. In elderly AD patients with IgE allergies, total IgE levels range from 2500–10,000 IU/mL. The most common environmental allergen in elderly patients with AD is dust mites, followed by pollen. However, the prevalence of allergies to food, fungi, and animal dander is low.1 Guttman et al19 analyzed the molecular markers in the skin lesions and peripheral blood of patients with AD across different age groups. They found that levels of Th2-related cytokines such as interleukin (IL)-5, IL-13, and chemokine ligands CCL13, CCL18, and CCL26 decrease with age in the skin lesions of patients with AD. Moreover, levels of Th22 and IL-22 also decrease with age, whereas the expression of Th1/Th17-related cytokines increases. Similar changes in cytokine levels were observed in serum, with downregulation of Th2 cytokines and upregulation of Th1 cytokines in elderly patients with AD. Li et al analyzed the clinical features and molecular profiles of elderly patients with AD in China. They found that serum IgE levels and eosinophil counts in elderly patients with AD were significantly lower than those in other age groups. The serum levels of IL-4, TARC, IL-17A, IL-6, IL-22, IL-33, and thymic stromal lymphopoietin (TSLP) in elderly AD patients than were significantly higher than those in healthy control group, indicating a mixed Th2/Th17/Th22 inflammation.20 Furthermore, Guttman et al21 discovered that cardiovascular risk factors (including growth differentiation factor, myeloperoxidase, and serum soluble ST2) and risk factors associated with arterial-pulse cone-like hardening (such as CCL4, CCL7, and sorting protein 1) were higher in elderly patients with AD than those in young patients with AD. This suggests an increased risk of cardiovascular events in older patients with AD, indicating the potential benefits of cardiovascular disease screening and prevention in this population.
AD in the Elderly Often Presents with a Distinct Clinical Characteristic Known as the “Reverse Sign”
While the skin appearance of elderly AD is generally similar to that of adult AD, there is a difference in the localization of lichenoid eczema. In children and adults with AD, lichen-like lesions commonly appear in the flexural areas of the elbows and knees. However, in elderly AD, these areas are typically unaffected, and the lichen-like lesions appear on the extended sides of the elbows and knees. This reverse sign may be attributed to decreased sweat secretion in the elderly.2,16,22–24 In addition, elderly AD can manifest with other specific skin manifestations, including intractable facial erythema, lateral eyebrow shedding (Hertoghe’s sign), Dennie-Morgan infraorbital folds, and neck eczema with reticular, rippled, or pigmented skin.22,25
Diagnosis of AD in the Elderly is Difficult
The diagnosis of AD in the elderly can be challenging for several reasons. Firstly, elderly individuals often have other pruritic skin diseases such as contact dermatitis, nummular dermatitis, chronic pruritus, scabies, adverse drug reactions, and skin malignancies. Moreover, the presence of concomitant systemic diseases such as hypertension, cardiovascular and cerebrovascular diseases, gastrointestinal diseases, and diabetes can cause pruritus due to medical conditions or adverse drug-related effects, further complicating the diagnosis of AD in the elderly. The complex immune mechanisms involved in AD and the lack of clear clinical, histopathological, and laboratory diagnostic markers also contribute to the difficulty in diagnosis. Currently, there are no specific diagnostic criteria for AD in the elderly. The Hanifin and Rajka (H&R) diagnostic criteria, which are widely used for AD diagnosis, can also be applied to the diagnosis of AD in the elderly.26 Tanei et al1,4 indicated additional diagnostic points for elderly AD, including lichen eczema on the extremities with the fossa cubitalis and popliteal fossa being unaffected, personal and family history of atopic diseases, increased levels of serum total IgE and allergen-specific IgE, a disease course of >6 months, and exclusion of other pruritic skin diseases.4 Yue et al27 conducted a study to validate diagnostic criteria for elderly Chinese populations and proposed the Chinese criteria of atopic dermatitis for children as a diagnostic tool for elderly Chinese patients, particularly for cases of mild and moderate AD, considering the limitations in sensitivity of the H&R and UK Working Party criteria in this population.
