Back to Journals » Research and Reviews in Parkinsonism » Volume 8
Curcumin and an antioxidant formulation protect C57BL/6J mice from MPTP-induced Parkinson’s disease like changes: potential neuroprotection for neurodegeneration
Authors Muthian G, Mackey V, Prasad K, Charlton C
Received 12 September 2017
Accepted for publication 23 March 2018
Published 9 November 2018 Volume 2018:8 Pages 49—59
DOI https://doi.org/10.2147/JPRLS.S151452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Peter Hedera
Gladson Muthian,1 Veronica Mackey,1 Kedar Prasad,2 Clivel Charlton1
1Department of Biochemistry, Cancer Biology, Neuroscience and Pharmacology, Meharry Medical College, Nashville, TN, USA; 2Research and Development, Antioxidant Research Institute, Premier Micronutrient Corporation, Novato, CA, USA
Background: Degeneration of nigrostriatal (NS) dopamine (DA) neurons is the major neuropathological marker of Parkinson’s disease (PD). The cause for the disorder is unknown, but a prenatal sensitization stage and a postnatal precipitating stage may be involved. The sensitization stage is based on studies showing that prenatal exposure to low doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) during the birth of substantia nigra (SN) DA neurons reduced DA, its metabolites, tyrosine hydroxylase (TH), the number of NS neurons as well as locomotor activities of the offspring. The observation that motor activities of the toxin-exposed animals deteriorated during aging, produced the condition equated to the precipitating stage. Other studies suggest that curcumin may offer protection.
Purpose: For this project, we studied the protection offered by curcumin and an antioxidant formulation from MPTP-induced toxicity.
Methods: Four groups of adult mice were pretreated, every other day for 27 days, with curcumin, 25 μg or 50 μg per mice of 25 g average body weight (equated to 1 mg/kg or 2 mg/kg) or with 25 mg/kg or 50 mg/kg of the antioxidant formulation. A control group received saline. MPTP was administer at the 20th day of pretreatment and all treatments continued for 7 days, then the animals were studied for changes in motor activities, striatal DA and DA metabolites as well as striatal and midbrain TH. The changes are indicative of PD-like NS damage.
Results: The data showed that MPTP markedly reduced movements, as well as DA, its metabolites and TH. Curcumin and the antioxidant formulation blocked and ameliorated the toxic effects of MPTP. MPTP reduced DA to 49.1%. Curcumin restored DA to 87.3% and 84.8%, and the antioxidants restored and elevated DA to 132.1% and 121.2%. MPTP reduced striatal TH to 45.1%. The doses of curcumin restored TH to 60.9% and 75.1% and the antioxidants restored TH to 90.7% and 94.7%. Curcumin and the antioxidants reduced MPTP-induced death.
Conclusion: The results demonstrated that curcumin and the antioxidants blocked the PD-like toxic effects of MPTP, indicative of the potentials as preventative measure for PD.
Keywords: antioxidants, curcumin, dopamine, tyrosine hydroxylase, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is an age-related neurodegenerative disease, characterized by the loss of dopaminergic neurons with cell bodies within the substantia nigra (SN) pars compacta and with axons projecting to the striatum. The neuronal degeneration results in the reduction of tyrosine hydroxylase (TH) and dopamine (DA) in the nigrostriatal (NS) pathway,1,2 leading to motor dysfunction, including tremor, rigidity and bradykinesia.3 About 70%–80% loss of dopaminergic neurons occurred in the pars compacta region of the SN of PD patients, and that is accompanied by intercytoplasmic inclusions known as Lewy’s bodies.63 Acetylcholine neurotransmission4 is also increased. DA replacement via its precursor, levo-dopa as well as DA agonists are the most effective treatments for PD and are mostly effective during the early stages of the disease; however, long-term therapy has been associated with serious side effects that can be as serious and incapacitating as the symptoms of PD. Since both the symptoms of PD as well as the side effects of PD therapy are devastating, it means that finding interventions that prevent or delay the onset of PD symptoms are valuable endeavors in the effort of reducing the suffering caused by the disorder.
Ninety to 95% of PD cases are unknown and regarded as idiopathic, and 5%–10% of the cases are caused by genetic abnormalities, mostly involving the alpha–synuclein,5 ubiquitin,6 and apolipoprotein E7 genes. Parkin gene mutation,8 with about eight variants,9 is associated with juvenile PD. It should be noted that, for both genetic- and idiopathic-derived PD, the PD patients were normal during their early lives. For the genetic-based PD, the predisease time is about 40 years and for the idiopathic variant about 60 years. So, PD is age related, but old people do not develop the disorder as a rule, which is set against the fact that aging is accompanied by progressive reduction of motor functions, that include essential tremor, reduced arm swing, shorter strides, slower reaction time and bradykinesia. This means that, as a general biological rule, during aging the nigrostriatial DA neurons do not deteriorate to the point of causing PD. So, for PD to be expressed in mature and older individuals, as seen for genetic and idiopathic cases, it would suggest that PD patients are predisposed for the disorder. This further suggests that the NS neurons of the PD patients were sensitized or vulnerable, and that the stress of normal aging serves to precipitate the symptoms of PD. The random occurrence of idiopathic PD indicates that the changes that produce the vulnerability are due to chance, and may occur during the morphological changes or “birth” of precursor cells to produce the NS DA neurons,10–12 when the neurons are very responsive to toxins and other endogenous interventions. The functional normality that exists before the onset of the PD symptoms suggests that the prenatal sensitization, or the early neuronal insults, occur at subthreshold level, which is easy to comprehend, knowing that about 70% NS neuronal loss occurs before the onset of PD symptoms. For the genetic variant of PD, the gene changes may render the NS DA neurons frail or sensitive to stress and biological changes that occur during development, maturation and aging.
