Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Curcumin Alleviates Epidermal Psoriasis-Like Dermatitis and IL-6/STAT3 Pathway of Mice
Authors Cai Z, Zeng Y, Liu Z, Zhu R, Wang W
Received 1 June 2023
Accepted for publication 25 August 2023
Published 1 September 2023 Volume 2023:16 Pages 2399—2408
DOI https://doi.org/10.2147/CCID.S423922
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Zhenguo Cai,1,2,* Yibin Zeng,3,* Zhuohang Liu,3 Ruizheng Zhu,3 Wuqing Wang1,3
1Department of Dermatology, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China; 2Department of Dermatology, Changhai Hospital, Naval Medical University, Shanghai, 200433, People’s Republic of China; 3Department of Dermatology, Minhang Hospital, Fudan University/Central Hospital of Minhang District, Shanghai, 201199, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wuqing Wang; Ruizheng Zhu, Email [email protected]; [email protected]
Background: To further investigate why curcumin (CUR) can attenuate psoriasis-like dermatitis of mice.
Methods and Results: Sixteen mice were randomized into four groups. The control group used carrier cream, and the model and the CUR group were applied with topical 5% imiquimod in the naked mice skin once a day for 6 days (62.5 mg/day/mice). Meanwhile, the control and model mice were given the same dose of saline by oral means, while mice in the CUR groups received oral drug doses of 50 and 100 mg/kg once a day for 6 days, respectively. CUR could largely improve imiquimod-induced lesions of mice. By using the ELISA and qPCR, we found that the protein and mRNA levels of epidermal TNF-α and IL-6 were inhibited by CUR. The phosphorylation levels of STAT3 and its downstream associated protein levels (eg, Cyclin D1, Bcl-2 and Pim1) in skin tissues of different groups were also inhibited by CUR. Furthermore, the results of immunohistochemistry also showed the repressed effect of CUR for the expression of TNF-α, IL-6 and p-STAT3 in psoriasis-like lesions of mice.
Conclusion: CUR can effectively ameliorate the featured lesions of psoriasis mice, which may be closely associated with the involvement of IL-6/STAT3 signaling.
Keywords: curcumin, psoriasis, IL-6/STAT3, inflammation
Introduction
Psoriasis is a chronic, repeated inflammatory disorder involved by multiple factors.1 The common clinical manifestations of psoriasis are inflammation erythema, covered with silver-white scales, locally or widely distributed throughout the body.2 The patients of psoriasis vulgaris are much common in the human populations worldwide.3 Because of the long course and easy recurrence of psoriasis, it seriously disturbed the mental and physical health of patients.4,5 As a multifunctional cytokine, the expression of IL-6 at psoriatic lesions is significantly elevated and involved in the development of psoriasis. STAT3, one of the major effector mediators of IL-6, is an important regulator of cell proliferation and is essential for the pathogenesis and development of psoriasis. Therefore, IL-6/STAT3 pathway is of great importance in psoriasis pathogenesis.6 Nowadays, the pathogenic mechanism of psoriasis has not been fully understood, and there are no specific drugs to prevent the recurrence of psoriasis in clinical practice. The main therapy method of psoriasis is to alleviate the disease and delay the recurrence. Therefore, finding new drugs and new drug targets for psoriasis will be critical for the ameliorating psoriasis.
At present, psoriasis treatment drugs include methotrexate, cyclosporine, retinoic acid, vitamin D3 derivatives, biological agents and traditional Chinese medicine.7 Biological preparations are expensive, and their long-term use safety needs to be studied because of their short application time, so the application conditions are relatively strict. Considering the side effects of cyclosporine and retinoic acid systems, clinical psoriasis treatment is also relatively cautious.8,9 The curative effect of Chinese traditional medicine is quite different, due to its individual differences, while there exist some drug resistance problems after long-term application of vitamin D3 derivatives.10 Currently, there is no full therapy method for psoriasis. Therefore, the development of new drugs for psoriasis has always been the focus of attention and research direction.
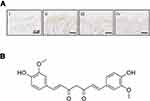
Curcuma longa is an important component extracted from Chinese herbal medicine rhizoma curcumae longae. It can be used to treat many diseases, such as neck and shoulder pain, rheumatic diseases, irregular menstruation and other diseases, and has the functions of blood ventilation, detumescence and pain relief.11 Curcumin (CUR), a plant polyphenol, is the main component of Curcuma longa, its chemical formula is C21H20O6 (Figure 1).12 It is reported that CUR has a promising application, including antioxidant, free radical scavenging, anti-virus, inhibiting tumor growth, analgesic treatment of rheumatism, anti-inflammatory, and protection of important organ functions, etc., which showed a good application prospect in the treatment of diseases,13,14 and has become a hot drug in scientific research of various disciplines. Previous reports have confirmed that CUR could prohibit the proliferation of human keratinocyte cell line (HaCaT) and induce apoptosis,15 but whether IL/STAT3 is involved in the process is still not fully studied. Therefore, the therapeutic mechanism of CUR for psoriasis deserves further study.