Basic Treatments for AD in the Elderly
To date, there are no guidelines published on the treatment of AD in the elderly. However, proper treatment and management of senile AD can help alleviate symptoms and improve patients’ quality of life. The management and treatment of AD in the elderly typically involve basic and systemic approaches. Basic treatments focus on identifying and avoiding exacerbating factors, maintaining the skin barrier through the use of moisturizers and emollients, implementing anti-inflammatory measures with topical corticosteroids, topical calcineurin inhibitors, PDE4 inhibitors, and providing oral antihistamines to relieve pruritus.28–32
Use of Topical Moisturizers and Emollients to Maintain the Skin Barrier
AD occurs due to potential skin barrier dysfunction, which is further aggravated in the elderly due to skin inflamm-ageing. This is characterized by an imbalance of skin flora colonization, reduced sweating, and a relative lack of ceramide in the epidermal stratum corneum.33,34 Therefore, proper bathing practices and regular application of moisturizers are particularly crucial for the AD in the elderly and form the foundation of any treatment plan. It is advisable for elderly individuals with poor compliance to moisturizers to avoid hot water and reduce the frequency of baths to minimize damage to the skin barrier. Fragrance-free and allergen-free non-soapy cleansers with a neutral to low pH can be used. Taking a warm bath (32°C–37 °C) for 5–10 min once a day or every other day is recommended. Elderly patients with AD should choose suitable moisturizing emollients and apply them multiple times a day immediately after bathing to minimize water evaporation and help restore and maintain the skin barrier function.35
Anti-Inflammatory Measures Including Topical Corticosteroids, Topical Calcineurin Inhibitors, and Topical PDE4 Inhibitors
Topical corticosteroids are the first-line anti-inflammatory treatment for AD, which can effectively reduce inflammation and preventing disease progression.36 When initiating topical corticosteroid treatment, factors such as potency, formulation, severity, and body area to be treated should be considered. Clinicians should be alert to local skin adverse reactions (such as skin atrophy, telangiectasia, and purpura) related to long-term topical corticosteroids, particularly in the elderly with fragile skin.37 Elderly patients with AD may face challenges when applying various topical medications due to lower self-care ability.38 It may be necessary to prescribe a combined preparation of topical corticosteroids and moisturizers to enhance the treatment compliance in older patients with AD, even though the stability of the drugs in such formulations might be compromised.39
Topical calcineurin inhibitors (tacrolimus and pimecrolimus) act by inhibiting cytokine production in T cells through intracellular calcineurin inhibition, which is different from the mechanism underlying topical corticosteroids. Topical calcineurin inhibitors are suitable for treating AD that involve with the face, neck, forearms, and hands of older patients. The most common adverse event is irritative symptoms (such as transient burning sensation and hot flushes) at the site of application during the first few days of treatment. The AD in the elderly may discontinue use of topical tacrolimus due to these adverse effects, highlighting the importance of prior explanation to improve treatment compliance. Low dose of tacrolimus ointment mixed with an emollient can minimize the irritation, which can be beneficial during the initial stage of treatment. Currently, there is no existing evidence indicating increased risk of cutaneous malignancies, lymphoma, or other malignancies with appropriate application of topical tacrolimus.28,31
Crisaborole is the firstly topical phosphodiesterase 4 (PDE4) inhibitor approved for treating mild to moderate AD. It is considered a second-line option, similar to the topical calcineurin inhibitors, for patients who are unresponsive to topical corticosteroids.40 Crisaborole has demonstrated safety and efficacy in the treatment of mild to moderate AD in patients aged 2 years and older,41,42 with improvement in symptoms such as pruritus, erythema, exudation, excoriation, induration/papulation, and lichenification. The results of two large clinical trials showed a treatment success rate of approximately 32%.43,44 While it is an effective treatment option with a low incidence of adverse effects, it may be expensive for elderly patients with AD. The elderly population often prefers topical corticosteroids due to their rapid efficacy and lower cost.
Systemic Treatments for AD in the Elderly
Due to age-related physical limitations and reduced mobility, elderly patients often struggle with adhering to treatment plans involving moisturizing emollients, avoiding triggers, and applying topical medications such as glucocorticoids and calcineurin inhibitors. This can lead to persistent or worsening symptoms, necessitating systemic medications to control pruritus and skin lesions. Traditional systemic therapies for AD include oral antihistamines/anti-allergic drugs, systemic corticosteroids, oral immunosuppressants, and light therapy (narrow-band ultraviolet UVB), etc. However, these treatments have limitations when it comes to elderly patients with AD. Oral antihistamines/anti-allergic drugs are only applied to alleviate the AD-related pruritus temporarily but with limited efficacy for long-term disease control. Oral corticosteroids require careful monitoring and carry the risk of adverse events including hypertension, diabetes, infections, peptic ulcer disease, cataracts, osteoporosis, adrenal dysfunction, and steroid purpura. Long-term use of oral immunosuppressants like cyclosporine increases the risk of malignancies (such as non-melanoma skin cancer or lymphoma) and organ toxicity, particularly in the cardiovascular system and kidneys. Regular visits to the hospital every week or every other week to receive continuous UVB exposure can be burdensome for elderly patients with AD. Lam et al45 reported a systematic review highlighting the underrepresentation of the older group in randomized clinical trials (RCTs) of systemic immunomodulatory treatments for AD, causing no sufficient evidence to support their safe clinical application in this population.