The idea of a sensitization stage and a precipitating stage for PD suggests that there ought to be interventions that can help to maintain healthy basal ganglia or maintain the condition of the neurons at a stage that is subthreshold for the symptoms of genetic and idiopathic PD. Such interventions may depend on finding drugs or other treatments, such as nutritional, exercise and other wellness programs that support the health of the NS neurons and delay neuronal death and the onset of PD. Studies showed that oxidative stress and mitochondrial dysfunction are major events that occur during neuronal death in PD and other neurodegenerative disorders.13,14 This may involve the depletion of glutathione in the dopaminergic cells, leading to mitochondrial dysfunction.15 Accordingly, therapy geared to prevent or to delay the onset and progression of PD could include the modulation of oxidative stress. Phenolic antioxidants, including flavonoids, may help to delay the onset and progression of PD.
Phenolic antioxidants are an extensive group of naturally occurring compounds that are widely distributed in plants as constituents of various fruits, nuts, leaves and other parts.16,17 Flavonoids, as examples, are potent antioxidants and free-radical scavengers, with efficacy that may exceed the antioxidant capacity of vitamins C and E.18–21 Flavonoids are capable of chelating metal ions, modifying the activity of cellular antioxidants and antioxidant enzymes, such as catalase and GSH.22 In vitro studies showed that flavonoids have the capability of modulating nitric oxide production, the secretion of tumor necrosis factor alpha and nuclear factor kB dependent gene expression.23 Flavonoids also have anti-inflammatory properties, inhibiting the activities of lipoxygenase and cycloxygenase. Moreover, flavonoids show very little toxicity, making these agents suitable for long-term utility, serving, eg, to slow down the rate of nigral cell loss.24
Curcumin falls into the category of flavonoids, and it is utilized as a flavor and for coloring food. So, curcumin may serve as a readily available source of food-related therapy. Curcumin is a naturally occurring yellow pigment isolated from turmeric, produced from the rhizomes of the plant, Curcuma Longa, and found mostly in South Asia.25,26 Turmeric has been used in India for centuries as a food preservative and for medicine; eg, for the treatment of human disease associated with injury and inflammation.27,28 In the laboratory, studies showed that curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium (MPP+) neurotoxicity, through anti-apoptotic and antioxidative properties.29 Curcumin may cross the blood–brain barrier and enter the central nervous system,30 a property that will support the use of curcumin as a therapeutic agent for neurodegenerative disorders. Curcumin appears to be nontoxic or it possess low toxicity, as doses as high as 12,000 mg have shown very little or no toxic effect.31 It is of interest also that dozens of biological molecules serve as targets for curcumin,32 and various ligands have been shown to physically interact with curcumin.32 These include amyloid protein,33 ATPase,34,35 glutathione,36 lipoxygenase,37,38 microtubulin,39 nucleic acid40 and protein kinase A and C.41
Antioxidants donate an electron to a free radical, converting it to harmless molecule, are regarded as micronutrients and include vitamins, such as vitamins B and D. Each may affect different pathways, by activating nuclear transcription factor that increases antioxidant enzymes by upregulating antioxidant genes via antioxidant response element, and some act via a reactive oxygen species (ROS)-independent mechanism, therefore a combination of antioxidants may offer more in reducing oxidative stress and inflammation. On that basis, we tested an antioxidant formulation (AOF) obtained from Premier Micronutrient Corporation, Narvato, CA, USA that contains: vitamin A, natural beta carotene, d-alpha tocopheryl acetate and d-alpha tocopheryl succinate (vitamin E), vitamin C, N-acetylcysteine, R-alpha lipoic acid, coenzyme and selenomethionine.
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyramidine (MPTP) has been shown to cause a PD like syndrome in laboratory animals and in humans and its toxicity has been well accepted as a model for PD.42,43 The neurotoxin, MPTP, is converted to MPP+ by the brain monoamine oxidase B (MAO-B), MPP+ is transported into dopaminergic neurons via the DA transporter and it accumulates in the mitochondria, to cause cell death by disrupting respiratory enzymes and inducing oxidative damage.44
The present study was designed to determine whether curcumin and an AOF counteract the PD-like toxic effects of MPTP in C57BL/6J mice. The study outcomes show that MPTP causes NS, dopaminergic and behavioral changes that are consistent with the well-established MPTP PD model and that curcumin and the AOF ameliorate the toxic effects of MPTP. So the outcomes are indicative of neuroprotective actions for curcumin and for the AOF. Thus, these agents may find utility in protecting the brain from harmful neurotoxic exposures and may slow the progression of PD symptoms. Some cases of idiopathic PD may have a fetal basis, as there is evidence that subtle NS impairments that were induced in the fetus can develop into PD-like changes during the course of aging,11 thus the practice of eating curcumin may serve as a healthy protector of the NS system.
