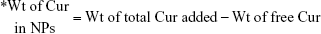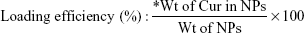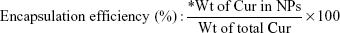Back to Journals » International Journal of Nanomedicine » Volume 13 » T-NANO 2014 Abstracts
Curcuminoid-loaded poly(methyl methacrylate) nanoparticles for cancer therapy
Authors Sahu A, Solanki P, Mitra S
Received 6 October 2016
Accepted for publication 2 January 2017
Published 15 March 2018 Volume 2018:13(T-NANO 2014 Abstracts) Pages 101—105
DOI https://doi.org/10.2147/IJN.S124021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Abhispa Sahu,1 Pratima Solanki,2 Susmita Mitra1
1Amity Institute of Nanotechnology, Amity University, Noida, 2Special Centre for Nanosciences, Jawaharlal Nehru University, New Delhi, India
Abstract: Curcuminoids (Curs), oleoresins from Curcuma longa L., have known anticarcinogenic and anti-inflammatory properties, but high toxicity, poor aqueous solubility and susceptibility to degradation in body fluids are deterrents to their clinical administration. Poly(methyl methacrylate) nanoparticles (PMMA-NPs) are biocompatible and resilient and can entrap hydrophobic drugs. The present investigation is related to solubilizing Curs by incorporating them in these nanoparticles (NPs) and is related to a study comparing the anticarcinogenic effect of drug-loaded NPs with free Cur using lung cancer (A549) cell line. Freshly extracted oleoresins were post loaded in PMMA-NPs prepared using emulsion polymerization. The presence of the three components of oleoresins was confirmed by thin-layer chromatography. The size and morphology of void and loaded NPs were determined by dynamic light scattering, scanning electron microscopy and transmission electron microscopy. The NPs were spherical with diameters of 192.66±5 nm (void) and 199.16±5 nm (loaded). Drug loading and encapsulation efficiency were 6% and 93%, respectively. From the Fourier transform infrared spectroscopy spectra, the characteristic absorption vibration of poly(methyl methacrylate) and the bands at 1,383, 1,233 and 962 cm−1 for Cur moiety were observed. Drug release up to 10 days was estimated in buffer, saline and serum. The highest release of ~55% in ~3 days was noted in buffer that exhibited the highest bioavailability. The in vitro anticancer activity of loaded drug was evaluated up to 72 hours by MTT assay using A549 cell line. Cellular uptake of dye-loaded NPs was visualized within 30 minutes of incubation. The results revealed that the dose- and time-dependent cell death in case of loaded PMMA-NPs was comparable to that of free Cur. According to the study, the drug-loaded PMMA-NPs appear to be highly suitable for effective, localized and safe chemotherapy.
Keywords: polymeric nanoparticle, squamous cell carcinoma, A549 cell line, anticarcinogenic
Introduction
Cancer therapy using nanomaterials has progressed significantly over the years. Recently, nanoparticles (NPs) have made inroads in the therapy of different types of squamous cell carcinoma (SCC), which is an epithelial malignancy, and the majority of the cases comprise non-melanoma skin cancer, head and neck cancer, oral and esophageal cancer and non-small cell lung cancer.1 Surgery, radiation therapy, chemotherapy or combination of these is used to deal with the serious threats of malignancy. Photodynamic therapy is also playing an important role, specifically in superficial SCC, but oral cancer and esophageal cancer need the intervention of localized therapy on exposed lesions. In this context, the use of drug-entrapped NPs can assist in the therapeutic process by controlling the availability of drug at the required site.
Several drugs have been reported for the therapy of carcinoma, of which curcumin, a natural product, has been associated with the regression of pre-malignant lesions of the bladder, soft palate, gastrointestinal tract, cervix, lungs and the skin, with treatment responses in established malignancy.2 The molecular entity curcuminoid (Cur) is obtained from the rhizome of turmeric. Cur is composed of three fractions (curcumin, demethoxycurcumin and bisdemethoxycurcumin); while all the fractions have reported anticancer activity, demethoxycurcumin has reported additional anti-inflammatory effect3 and hence could be used in chemotherapy ensuring dual benefits. However, due to several difficulties in the clinical administration of Cur such as low water solubility and degradation under physiological conditions, its therapeutic efficacy is inadequate. Polymeric nanocarriers with hydrophobic core or shell help in the dissolution of hydrophobic drugs and the preparation of effective safe formulations.4 Of the different types of hydrophobic polymers, the biocompatible polyester poly(methyl methacrylate) (PMMA) has been widely used in drug delivery applications since it has some desirable characteristics such as being resistant to chemical hydrolysis, achiral, high permeability for many drugs and lack of toxicity.5 In our present investigation, poly(methyl methacrylate) nanoparticles (PMMA-NPs) were considered for loading Cur with dual benefits, primarily due to the fact that these NPs are inert and would be resistant to the pH and chemical conditions in the vicinity of the superficial and exposed cancerous lesions of the squamous epithelial cells. To further enhance and prolong the drug effect, the surface of the NPs was modified with the bioadhesive polymer poly(acrylic acid) (PAA) to ensure longer residence time of the NPs at the disease site.