Oral Antihistamines/Anti-Allergic Drugs
The use of antihistamines in AD remains controversial, but many studies support their role in alleviating AD-related pruritus, especially in patients with comorbid allergies such as urticaria and allergic rhinitis.1,46,47 For elderly patients with AD, taking first-generation antihistamines before bedtime can improve sleep quality and control itching due to their sedative effect on the central nervous system.48 However, it is important to be aware of the potential side effects of first-generation antihistamines in the elderly, including dizziness, drowsiness, cognitive dysfunction, urinary retention, constipation, and postural hypotension.49 Second-generation antihistamines have fewer side effects than first-generation ones, but caution should still be exercised, considering the elderly population’s underlying health conditions, such as cardiac and renal issues. Adjusting the dosage may be necessary due to age-related metabolic changes.50
Systemic Corticosteroids
Systemic corticosteroids can be used for patients with moderate to severe AD at a dose of 5–15 mg/day or 0.1–0.2 mg/(kg·d). Retrospective studies have shown that combining systemic glucocorticoid therapy with basic treatments is relatively effective for elderly AD.4,16 However, systemic use of glucocorticoids in elderly patients can lead to electrolyte imbalances, hypertension, osteoporosis, and other adverse reactions. Additionally, their long-term efficacy is limited, and relapse or rebound may occur after discontinuation. Clinicians should monitor and prevent the occurrence of these adverse events.
Oral Immunosuppressants
Cyclosporine inhibits the production of T cell-dependent cytokine and activates the immune pathway by blocking nuclear factors in T cells. Oral cyclosporine is effective in treating severe AD in elderly patients when administered at optimal and full doses (eg, 3 mg/kg/day).28 However, clinicians need to be cautious about the potential increased risk of malignancies including non-melanoma skin malignancies or lymphoma, as well as organ toxicity, especially in the cardiovascular system and kidneys, related to long-term use of cyclosporine for the AD in the elderly. The course of oral cyclosporine treatment for AD in the elderly should not exceed 12 weeks, although intermittent restarts of >2 weeks can be considered. Elderly individuals may be more concerned about the adverse effects of cyclosporine due to the increased risk of cancer and higher prevalence of renal insufficiency in this age group. Discontinuation of oral cyclosporine often leads to the recurrence of symptoms in elderly patients with AD. Moreover, the cost of cyclosporine is significantly higher than that of oral corticosteroids, which limits its application to treat the AD in the elderly. Methotrexate is primarily used for refractory elderly AD at relatively low doses of 10–15 mg per week. Azathioprine is mostly prescribed for severe or refractory cases in elderly patients with AD at a daily dose of 50–100 mg, with mercaptopurine methyltransferase genotyping recommended prior to medication. Tripterygium vine may be an important option to treat the elderly patients with moderate to severe AD, as it does not have adverse effects on fertility and can be administered at a daily dose of 1.0–1.5 mg/kg. When using immunosuppressants such as cyclosporine, methotrexate, azathioprine, and tripterygium, careful attention should be given to potential adverse reactions, including bone marrow suppression and hepatic and renal insufficiency in elderly patients.
Phototherapy
Ultraviolet light is beneficial for AD, particularly for patients with moderate to severe symptoms.51 Narrow-band UVB phototherapy exerts immunomodulatory effects by suppressing the immune pathways of Th2, Th22, and Th1 cells, as well as their associated cytokines in the affected skin of individuals with AD. As an adjuvant therapy, narrow-spectrum UVB treatment with an irradiation dose ranging from ~0.35–0.70 J/cm2 can be beneficial for elderly patients with AD.52 However, it is important to note that narrow-spectrum UVB therapy may not effectively address acute episodes of AD and excessive exposure can potentially trigger eczema flare-ups. Additionally, narrow-band UVB phototherapy should not be used in combination with oral cyclosporine or topical calcineurin inhibitors, as this will increase the risk of skin malignancies. Adverse events of phototherapy may consist of mild irritation, diffuse pigmentation, or solar lentigo.