Materials and methods
Chemicals
Curcumin (1,7-bis 4hydroxy-3-methoxyphenyl)–1,6-heptadiene-3,5-dione) was purchased from Calbiochem, La Jolla, CA. USA. The dosage of curcumin was based on previous trials and other studies,45 and the levels used show no acute behavioral toxicity. The AOF was obtained from Premier Micronutrient Corporation and contains: vitamin A, natural beta carotene, d-alpha tocopheryl acetate and d-alpha tocopheryl succinate (vitamin E), vitamin C, N-acetylcysteine, R-alpha lipoic acid, coenzyme and selenomethionine. The formulation was designed for human consumption and is commercially available. Polyclonal TH antibody was purchased from Chemicon, San Diego, CA, USA.
Experimental design
Animals
The C57BL/6J male animals were purchased from Jackson Laboratory, Bar Harbor, MN, USA. The animals were about 6 weeks at purchase and allowed to acclimatize in the animal care facility for 5 days. The experimental animals were pretreated with curcumin, antioxidants or vehicle as shown in Table 1, and which are adaptations from previous studies.12,45 MPTP treatment was administered at 30 mg/kg body weight once a day for 7 days. PBS was used as the vehicle for MPTP and to treat the control mice. All medications were injected by the intraperitoneal route. The putative neuro-protectant, curcumin, was given at 25 µg/mouse or 50 µg/mouse, equivalent to 1 mg/kg or 2 mg/kg, and the AOF at 5 mg/kg or 25 mg/kg. Curcumin and the antioxidants were dissolved in PBS containing 0.1% Tween-80. The PBS-Tween-80 was also used for control treatment. The mice were injected every other day for 28 days. For seven more days, the treatments continued but were accompanied with MPTP, administered once a day for the remaining 7 days (Table 1). Six animals per group for the six groups were used for the behavioral studies and for the biochemical analysis. Three animals per group for the six groups were used for Western blot determination of TH. Thus, the total (N) animals used was 54 mice.
Seven days after the last injection, the motor activity of the mice were determined. A subgroup was sacrificed by decapitation, their brains dissected and the striatum and midbrains were used for Western blot analysis for TH and for the high performance liquid chromatographic (HPLC) determination of DA and its metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and 3-methoxytyramine (3-MT).
The level of MPTP used for treatment is well known to produce PD-like changes, including poverty of movements, loss of NS DA and TH and NS cell loss. For the study the animals were divided into the following groups: 1) normal negative control mice treated with PBS-0.1% Tween 80; 2) positive control treated with 30 mg MPTP/kg body weight; 3) MPTP + curcumin at 1 mg/kg; 4) MPTP + 2 mg/kg curcumin; 5) MPTP + 25 mg/kg antioxidants; 6) MPTP + 50 mg/kg antioxidants (Table 1). All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health) and were approved by the Institutional Animal Care and Use Committee at Meharry Medical College.
Measurement of locomotor activities
Seven days after the last treatment the mice locomotor activity was assessed in a locomotor activity monitor (Versamax Analyzer, Accusccan Instruments Inc., Columbus, OH, USA), as reported previously.11 The animals were placed in the activity cage and allowed 3 minutes to acclimatize to the cage environment. Measurements were started and continued for 10 minutes. Motor activities were measured in a quiet isolated room with dim lighting. Movement time (MT), total distance (TD) traveled and the number of movements made (NM) were determined over the 10-minute period. The computer, with software that came with the instrument, records the activities for each animals and the mean and standard error were calculated.
HPLC–electrochemical (EC) determination of DA and its metabolites, DOPAC and 3-methoxytyramine (3-MT)
The isolated striatal tissues were homogenized in 750 µL of 0.1 M trichloro-acetic acid (TCA), which contains 10−2 M sodium acetate, 10−4 M EDTA and 10.5% methanol (pH 3.8) using a tissue dismembrator (Thermo Fisher Scientific, Waltham, MA, USA). Samples were spun in a micro-centrifuge at 10,000 × g for 20 minutes. Samples of the supernatant were then analyzed for DA and its metabolites at the core analytical facility at Vanderbilt University. The detection limit was reported to be 2 pg.
DA and its metabolites were determined by specific HPLC assay utilizing an Antec Decade 11 (oxidation: 0.5) electrochemical detector operated at 33°C. Supernatant samples of 20 µL were injected, using a Water’s 717 + autosampler, onto a Phenomenex Nucleosol (5U, 100 A) C18 HPLC column (150 × 4.60 mm). Biogenic amines were eluted with a mobile phase consisting of 89.5% 0.1 M TCA, 10-2 M sodium acetate, 10-4 M EDTA and 10.5% methanol (pH 3.8). Solvent is delivered at 0.8 mL/min using Water’s 515 HPLC pump. Using this HPLC solvent, the following biogenic amines elute in the following order: noradrenaline, adrenaline, DOPAC, DA, 5-hydroxyindole acetic acid (5-HIAA), 5-hydroxytryptamine (5-HT) and 3-methoxytyramine (3-MT). HPLC control and data acquisition were managed by Water’s Empower software.