Materials and methods
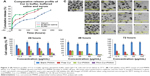
Methyl methacrylate, PAA, sodium dodecyl sulfate (SDS) and 2,2′-azoisobutyronitrile (AIBN) were purchased from Sigma (St Louis, MO, USA). Chemicals for cell culture and MTT assay were purchased from Sigma. All other chemicals of analytical reagent grade were obtained locally. The cell line A549 was purchased from the cell repository of the National Centre for Cell Science, Pune, India. Cur was extracted by hot solvent method from dried and powdered turmeric, and the presence of all three fractions of the Cur was confirmed by thin-layer chromatography (Figure 1A).
NP synthesis by microemulsion polymerization
A total of 26.6 mg of AIBN and 7.766 mg of SDS were mixed in 20 mL of Milli-Q water in a three-necked round bottomed flask under constant agitation and nitrogen feed at 70°C. The monomer was added drop wise, and the above reaction parameters were maintained overnight for the polymerization process.
Drug loading of PMMA-NPs (Cur-PMMA)
The dispersion of NPs was diluted 10-fold, and 2 mL was taken in a dialysis membrane (12 kD) to which Cur was gradually added till free drug was obtained in the membrane bag. The free drug content was estimated from the UV–vis absorption peak at 420 nm and used to determine loading and encapsulation efficiency using the following equations:
|
|
|
Surface modification with PAA of Cur-loaded PMMA-NPs (PAA-Cur-PMMA)
Drug-loaded NPs were surface functionalized by adding 0.01% (w/w) PAA to NP dispersion under mild stirring and were maintained at 25°C for 4 hours.
Characterization techniques used
Dynamic light scattering (DLS) using Zetasizer Nano ZS90 (Malvern Instruments Ltd, Malvern, UK), scanning electron microscopy (SEM; Zeiss, Oberkochen, Germany), transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FTIR; RX1; PerkinElmer Inc., Waltham, MA, USA) were performed for void, loaded and surface-functionalized NPs.
In vitro drug release
The release was checked in falcon tubes where 1.5 mL of Cur-PMMA-NP dispersion was added to 1.5 mL of each of buffer (pH 7.4), buffered saline and bovine fetal serum in triplicate and incubated at 37°C up to 10 days. After periodic intervals, the samples were centrifuged at 3,000 rpm, the supernatant was discarded and the Cur pellet was dissolved in 3 mL ethanol and the absorbance spectra at 420 nm were recorded.
Cellular uptake
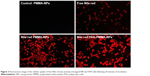
To determine the cellular uptake by A549 cell line, cells were placed on a cover slip in a six-well tissue culture plate and incubated at 37°C until they reached sub-confluent levels. They were then exposed to 50 μg/mL concentrations of PMMA-NPs, only Nile red dye, Nile red-labeled PMMA-NPs and PAA-PMMA-NPs and determined under fluorescence microscopy.
In vitro cell viability assay
To determine the effect of free Cur, PMMA-NPs, Cur-PMMA-NPs and PAA-Cur-PMMA-NPs on cell viability, MTT assay was carried out using lung cancer cell line (A549). For the assay, 2×103 cells/well were plated in a 96-well plate and treated with 12.5, 25, 50 and 100 μg/mL concentrations of free Cur and equivalent doses of Cur-PMMA-NPs and PAA-Cur-PMMA-NPs; the calculated concentration of PMMA-NPs was also added. The assay was performed for 24, 48 and 72 hours duration, and relative growth inhibition compared to untreated control cells was estimated.