Biologics
In recent years, several biologic medications have been developed and approved or are undergoing clinical trials under the supervision of regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) (refer to Table 1 for an overview). These biologics have demonstrated high efficacy in patients with AD. Dupilumab, as a human monoclonal IgG4 antibody, targets the alpha subunit of the IL-4 receptor. By suppressing the IL-4 and IL-13 signaling pathways, it effectively controls the Th2 response, leading to reduced levels of CCL17, a key regulator of Th2 response and an AD biomarker. Dupilumab was approved in 2017 for the treatment of moderate to severe AD in adults, demonstrating significant improvements in pruritus and inflammation without dose-limiting toxicity.53–56 Studies have also investigated the efficacy and safety of dupilumab in elderly patients with severe AD. A multicenter retrospective observational study evaluated 276 patients aged ≥65 years and revealed significant improvements in Eczema Area and Severity Index (EASI) scores, pruritus, sleep quality, and overall quality of life after 16 weeks of treatment with dupilumab. The efficacy of dupilumab in older AD patients was comparable to that in younger patients. Adverse reactions were observed in 22.51% of elderly AD patients treated with dupilumab, with conjunctivitis being the most common adverse event, although it was often pre-existing and did not worsen significantly during treatment. Only 1 patient (0.36%) stopped taking the drug due to adverse reactions.57 Silverberg et al58 pooled the data from four RCTs on dupliumab59–61 and based on age-related stratifications (<60 [N =2261] and ≥60 [N = 183] years), dupilumab efficacy and safety were noted in patients aged ≥60 years with moderate to severe AD. The results revealed that dupilumab improved the signs and symptoms in of AD in patients aged ≥60 years, and this improvement was comparable to that observed in patients aged <60 years. The safety was noted to be consistent with the known dupilumab safety profile. However, the generalizability of these findings is limited by the small number of patients in the ≥60-year-old group. This limitation can be attributed to the pain of injections and regular treatment visits every 2 weeks.62 Future studies should explore the safety of dupilumab in patients aged ≥70 years. In 2021, tralokinumab was approved by EMA for the treatment of moderate to severe AD in adults, becoming the world’s first monoclonal antibody targeting IL-13 approved for the treatment of AD.63–65 Several other biologics are still being examined in clinical trials, exhibiting good efficacy and safety data.
 |
Table 1 Biologics Therapies for AD |
JAK Inhibitors
The JAK-STAT signaling pathway plays an essential role to mediate the effects of various key cytokines that bind to keratinocytes, immune cells, and peripheral sensory neurons to exacerbate the inflammation and itching, such as IL-4, IL-5, IL-13, IL-31, IL-22, and TSLP, which are implicated in the pathogenesis of AD. The therapeutic potential of JAK inhibitors was initially recognized in researches of autoimmune and autoinflammatory diseases characterized by JAK-STAT polymorphisms or heightened JAK-STAT signaling. Recently, JAK inhibitors have emerged as a novel treatment approach for AD due to the significance of JAK-STAT signaling, particularly JAK1, in regulating Th2 cytokines including IL-4, IL-13, and IL-31 (refer to Table 2 for an overview).66–68 Baricitinib, upadacitinib, and abrocitinib have received FDA or EMA approval for the oral treatment of moderate to severe AD.69–74 In three randomized Phase III trials (Measure Up1, Measure Up2, and AD Up) evaluating the efficacy and safety of upadacitinib in moderate-to-severe AD, elderly patients (≥65 years and ≤75 years) treated with upadacitinib 15 mg and 30 mg QD achieved comparable or higher percentages of EASI 75 and vIGA-AD 0/1 (a validated Investigator’s Global Assessment of AD 0/1 score) at week 52 compared to the placebo group. The incidence of adverse events (AEs) adjusted for exposure was higher in patients aged ≥65 years receiving upadacitinib 30 mg than in patients aged ≥65 years receiving upadacitinib 15 mg or patients aged <65 years receiving upadacitinib 15 mg or 30 mg.75,76 Cork et al,77 analyzed data from one phase IIB study and six phase III studies of abrocitinib in the treatment of moderate to severe AD.69,78–82 The study investigated the long-term safety of abrocitinib in a large sample of patients across different age groups, with data collected up to July 24, 2020. The analysis was conducted in two cohorts: a placebo-control cohort comprising adolescent patients aged 12–17 years and a full analysis cohort consisting of patients who received at least one dose of abrocitinib (100 mg or 200mg). The patients were divided into four groups based on age: 12–17 years old, 18–39 years old, 40–64 years old, and ≥65 years old. In the full analysis cohort, approximately 5% of patients were aged ≥65 years. Overall, a higher proportion of patients aged ≥65 years reported serious AEs, severe AEs, and AEs leading to treatment discontinuation compared to other age groups. The analysis also observed an increased incidence of low platelet counts (<75 × 103/mm3), low absolute lymphocyte counts (<0.5 × 103/mm3), and opportunistic shingles infection with age and dose. Consequently, patients aged ≥65 years may require closer monitoring when treated with abrocitinib. Ruxolitinib cream, approved by the FDA in 2022 for the treatment of moderate to severe AD, selectively inhibits JAK1 and JAK2 to deliver the drug directly to the affected skin, resulting in faster onset of action and reduced potential for AEs typically associated with oral administration.83 Delgocitinib ointment, which was approved by Japan in 2020 for the treatment of mild and moderate AD, exhibits broad inhibition of signaling pathways associated with inflammatory cytokines related to the pathogenesis of AD. It also has the potential to restore the skin barrier function by increasing the production of proteins involved in terminal differentiation, like filaggrin.84 Daniel et al85 analyzed 32 Phase II and phase III clinical trials involving five JAK inhibitors (abrocitinib, baricitinib, upadacitinib, ruxolitinib, and delgocitinib) in adult patients.83,84,86,87 Among these trials, the proportion of participants aged over 65 years ranged from 2.1%–8.0%. In the majority of trials (7 of 9), older adults constituted 5% of the study population. Several trials established upper age limits (65, 70, or 75 years), and most trials (87.5%) included statements indicating that certain unspecified laboratory abnormalities or medical conditions could lead to exclusion at the discretion of the researchers. These criteria may inadvertently exclude many older adults. While majority of trials did not explicitly exclude older patients based on age, the limited representation of individuals aged >65 suggests that indefinite exclusion criteria or challenges in recruiting and retaining older patients might contribute to the underrepresentation. Since the prevalence of exclusionary diseases tends to increase with age, a number of older adults may be excluded from trials even when there is no specified upper age limit. While it is important to avoid unnecessary risks for patients in clinical trials, the use of common comorbidities and low age thresholds can be overly cautious. Furthermore, JAK inhibitors have been associated with black box warnings for serious infections, malignancies, thrombosis, major cardiovascular events, and death.88 The elderly population is particularly susceptible to these disorders. As a result, the EMA recommends that patients aged 65 and older, who have an increased risk of major cardiovascular problems and cancer, and who smoke, should only be considered for JAK inhibitors if no suitable alternative treatment options exist. To establish the safety and efficacy of JAK inhibitors, aggressive efforts are needed to recruit and retain older adults in clinical studies. Without such studies, oral JAK inhibitors may not become the first-line therapies for AD in the elderly.