Western blot analysis of TH protein expression
Immunoblotting, as performed in previous studies,11,12,45 was used to quantify the amount of TH and LAAD proteins in the midbrain. After decapitation, the brains were dissected and the sections were homogenized in lysis buffer (Ambion, Austin, TX, USA). Protein concentration was determined using Bio-Rad protein reagent (Bio-Rad, Hercules, CA, USA). The proteins were precipitated by adding 100% methanol and centrifuged at 10,000 rpm for 10 minutes in a Sorvall refrigerated centrifuge. Then the supernatants were decanted and the precipitates washed with 90% methanol for 10 minutes. Proteins were dissolved in Lamelli sample buffer and transferred to nitrocellulose membrane. The membranes were blocked in 5% BSA for 1 hour. Membranes were incubated with primary rabbit TH (1:2,000, Chemicon International Inc., Temecula, CA, USA) and then exposed to the secondary antibody 1:10,000 (HRP conjugated anti-rabbit IgG, Sigma Chemical Co., Saint Louis, MO, USA) and visualized by chemiluminescence. All the experiments were repeated three times in different animals from the same group to confirm the results.
Statistical analysis
All data are reported as mean ± SEM and analyzed by one-way analysis of variance followed by Newman–Keuls test. P-values <0.05 were considered significant (PRISM, GraphPad Software, Inc., La Jolla, CA, USA).
Results
Postnatal effect of curcumin and antioxidants on locomotor activities in PBS and MPTP treated mice
The data show that the control animals move vigorously during the measurement period of 10 minutes. Each mouse travels about 750 cm distance, actively moving for about 50 seconds, which represents 1/12th of the observation time. However, stereotype movements, which are defined as nonlocomotion repetitive body movements, were prominent, since each mouse registers about 75 mixed locomotive and stereotype-like movements. Here, measures are reported mostly as percentages, which allow the real numbered changes in the figures to be easily assessed.
Effects of MPTP
The study shows that MPTP treatments significantly decreased all the measures of movements when compared with the control (Figure 1, column (C)1 vs C2). MPTP reduced the TD traveled to 32.0% of the control value (Figure 1, TD, C1 vs C2). The NM was reduced to 33.4% (Figure 1, NM, C1 vs C2) and movement time (MT) was reduced to 46.4% (Figure 1, MT, C2 vs C1) following MPTP.
Effects of curcumin
Curcumin had an ameliorating effect on movements. For TD, the 25 mg/kg (1 mg/kg) dose level of curcumin partially reversed the effect of MPTP on TD from the 32.0% reduction caused by MPTP to 59.4% level of the control (Figure 1, TD, C3 vs C1). The 50 µg/mouse (2 mg/kg) of curcumin restored TD and enhanced it to 136% of control (Figure 1, TD, C3 vs C1). For the NM, the 25 mg/kg curcumin reversed the 33.4% reduction caused by MPTP to 61.8% of control (Figure 1, NM, C2 vs C3). The 50 µg/mouse (2 mg/kg) of curcumin restored NM and enhanced it to 146.5% of control (Figure 1, NM, C3 vs C1). For MT, the 25 mg/kg curcumin reversed the 46.4% reduction caused by MPTP to 71.1% of control (Figure 1, MT, C2 vs C3). The 50 µg/mouse (2 mg/kg) of curcumin restored MT and enhanced it to 156.6% of control (Figure 1, MT, C3 vs C1).
Effects of the AOF
The pre- and coadministration of the AOF with MPTP restored and enhances the reducing effects of MPTP on locomotor activities. For TD, the reduction of TD to 32% by MPTP was restored to 94.6% by 25 mg/kg AOF (Figure 1, TD, C2 vs C5) and the 50 mg/kg formulation fully restores and enhances the TD to 142% of the control (Figure 1, TD, C2 vs C6). For NM, the MPTP reduction of NM to 33.4% was restored and enhanced to 132.3% of the control by the 25 mg/kg of the AOF (Figure 1, NM, C2 vs C5) and the 50 mg/kg of AOF restored and enhanced the NM to 143% (Figure 1, NM, C2 vs C6). MT was restored and enhanced from the MPTP reduction of 46.4% (Figure 1, MT, C2) to 126% by the 25 mg/kg of AOF (Figure 1, MT, C2 vs C5) and doubling the dosage of the AOF to 50 mg/kg further enhanced MT to 161.2% of control (Figure 1, MT, C2 vs C6).
Postnatal effect of curcumin and antioxidants on DA, DOPAC and 3-MT in mice treated with MPTP vs PBS control
Effects of MPTP
Figure 2 shows that MPTP treatments significantly decreased DA to 46.4% (28.4 ± 3.4 ng/mg), compared with 58.4 ± 5.3 ng/mg (100%) for the PBS group (Figure 2, DA, C2 vs C1). Figure 2 also shows that treatments with MPTP reduced DOPAC to 74.1% (51.2 ± 2.8 ng/mg) of the control value of 70.0 ± 3.86 ng/mg (Figure 2, DOPAC, C2 vs C1). The methyl metabolite of DA, 3-MT, was also reduced to 37.6% (7.9 ± 0.7 ng/mg) by MPTP from the control value of 22.7 ± 2.0 ng/mg (Figure 2, 3-MT, C2 vs C1).