Results and discussion
NPs were optimized using various reaction parameters, and the resulting void and loaded NPs were sonicated prior to size and morphology determination. The optimum batch of NPs and loaded NPs were of size 192.66±5 nm (void) and 199.16±9 nm (diameter) as confirmed by DLS and were well dispersed in aqueous media with no aggregates. The size of PAA-Cur-PMMA-NPs was 205±7nm (Figure 1B). The corresponding loading and encapsulation efficiency of Cur in the nanospheres were found to be 6% and 93%, respectively. As opposed to free Cur, which exhibits poor aqueous solubility, the loading of the drug in the NPs resulted in high solubility as the encapsulation efficiency of the polymerized particles was very high. According to the SEM image of loaded PMMA-NPs (Figure 1C) and TEM images (Figure 1D) of PMMA-NPs, Cur-PMMA-NPs and PAA-Cur-PMMA-NPs, the particles were of spherical morphology and of similar size as DLS data. The fuzzy coating was visible following PAA coating.
The physical entrapment of the drug in the polymer was corroborated from the FTIR spectra (data not shown) of the free drug, void polymer matrix and the loaded NPs. The spectrum of Cur-PMMA-NPs revealed the characteristic absorption vibration of PMMA at 1,046 and 840 cm−1, as well as the peak at 1,723 cm−1 that represents the acrylate carboxyl group. Similarly, the bands 2,920, 1,461 and 1,024 cm−1, which were due to vibrations of aliphatic C–H stretches and mixed vibrations of CH3, aromatic CCC and CCH of Cur, were also present in the loaded NPs. The presence of bands at 1,383, 1,233 and 962 cm−1 that represent the in-plane bending of −OH of the two phenolic and an enolic group, respectively, indicates the presence of the intact Cur moiety in the Cur-PMMA-NPs.
Drug release from PMMA nanospheres in phosphate buffer, buffered saline and serum was analyzed (Figure 2A). Burst release of Cur in buffer (40%) and buffered saline (20%) was noted within 5 hours, following which the release was more gradual with 83% release in 185 hours. In serum, highly sustained and controlled release was observed with ~40% being released within 150 hours and 75% in 240 hours.
Cellular uptake results (Figure 3) revealed rapid internalization of Nile red-labeled NP samples as compared to free dye. Within 30 minutes, bright fluorescence was observed within the cells treated with dye-PMMA-NPs and dye-PAA-PMMA-NPs, while in comparison with free dye, very low fluorescence was visualized. Control cells with blank NPs showed no fluorescence.
The in vitro anticarcinogenic activity of surface-functionalized drug-loaded NPs, only drug-loaded NPs, free drug and void NPs was evaluated up to 72 hours. The results revealed that dose- and time-dependent cell death was much higher within a given time duration in case of loaded PMMA-NPs as compared to free Cur. PAA-Cur-PMMA-NPs showed maximum cell death within 24 hours with complete cell death beyond 24 hours (Figures 2B and C).
Conclusion
In this study, the PMMA-NPs exhibited high drug loading and encapsulation efficiency. The sustained release of Cur in serum can result in a consistent availability of the drug at the required site. The uptake of drug-loaded NPs would ensure intracellular release, thus fast and efficient cell death. The cell viability assays also demonstrated better affectivity of loaded NPs in comparison to free Cur. According to the study, the NP formulation appears to be highly suitable for localized and safe chemotherapy.
Acknowledgment
The authors thank Dr RP Singh and Dr LM Bharadwaj for their kind support throughout the project.
Disclosure
The authors report no conflicts of interest in this work.
References
Wang ZQ, Liu K, Huo ZJ, et al. A cell-targeted chemotherapeutic nanomedicine strategy for oral squamous cell carcinoma therapy. J Nanobiotechnology. 2015;13(63):1–10. | ||
Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res (Phila). 2011;4(8):1158–1171. | ||
Guo LY, Cai XF, Lee JJ, et al. Comparison of suppressive effects of demethoxycurcumin and bisdemethoxycurcumin on expressions of inflammatory mediators in vitro and in vivo. Arch Pharm Res. 2008;31(4):490–496. | ||
Mukherjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29(10):3867–3876. | ||
Elvira C, Fanovich A, Fernandez M, Fraile J, San Román J, Domingo C. Evaluation of drug delivery characteristics of microspheres of PMMA–PCL–cholesterol obtained by supercritical-CO2 impregnation and by dissolution–evaporation techniques. J Control Release. 2004;99(2):231–240. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.