 |
Table 2 JAK Inhibitors Therapies for AD |
Conclusion and Future Perspectives
The incidence of AD is gradually increasing among the elderly population. Although type 2 inflammation still plays a crucial role involved into the pathogenesis of AD in the elderly, it exhibits unique features known as inflamm-ageing. Elderly patients with AD often have other concurrent pruritic skin conditions, and there are no specific clinical, histopathological, or laboratory diagnostic markers. Thus, clinicians need to exercise caution to ensure an accurate diagnosis and exclude various differential diagnoses. The management and treatment of AD in the elderly primarily involve basic and systemic therapies. Elderly individuals often exhibit reduced adherence to basic treatments, especially topical drugs, which can result in delayed relief or even worsening of the disease. Additionally, the elderly population has a higher incidence of cardiovascular diseases, malignant tumors, and other systemic conditions. Therefore, it is crucial to select an effective, safe, and well-tolerated therapeutic approach for AD in the elderly. Early initiation of systemic therapy is critical, even in cases of mild to moderate AD in the elderly, to alleviate symptoms and improve their quality of life. Traditional systemic therapies have limitations due to adverse events or the need for continuous monitoring of laboratory indicators. Novel systemic therapies, particularly biologics and JAK inhibitors, play significant roles in the treatment of AD in the elderly. Dupilumab, the first biologic approved by the FDA for AD, has demonstrated efficacy and safety across all age groups. Despite limited evidence, the efficacy and safety of dupilumab in treating AD in the elderly are comparable to those in younger patients. However, JAK inhibitors cannot be considered first-line therapy for AD in the elderly due to the risk of certain adverse events such as infection and cardiovascular issues, despite their good and rapid efficacy. Importantly, there is limited clinical data available on the application of JAK inhibitors in elderly patients with AD, similar to biologics. Overall, despite the increasing evidence of rising AD incidence among older adults, the development of effective and safe treatment guidelines for AD in the elderly is still underestimated. More population-specific data is necessary to establish evidence-based treatments tailored to this age group.