Effects of curcumin
Curcumin administration of 1 mg/kg had an ameliorating effect on DA, by restoring the neurotransmitter from the 46.4% reduction (28.4 ± 3.4 ng/mg) caused by MPTP to the 87.3% (51.4 ± 7.2 ng/mg) level of the control value (Figure 2, DA, C2 vs C3). Thus, the 1 mg/kg curcumin caused 40.9% gain over the MPTP-induced 46.4% reduction of DA. Treatment with 2 mg/kg of curcumin also restored DA to 50.4 ± 7.2 ng/kg or 84.8% of control (Figure 2, DA, C4). DOPAC levels were not improved following treatment with 1 µg/mouse (25 mg/kg) of curcumin, but slight increase of 11.5% occurred following the 2 mg/kg curcumin treatment. The 1 mg/kg dose of curcumin partially reversed the MPTP-induced reduction of 3-MT to 16.5 ± 2.5 ng/mg or 72.0% of the control (Figure 2, 3-MT, C1 vs C3) and 2 mg/kg curcumin restored 3-MT to 20.4 ± 3.2 ng/mg or 90.4% of control (Figure 2, 3-MT, C1 vs C4).
Effects of the AOF
The 25 mg/kg as well as the 50 mg/kg of the AOF fully restored the level of DA and enhanced it to 78.0 ± 6.3 ng/mg and 72.2 ± 6.8 ng/mg or 32.1% and 21.2% above the control (Figure 2, DA, C2 vs C5 and C6). Interestingly, DOPAC was restored from 51.2 ± 2.3 ng/mg (74.1%) reduction caused by MPTP to 72.6 ± 2.2 ng/mg (103.5%) and 76.0 ± 6.3 ng/mg (108.2%) levels following the 25 and 50 mg/kg doses of AOF. As shown for DA, the 25 mg/kg and the 50 mg/kg of AOF fully restored and elevated the 3-MT level to 117.2% and 143.6% of the control value (Figure 2, 3-MT, C2 vs C5 and C6).
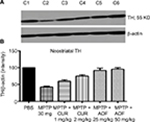
Postnatal effects of curcumin and antioxidants on TH expression in PBS and MPTP-treated mice
Striatal TH
Effects of MPTP
Figure 3 shows that MPTP administration significantly decreased striatal TH expression to 42.9% of the PBS control level (Figure 3, C2 vs C1).
Effects of curcumin
The administration of 1 mg/kg of curcumin has a slight ameliorating effect, by restoring TH to 60.3% of the control: a restoration of 17.4% above the MPTP value (Figure 3, C3 vs C1). The dose of 2 mg/kg of curcumin elevated TH to 74.8% of the control, which is a restoration of 31.9% (Figure 3, C4 vs C2). Thus, a dose-related effect of curcumin is shown.
Effects of AOF
The 25 mg/kg the AOF restored striatal TH from the MPTP reduction of 42.9% to 91.4% of control, thus showing a net positive effect of 48.5%, and a 50 mg/kg dose of AOF restored TH to 95%, which is a net positive effect of 52.1% (Figure 3, C5 and C6 vs C2).
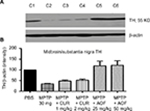
Midbrain (SN) TH
Effects of MPTP
Figure 4 shows that MPTP administration significantly decreased the midbrain TH expression to 35.7% of the PBS control (Figure 4, C2 vs C1).
Effects of curcumin
Curcumin administration of 1 mg/kg had a slight ameliorating effect, by restoring midbrain TH by 12.9% above that caused by MPTP. The 2 mg/kg curcumin showed similar effect of 14.2% improvement (Figure 4, C4 vs C2).
Effects of the AOF
The 25 mg/kg AOF restored midbrain TH from the MPTP reduction of 35.7% to 115.4% of the control and the 50 mg/kg formulation restored TH to 118.6% of control, from the MPTP reduction of 35.7% (Figure 4, C5 and C6 vs C2).
Discussion
The objective of the present study was to investigate the effects of curcumin and an AOF on MPTP-induced changes in the brain of C57BL/6J male mice. MPTP is known to produce models of PD, by causing NS dopaminergic neuronal degeneration, depletion of DA, loss of TH and reduction in motor activities,12,45 so we analyzed most of these toxic features of MPTP in mice and determined whether curcumin and an AOF can ameliorate the toxic effects of MPTP.
Curcumin belongs to a class of polyphenolic pigments found in the spice turmeric produced from the rhizomes of the plant Curcuma longa. Curcumin is well documented for its medicinal properties in the traditional Indian and Chinese systems of medicine.46 The efficacy of curcumin may be provided by its antioxidant or its free-radical scavenger action that offers neuroprotection. Since oxidants and free radicals are believed to play important roles in the cause of neurodegeneration, including Alzheimer’s disease (AD) and PD, it becomes very meaningful and relevant that several epidemiological studies described the reduced prevalence of AD and PD in India,47–49 where about 60–200 mg of dietary curcumin is consumed daily.47 Moreover, it was shown that those who consume turmeric or curry, that is produced from curcumin, occasionally, often or very often, scored significantly better on the Mini-mental State Examination than did those who never or rarely consumed curry.50 The Mini-mental State Examination is an established measure of cognitive function.
The AOF utilized in this study contains vitamin A (natural beta carotene), vitamin E (d-alpha tocopheryl acetate and d-alpha tocopheryl succinate), vitamin C, N-acetylcysteine, R-alpha lipoic acid, coenzyme and selenomethionine. Although individual functions are associated with the vitamins, the formulation is promoted mostly for antioxidant properties. Accordingly, both curcumin and the formulation are projected to be antioxidants. Both dosages of the AOF utilized in this study show higher magnitude of effects than does curcumin, but it should be noted that the respective dosage of the antioxidants were about 25 times higher than curcumin.
The administration of MPTP in this study resulted in a marked reduction in the measures of motor activities that were examined. MPTP also depleted DA as well as the levels of the metabolites of DA, DOPAC and 3-MT, and MPTP correspondingly reduced TH in the striatum and the midbrain: noting that the striatum contains the terminals for DA neurons with cell bodies located in the SN, a region that contributes most of the TH found in the midbrain. Interestingly, all measures that we examined were down regulated by MPTP, and curcumin and the AOF reduced the toxic effects of MPTP or restored the changes caused by MPTP. The parallel changes caused by both curcumin and the AOF suggest that both have similar mechanism of action, and that curcumin may also serve as an antioxidant. Curcumin clearly showed dose-response effects; however, at the higher dose-level of curcumin, the changes in behaviors exceeded the control values (Figure 1), which suggests that curcumin by itself will enhance performance. The antioxidants may enhance performance, because the formulation does not merely reverses the toxic effects caused by MPTP, but it elevates TD, NM, MT as well as midbrain TH above the 100% control values. The patterns of the changes caused by both curcumin and the AOF indicate that both treatments may modulate the same pathway, which may entail modulating the expression of TH protein, the rate-limiting enzyme for DA biosynthesis.51–54 So, apparently, the increased TH drives the synthesis of DA that serves to modulate locomotor activity. Thus, curcumin and AOF may serve to protect against MPTP neurotoxicity by activating the dopaminergic pathway. It should be realized, though, that curcumin has been shown to modulate various other systems and pathways32 and to affect multiple cellular targets.32 For example, it has been shown that curcumin inhibits c-jun N-terminal kinase and prevents the loss of dopaminergic neurons.55 Oxidative stress plays a major role in aging and in age-related neurodegenerative diseases, including PD,56 which may explain the therapeutic or preventative role for antioxidants, such as curcumin and AOF. Moreover, epidemiological studies show that exposure to environmental agents, such as neurotoxins and pesticides,57,58 increase oxidative damage to cells, by suppressing mitochondrial complex-1 activity and reducing glutathione levels.59 This suggests that early and long-term utility of curcumin may help to counteract the harmful effects of environmental toxins in causing PD-like changes,45 and other human disorders.32
Further studies are necessary to explore the effects of curcumin and the antioxidants on various targets in various animal models and, perhaps, in human neurodegenerative diseases. The data show that curcumin is protective, but it is not known whether curcumin will slow or stop the progression of PD after the symptoms are well developed, so further studies are necessary including treatment at various stages of the disorders, when substantial degeneration has already occurred. There is a global interest in finding new and safe agents to prevent oxidative stress related to neurodegeneration and a host of other disorders. Since curcumin is already widely used as food flavor and has been reported to be anti-inflammatory,49 and to serve as an antioxidant,32,60 curcumin may be exploited as a neuroprotective and preventative agent for neurodegenerative disorders. This could be seen as very practical, since curcumin is well tolerated,61,62 on the basis that dose levels as high as 12,000 mg of curcumin have been taken by human volunteers.31 Future studies will examine the mechanism of action of curcumin and the vitamin AOF by measuring and studying various markers, including catalase, lipid peroxidation, protein carbonylation, dihydroethidium staining and superoxide dismutase.
Conclusion
The degeneration of SN dopaminergic neurons projecting to the striatum results in PD. DA replacement therapy represents the major treatment, but the toxic side effects of the major drugs used for PD can be severe and incapacitating, as much as the disease itself. There is no cure for PD and no intervention slows the progression of the disease. PD occurs during the adult years of life, about the age of 40 years for the genetic variant and 60–65 years for the idiopathic variant. This means that a window for preventing the disorder may exist. Accordingly, preventative therapy such as food, flavors, vitamins, other antioxidants and healthful lifestyle may be of value in blocking or slowing the onset of PD. Accordingly, presymptomatic interventions that may include curcumin and AOF may help to maintain the functionality of the basal ganglia neurons. This idea is of particular interest since age-related changes are proposed to be precipitating factors for PD10 by pairing with early genetic and/or idiopathic sensitization or with predispositions.10 This means that the age-related precipitating stage may be controllable and perhaps delayed by antioxidants and other neuroprotective agents. Positive lifestyle, including improved diets and access to health care, is known to markedly improve human health and increased longevity; accordingly, treatment-based interventions coupled with exercise and other wellness programs may delay neuronal death and the onset of PD. Indeed, this may offer the best hope, since in spite of enormous endeavors and excellent research, there is no curative treatment for PD.
Acknowledgments
This work was supported by National Institute of Health (NIH) RO1NS041674 and NIH R21NS049623. The authors acknowledge financial support from Dr. Bernard Crowell, Pinnacle Orthopedics, PA., 13100 Chenel Parkway, Little Rock, AR 72223.
Disclosure
The authors report no conflicts of interest in this work.