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Funding
The research was supported by Natural Science Foundation of China (82073453) and the Outstanding Young People Fund in Zhejiang Provincial People’s Hospital (ZRY2020C008).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tanei R. Atopic dermatitis in older adults: a review of treatment options. Drugs Aging. 2020;37(3):149–160. doi:10.1007/s40266-020-00750-5
2. Tanei R, Katsuoka K. Clinical analyses of atopic dermatitis in the aged. J Dermatol. 2008;35(9):562–569. doi:10.1111/j.1346-8138.2008.00524.x
3. Bieber T, D’Erme AM, Akdis CA, et al. Clinical phenotypes and endophenotypes of atopic dermatitis: where are we, and where should we go? J Allergy Clin Immunol. 2017;139(4s):S58–S64. doi:10.1016/j.jaci.2017.01.008
4. Tanei R, Hasegawa Y. Atopic dermatitis in older adults: a viewpoint from geriatric dermatology. Geriatr Gerontol Int. 2016;16(Suppl 1):75–86. doi:10.1111/ggi.12771
5. Bozek A, Fisher A, Filipowska B, et al. Clinical features and immunological markers of atopic dermatitis in elderly patients. Int Arch Allergy Immunol. 2012;157(4):372–378. doi:10.1159/000329150
6. Muto T, Hsieh SD, Sakurai Y, et al. Prevalence of atopic dermatitis in Japanese adults. Br J Dermatol. 2003;148(1):117–121. doi:10.1046/j.1365-2133.2003.05092.x
7. Wolkewitz M, Rothenbacher D, Löw M, et al. Lifetime prevalence of self-reported atopic diseases in a population-based sample of elderly subjects: results of the ESTHER study. Br J Dermatol. 2007;156(4):693–697. doi:10.1111/j.1365-2133.2006.07659.x
8. Chello C, Carnicelli G, Sernicola A, et al. Atopic dermatitis in the elderly Caucasian population: diagnostic clinical criteria and review of the literature. Int J Dermatol. 2020;59(6):716–721. doi:10.1111/ijd.14891
9. Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27(2):78–88. doi:10.18176/jiaci.0138
10. Pietschmann P, Gollob E, Brosch S, et al. The effect of age and gender on cytokine production by human peripheral blood mononuclear cells and markers of bone metabolism. Exp Gerontol. 2003;38(10):1119–1127. doi:10.1016/S0531-5565(03)00189-X
11. Bocheva GS, Slominski RM, Slominski AT. Immunological aspects of skin aging in atopic dermatitis. Int J Mol Sci. 2021;22(11):5729. doi:10.3390/ijms22115729
12. Cevenini E, Monti D, Franceschi C. Inflamm-ageing. Curr Opin Clin Nutr Metab Care. 2013;16(1):14–20. doi:10.1097/MCO.0b013e32835ada13
13. Minciullo PL, Catalano A, Mandraffino G, et al. Inflammaging and anti-inflammaging: the role of cytokines in extreme longevity. Arch Immunol Ther Exp. 2016;64(2):111–126. doi:10.1007/s00005-015-0377-3
14. Ventura MT, Casciaro M, Gangemi S, et al. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15(1):21. doi:10.1186/s12948-017-0077-0
15. Yatagai T, Shimauchi T, Yamaguchi H, et al. Sensitive skin is highly frequent in extrinsic atopic dermatitis and correlates with disease severity markers but not necessarily with skin barrier impairment. J Dermatol Sci. 2018;89(1):33–39. doi:10.1016/j.jdermsci.2017.10.011
16. Tanei R. Clinical characteristics, treatments, and prognosis of atopic eczema in the elderly. J Clin Med. 2015;4(5):979–997. doi:10.3390/jcm4050979
17. Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58(1):1–7. doi:10.1016/j.jdermsci.2010.02.008
18. Leung DYM, Boguniewicz M, Howell MD, et al. New insights into atopic dermatitis. J Clin Invest. 2004;113(5):651–657. doi:10.1172/JCI21060
19. Dubin C, Del Duca E, Guttman-Yassky E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev Clin Immunol. 2021;17(8):835–852. doi:10.1080/1744666X.2021.1940962
20. Wang S, Zhu R, Gu C, et al. Distinct clinical features and serum cytokine pattern of elderly atopic dermatitis in China. J Eur Acad Dermatol Venereol. 2020;34(10):2346–2352. doi:10.1111/jdv.16346
21. He H, Li R, Choi S, et al. Increased cardiovascular and atherosclerosis markers in blood of older patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2020;124(1):70–78. doi:10.1016/j.anai.2019.10.013
22. Tanei R. Atopic dermatitis in the elderly. Inflamm Allergy Drug Targets. 2009;8(5):398–404. doi:10.2174/1871528110908050398
23. Kawashima T, Kobayashi S, Miyano M, et al. [Senile type atopic dermatitis]. The Japanese journal of dermatology. 1989;99(10):1095–1103.
24. Silverberg JI, Margolis DJ, Boguniewicz M, et al. Distribution of atopic dermatitis lesions in United States adults. J Eur Acad Dermatol Venereol. 2019;33(7):1341–1348. doi:10.1111/jdv.15574
25. Nishioka K. Atopic eczema of adult type in Japan. Australas J Dermatol. 1996;37(Suppl 1):S7–S9. doi:10.1111/j.1440-0960.1996.tb01088.x