References
Nagatsu T, Kato T, Nagatsu I. Catecholamine-related enzymes in the brain of patients with parkinsonism and Wilson’s disease. In: Poirer LJ, Sourkes TL, Bedard PJ, editors. Advances in Neurology, Vol. 24. New York: Raven Press; 1979:283–292. | ||
Heikkila RE, Sonsalla PK. The MPTP-treated mouse as a model of parkinsonism: how good is it? Neurochem Int. 1992(20 Suppl):299–303. | ||
Forno LS, Norville RL. Ultrastructure of Lewy bodies in the stellate ganglion. Acta Neuropathol. 1976;34(3):183–197. | ||
Yahr MD, Bering EA. Parkinson’s disease. Present Status and Research Trends. Yahr MD, Bering EA, editors. US-DHEW PP47, 1968. | ||
Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. | ||
Leroy E, Anastasopoulos D, Konitsiotis S, Lavedan C, Polymeropoulos MH. Deletions in the Parkin gene and genetic heterogeneity in a Greek family with early onset Parkinson’s disease. Hum Genet. 1998;103(4):424–427. | ||
Krüger R, Vieira-Saecker AM, Kuhn W, et al. Increased susceptibility to sporadic Parkinson’s disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann Neurol. 1999;45(5):611–617. | ||
Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. | ||
Lansbury PT, Brice A. Genetics of Parkinson’s disease and biochemical studies of implicated gene products. Curr Opin Cell Biol. 2002;14(5):653–660. | ||
Charlton CG. Fetal and environmental basis for the cause of Parkinson’s disease. In: Barrios FA, Bauer C, editors. Basal Ganglia: An Integrative View. Vol. 64. InTECH, Rijeka, Croatia. http://dx.doi.org/10.5772/2976. Chapter 2: 1212;31–64. | ||
Muthian G, Mackey V, King J, Charlton CG. Modeling a sensitization stage and a precipitation stage for Parkinson’s disease using prenatal and postnatal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Neuroscience. 2010;1691093(3):1085–1093. | ||
Muthian G, King J, Dent L, Smith M, Mackey V, Charlton C. Prenatal and postnatal exposures to 1-methyl-4-phenyl-1,2,3,6-tetra hydropyridine (MPTP) impaired mouse midbrain dopamine system and may produce a predisposing and inducing model for Parkinson’s disease. J Behav Brain Sci. 2012;02(04):485–494. | ||
Beal MF. Oxidative metabolism. Ann N Y Acad Sci. 2000;924:164–169. | ||
Przedborski S. Pathogenesis of nigral cell death in Parkinson’s disease. Parkinsonism Relat Disord. 2005;11(Suppl 1):S3–S7. | ||
Jha N. Jurma O, Lalli G, et al. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex 1 activity: implications for Parkinson’s disease. J Biol Chem. 2000;275:260996–26101. | ||
Kühnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–119. | ||
Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer. 1993;20(1):21–29. | ||
Vinson JA, Dabbagh YA, Serry MM, Jang J, Jang J, Cai S. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J Agric Food Chem. 1995;43(11):2800–2802. | ||
Wiseman SA, Balentine DA, Frei B. Antioxidants in tea. Crit Rev Food Sci Nutr. 1997;37(8):705–718. | ||
Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med. 1995;19(4):481–486. | ||
Middleton E, Andaswami CK, Theoharides T. The effect of plant flavonoids on mammalian cells. Pharmacol Rev. 2000;52:673–751. | ||
Sudheesh S, Sandhya C, Sarah Koshy A, Vijayalakshmi NR. Antioxidant activity of flavonoids from Solanum melongena. Phytother Res. 1999;13(5):393–396. | ||
Park YC, Pae HO, Yoo JC, Choi BM, Jue DM. Chloroquine inhibits inducible nitric oxide synthase expression in murine peritoneal macrophages. Pharmacol Toxicol. 1995;4:188–191. | ||
Hoult JR, Moroney MA, Payá M. Actions of flavonoids and coumarins on lipoxygenase and cyclooxygenase. Methods Enzymol. 1994;23454:443. | ||
Lodha R, Bagga A. Traditional Indian systems of medicine. Ann Acad Med Singapore. 2000;29(1):37–41. | ||
Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25(6):447–452. | ||
Tobinai M, Shimoyama S, Inoue S, et al. Phase I study of YK-176 (2′deoxycoformycin) in patient with adult T-cell leukemia-lymphoma. Jpn J Clin Oncol. 1992;22:164–171. | ||
Hanada US, Ohno N, Ishitsuka K, et al. Combination chemotherapy (RCM Protocol) for the acute or lymphoma type adult T-cell leukemia, Leuk. Lymph. 1995;18:317–321. | ||
Chen J, Tang XQ, Zhi JL, et al. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11(6):943–953. | ||
Quin XY, Cheng Y, Cui J, Zhang Y, Lc Y. Potential protection of curcumin against amyloid beta-induced toxicity on cultured rat prefrontal cortical neurons. Neurosci Lett. 2009;463:158–161. | ||
Lao CD, Normolle D, Heath DD, et al. Ruffin iv, MTDose escalation of curcuminoid formulation. BMC Complement Altern Med. 2006;6(10):6–10. | ||
Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. | ||
Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–5901. | ||
Logan-Smith MJ, Lockyer PJ, East JM, Lee AG. Curcumin, a molecule that inhibits the Ca2+-ATPase of sarcoplasmic reticulum but increases the rate of accumulation of Ca2+. J Biol Chem. 2001;276(50):46905–46911. | ||