26. Sharma L. Diagnostic clinical features of atopic dermatitis. Indian J Dermatol Venereol Leprol. 2001;67(1):25–27.
27. Yue W, Cheng D, Sun Z, et al. Validation of diagnostic criteria for atopic dermatitis and proposal of novel diagnostic criteria for adult and elderly Chinese populations: a multicentre, prospective, clinical setting-based study. Br J Dermatol. 2023;188(3):420–426. doi:10.1093/bjd/ljac097
28. Katoh N, Ohya Y, Ikeda M, et al. Clinical practice guidelines for the management of atopic dermatitis 2018. J Dermatol. 2019;46(12):1053–1101. doi:10.1111/1346-8138.15090
29. Katayama I, Aihara M, Ohya Y, et al. Japanese guidelines for atopic dermatitis 2017. Allergol Int. 2017;66(2):230–247. doi:10.1016/j.alit.2016.12.003
30. Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4s):S49–S57. doi:10.1016/j.jaci.2017.01.009
31. Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682. doi:10.1111/jdv.14891
32. Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878. doi:10.1111/jdv.14888
33. De Martinis M, Sirufo MM, Ginaldi L. Allergy and aging: an old/new emerging health issue. Aging Dis. 2017;8(2):162–175. doi:10.14336/AD.2016.0831
34. Yosipovitch G. Dry skin and impairment of barrier function associated with itch - new insights. Int J Cosmet Sci. 2004;26(1):1–7. doi:10.1111/j.0142-5463.2004.00199.x
35. Irawan Y, Rihatmadja R, Legiawati L, et al. Atopic dermatitis in the elderly. J Gen Proced Dermatol Venereol Indonesia. 2016;1(2):20–24. doi:10.19100/jdvi.v1i2.32
36. Simon D, Bieber T. Systemic therapy for atopic dermatitis. Allergy. 2014;69(1):46–55. doi:10.1111/all.12339
37. Katoh N, Tennstedt D, Abellan van Kan G, et al. Gerontodermatology: the fragility of the epidermis in older adults. J Eur Acad Dermatol Venereol. 2018;32(Suppl 4):1–20. doi:10.1111/jdv.15253
38. Brunner PM, Khattri S, Garcet S, et al. A mild topical steroid leads to progressive anti-inflammatory effects in the skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2016;138(1):169–178. doi:10.1016/j.jaci.2015.12.1323
39. Suzuki T, Uchino T, Miyazaki Y, et al. The effect of storage time on the release profile of dexamethasone dipropionate from admixtures of steroid and heparinoid ointments. Pharmazie. 2014;69(2):104–108.
40. Papier A, Strowd LC. Atopic dermatitis: a review of topical nonsteroid therapy. Drugs Context. 2018;7:212521. doi:10.7573/dic.212521
41. Zane LT, Kircik L, Call R, et al. Crisaborole topical ointment, 2% in patients ages 2 to 17 years with atopic dermatitis: a Phase 1b, Open-Label, Maximal-Use Systemic Exposure Study. Pediatr Dermatol. 2016;33(4):380–387. doi:10.1111/pde.12872
42. McDowell L, Olin B. Crisaborole: a novel nonsteroidal topical treatment for atopic dermatitis. J Pharm Technol. 2019;35(4):172–178. doi:10.1177/8755122519844507
43. Bissonnette R, Pavel AB, Diaz A, et al. Crisaborole and atopic dermatitis skin biomarkers: an intrapatient randomized trial. J Allergy Clin Immunol. 2019;144(5):1274–1289. doi:10.1016/j.jaci.2019.06.047
44. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494–503.e6. doi:10.1016/j.jaad.2016.05.046
45. Lam M, Zhu JW, Maqbool T, et al. Inclusion of older adults in randomized clinical trials for systemic medications for atopic dermatitis: a systematic review. JAMA Dermatol. 2020;156(11):1240–1245. doi:10.1001/jamadermatol.2020.2940
46. Butler JM, Chan SC, Stevens S, et al. Increased leukocyte histamine release with elevated cyclic AMP-phosphodiesterase activity in atopic dermatitis. J Allergy Clin Immunol. 1983;71(5):490–497. doi:10.1016/0091-6749(83)90467-0
47. Ikoma A, Rukwied R, Ständer S, et al. Neuronal sensitization for histamine-induced itch in lesional skin of patients with atopic dermatitis. Arch Dermatol. 2003;139(11):1455–1458. doi:10.1001/archderm.139.11.1455
48. Kawashima M, Tango T, Noguchi T, et al. Addition of fexofenadine to a topical corticosteroid reduces the pruritus associated with atopic dermatitis in a 1-week randomized, multicentre, double-blind, placebo-controlled, parallel-group study. Br J Dermatol. 2003;148(6):1212–1221. doi:10.1046/j.1365-2133.2003.05293.x
49. Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–1241. doi:10.1152/physrev.00043.2007
50. Ventura MT, Scichilone N, Paganelli R, et al. Allergic diseases in the elderly: biological characteristics and main immunological and non-immunological mechanisms. Clin Mol Allergy. 2017;15(1):2. doi:10.1186/s12948-017-0059-2
51. Tanei R, Oda A, Hasegawa Y. Narrow-band ultraviolet B is a useful adjunctive treatment for atopic dermatitis in older adults: case reports. Geriatr Gerontol Int. 2016;16(Suppl 1):75–86.
52. Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128(3):583–593.e4. doi:10.1016/j.jaci.2011.05.042
53. Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139. doi:10.1056/NEJMoa1314768
54. Hamilton JD, Ungar B, Guttman-Yassky E. Drug evaluation review: dupilumab in atopic dermatitis. Immunotherapy. 2015;7(10):1043–1058. doi:10.2217/imt.15.69
55. Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134(6):1293–1300. doi:10.1016/j.jaci.2014.10.013
56. Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):155–172. doi:10.1016/j.jaci.2018.08.022
57. Patruno C, Napolitano M, Argenziano G, et al. Dupilumab therapy of atopic dermatitis of the elderly: a multicentre, real-life study. J Eur Acad Dermatol Venereol. 2021;35(4):958–964. doi:10.1111/jdv.17094
58. Silverberg JI, Lynde CW, Abuabara K, et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023;24(3):469–483. doi:10.1007/s40257-022-00754-4
59. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi:10.1056/NEJMoa1610020
60. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi:10.1016/S0140-6736(17)31191-1
61. de Bruin-Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178(5):1083–1101. doi:10.1111/bjd.16156
62. Eichenfield LF, Bieber T, Beck LA, et al. Infections in dupilumab clinical trials in atopic dermatitis: a comprehensive pooled analysis. Am J Clin Dermatol. 2019;20(3):443–456. doi:10.1007/s40257-019-00445-7
63. Wollenberg A, Blauvelt A, Guttman‐Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449. doi:10.1111/bjd.19574
64. Blair HA. Tralokinumab in Atopic Dermatitis: a Profile of Its Use. Clin Drug Investig. 2022;42(4):365–374. doi:10.1007/s40261-022-01135-9
65. Wollenberg A, Weidinger S, Worm M, Bieber T. Tralokinumab in atopic dermatitis. J Dtsch Dermatol Ges. 2021;19(10):1435–1442.
66. Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148(4):927–940. doi:10.1016/j.jaci.2021.08.009
67. He H, Guttman-Yassky E. JAK inhibitors for atopic dermatitis: an update. Am J Clin Dermatol. 2019;20(2):181–192. doi:10.1007/s40257-018-0413-2
68. Rodrigues MA, Torres T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J Dermatolog Treat. 2020;31(1):33–40. doi:10.1080/09546634.2019.1577549
69. Blauvelt A, Silverberg JI, Lynde CW, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. 2022;86(1):104–112. doi:10.1016/j.jaad.2021.05.075
70. Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157(6):691–699. doi:10.1001/jamadermatol.2021.1273
71. Silverberg JI, de Bruin-Weller M, Bieber T, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: week 52 AD up study results. J Allergy Clin Immunol. 2022;149(3):977–987.e14. doi:10.1016/j.jaci.2021.07.036
72. Crowley EL, Nezamololama N, Papp K, et al. Abrocitinib for the treatment of atopic dermatitis. Expert Rev Clin Immunol. 2020;16(10):955–962. doi:10.1080/1744666X.2021.1828068
73. Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884. doi:10.1016/j.jaci.2019.11.025
74. Melo A, Carrascosa JM, Torres T. Baricitinib for the treatment of atopic dermatitis. J Dermatolog Treat. 2022;33(5):2404–2413. doi:10.1080/09546634.2021.1967268
75. Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi:10.1016/S0140-6736(21)00588-2
76. Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi:10.1016/S0140-6736(21)00589-4
77. Cork MJ, Geng B. Abrocitinib treatment in patients with moderate-to-severe atopic dermatitis: safety of abrocitinib stratified by age. In:
78. Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a Phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–1379. doi:10.1001/jamadermatol.2019.2855
79. Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi:10.1016/S0140-6736(20)30732-7
80. Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi:10.1001/jamadermatol.2020.1406
81. Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N Engl J Med. 2021;384(12):1101–1112. doi:10.1056/NEJMoa2019380
82. Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157(10):1165–1173. doi:10.1001/jamadermatol.2021.2830
83. Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863–872. doi:10.1016/j.jaad.2021.04.085
84. Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment in pediatric patients with atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J Am Acad Dermatol. 2021;85(4):854–862. doi:10.1016/j.jaad.2021.06.014
85. Sreekantaswamy SA, Tully J, Edelman LS, et al. The underrepresentation of older adults in clinical trials of Janus kinase inhibitors in the treatment of atopic dermatitis. J Am Acad Dermatol. 2022;87(5):1174–1176. doi:10.1016/j.jaad.2022.02.051
86. Gong X, Chen X, Kuligowski ME, et al. Pharmacokinetics of ruxolitinib in patients with atopic dermatitis treated with ruxolitinib cream: data from Phase II and III studies. Am J Clin Dermatol. 2021;22(4):555–566. doi:10.1007/s40257-021-00610-x
87. Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82(4):823–831. doi:10.1016/j.jaad.2019.12.015
88. Elmariah SB, Smith JS, Merola JF. JAK in the [Black] Box: a dermatology perspective on systemic JAK inhibitor safety. Am J Clin Dermatol. 2022;23(4):427–431. doi:10.1007/s40257-022-00701-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