Bilmen JG, Khan SZ, Javed MH, Michelangeli F. Inhibition of the SERCA Ca2+ pumps by curcumin. Curcumin putatively stabilizes the interaction between the nucleotide-binding and phosphorylation domains in the absence of ATP. Eur J Biochem. 2001;268(23):6318–6327. | ||
Awasthi S, Pandya U, Singhal SS, et al. Curcumin-glutathione interactions and the role of human glutathione S-transferase P1-1. Chem Biol Interact. 2000;128(1):19–38. | ||
Jankun J, Aleem AM, Malgorzewicz S, et al. Synthetic curcuminoids modulate the arachidonic acid metabolism of human platelet 12-lipoxygenase and reduce sprout formation of human endothelial cells. Mol Cancer Ther. 2006;5(5):1371–1382. | ||
Skrzypczak-Jankun E, Zhou K, Mccabe NP, Selman SH, Jankun J. Structure of curcumin in complex with lipoxygenase and its significance in cancer. Int J Mol Med. 2003;12(1):17–24. | ||
Gupta KK, Bharne SS, Rathinasamy K, Naik NR, Panda D. Dietary antioxidant curcumin inhibits microtubule assembly through tubulin binding. Febs J. 2006;273(23):5320–5332. | ||
Zsila F, Bikadi Z, Simonyi M. Circular dichroism spectroscopic studies reveal pH dependent binding of curcumin in the minor groove of natural and synthetic nucleic acids. Org Biomol Chem. 2004;2(20):2902–2910. | ||
Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett. 1994;341(1):19–22. | ||
Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson’s disease. Bioessays. 2002;24(4):308–318. | ||
Grünblatt E, Mandel S, Youdim MB. MPTP and 6-hydroxydopamine induced neurodegeneration as models for Parkinson’s disease: neuroprotective strategies. J. Neurol. 2000;247:95–102. | ||
Nicotra A, Parvez SH. Cell death induced by MPTP, a substrate for monoamine oxidase B. Toxicology. 2000;153(1-3):157–166. | ||
Muthian G, Smith M, Dent L, et al. Curcumin prevents and ameliorates biochemical and behavioral toxicities of MPTP in C57Bl/6J mice: its potential use in preventing and treating parkinsonism. J Parkinsons Dis Alzheimer Dis. 2015;2(2):1–10. | ||
Maheshwari RK1, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;782087(18):2081. | ||
Commandeur JN, Vermeulen NP. Cytotoxicity and cytoprotective activities of natural compounds. The case of curcumin. Xenobiotica. 1996;26(7):667–680. | ||
Kelloff GJ, Crowell JA, Hawk ET, et al. Strategy and planning for chemopreventive drug development: clinical development plans II. J Cell Biochem Suppl. 1996;26:54–71. | ||
Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med. 2003;9(1):161–168. | ||
Tp N, Chiam PC, Lee T, Chua HC. Lim, L. and Kua, AE. Curry consumption and cognitive function in the elderly. Am J Epidemiol. 2006;164:889–906. | ||
Hefti F, Melamed E, Wurtman RJ. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res. 1980;195(1):123–137. | ||
Onn SP, Berger TW, Stricker EM, Zigmond MJ. Effects of intraventricular 6-hydroxydopamine on the dopaminergic innervation of striatum: histochemical and neurochemical analysis. Brain Res. 1986;376(1):8–19. | ||
Blanchard V, Anglade P, Dziewczapolski G, Savasta M, Agid Y, Raisman-Vozari R. Dopaminergic sprouting in the rat striatum after partial lesion of the substantia nigra. Brain Res. 1996;709(2):319–325. | ||
Blanchard V, Chritin M, Vyas S, et al. Long-term induction of tyrosine hydroxylase expression: compensatory response to partial degeneration of the dopaminergic nigrostriatal system in the rat brain. J Neurochem. 1995;64(4):1669–1679. | ||
Pan J, Li H, Ma J-F, et al. Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Transl Neurodegener. 2012;1(1):16–19. | ||
Jenner P. Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov Disord. 1998;13(Suppl 1):24–34. | ||
Kalivendi SV, Cunningham S, Kotamraju S, Joseph J, Hillard CJ, Kalyanaraman B. Alpha-synuclein up-regulation and aggregation during MPP+-induced apoptosis in neuroblastoma cells: intermediacy of transferrin receptor iron and hydrogen peroxide. J Biol Chem. 2004;279(15):15240–15247. | ||
Pettifer KM, Jiang S, Bau C, et al. MPP(+)-induced cytotoxicity in neuroblastoma cells: Antagonism and reversal by guanosine. Purinergic Signal. 2007;3(4):399–409. | ||
Jagatha B, Mythri RB, Vali S, Bharath MMS. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: Therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radic Biol Med. 2008;44(5):907–917. | ||
Srivastava, R. and Srimal, RC. Modification of certain inflammation-induced biochemical changes by curcumin. Indian J Med Res. 1985;81:215–223. | ||
Shankar TN, Shantha NV, Ramesh HP, Murthy IA, Murthy VS. Toxicity studies on turmeric (Curcuma longa): acute toxicity studies in rats, guineapigs and monkeys. Indian J Exp Biol. 1980;18(1):73–75. | ||
Qureshi S, Shah AH, Ageel AM. Toxicity studies on Alpinia galanga and Curcuma longa. Planta Med. 1992;58(2):124–127. | ||
Nester EJ, Hyman SE, Malenka RC. Control of movement. In: Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. New York: McGraw-Hill; 2001:312. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.