Thus, we tried to explore and elucidate the effect mechanism of CUR on imiquimod-induced psoriasis mice in vivo. Our experimental results provided a solid theoretical basis for the clinical application of CUR.
Methods and Materials
Materials and Reagents
Curcumin (purity > 98%) (Shanghai, Yuanye) and 5% imiquimod cream (Sichuan, Mingxin) were obtained from Chinese manufacturers. Vaseline was purchased from Shanghai Shangxi Weikang Pharmaceutical Co., Ltd. (Shanghai, China). Mouse TNF-α (3511-1A-6) ELISA kit was purchased from Mabtech (Sweden). IL-6 (KA3344) ELISA kit was purchased from Abnova (USA). Stat3 (124H6, #9139), phospho Stat3 (Tyr705, #9131) and cyclin D1 (92G2, #2978) were purchased from Cell Signaling Technology. Bcl-2 (C-2, sc-7382) and Pim-1 (12H8, sc-13513) antibody were gained from Santa Cruz Biotechnology. IL-6 (ab6672) antibody was purchased from Abcam.
TNF-α (60291-1-Ig) antibody was gained from Proteintech.
Animals
Sixteen male BALB/c mice (19 ± 3g) were raised by the experimental animal center of the Fudan University (Shanghai, China). All mice were fed under SPF conditions with the appropriate temperature and humidity. The light exposure time was maintained at 12h. During the experiment, the animals were exposed to adequate food and water, and quarantined for one week before experiment. All mice were maintained according to the National Institutes of Health Guidelines of the USA (National Research Council of USA, 1996).
Experimental Procedure
Sixteen male BALB/c mice were randomized into four groups: the control group, model group, and two dosage of CUR groups (50 and 100 mg/kg). All the mice were removed about 2 cm × 3 cm of the back hair. The control group used a vehicle to smear the back skin, and the model and the CUR group were applied with topical 5% imiquimod in the naked mice skin once a day for 6 days (62.5 mg/day/mice).16,17 Meanwhile, the control and model mice were given the same dose of saline by oral means, while mice in the CUR group received oral drug doses of 50 and 100 mg/kg, respectively. The animal experiments were completed on day 6, anesthetize all the mice with 4% isoflurane by the inhaled isoflurane anesthesia machine, and then harvest the skin tissues for marker detection. All the mice will be sacrificed by cervical dislocation. Then, the relevant material was collected.
The whole experiment was conducted at the experimental animal center of Fudan University (Shanghai, China). All experiments were reviewed and supported by the Institutional Animal Care and Use Committee on the Ethics of Animal Experiments of Fudan University (Protocol Number: YZ201709).
PASI Score of Skin Lesions
According to the PASI scoring standard, the erythema, scales and infiltration degree of the lesion in the back skin of the mice were recorded as 0–4 points, and the total score was these three added scores. The criteria are as follows: 0 points, no erythema, scales or even with the skin; 1 point, mild and light erythema, a little fine scales or the lesions of the skin slightly higher than the normal skin; 2 points, moderate and red plaques, skin lesions covered with scales or skin lesions in the form of flakes or moderate protuberance; 3 points, severe and deep red plaques, almost all skin lesions covered by the surface. There were thick layered scales or prominent skin lesions; 4 points, very severe and very deep red patches, all skin lesions were covered with very thick layered scales or skin lesions thickening prominence was very obvious.18
ELISA and Real-Time Quantitative PCR Assays
After ending the experiment, harvest the skin tissues from back sections of mice, stored at −80°C before use. The epidermal protein levels of TNF-α, IL-6 and p-STAT3 were examined according to the advice of the ELISA kit. RNA isolation and mRNA amplification were processed by the advice of manufacturing kit. Broken tissue was added to Trizol (Beyotime) for mixed concussion for full RNA extraction. Subsequently, the extracted RNA was reverse-transcribed into cDNA with the PrimeScriptTM reagent kit (Beyotime). Lastly, qPCR was continued to operate by the real-time PCR system. The whole process to be performed was as described previously.18 GAPDH mRNA was an endogenous control for all experiments. All the primer sequences we used are showed in Table 1.
 |
Table 1 All Sequences of the Primers Used in This Experiment |
Histopathological Analysis
In the center of the whole skin lesions (2 cm × 3 cm of the mice back), take about 0.5 cm of lesion skin and fix it in 10% paraformaldehyde as soon as possible for at least 24 hours. Main steps were conducted as described previously.19 Images were taken at original magnification of 200× (Olympus BX-50 Microscope, Japan, and Leica DMIL, Leica Microsystems, Germany).
Western Blot
The protein expression of Stat3 (1:1000), phosphor-Stat3 (1:1000), cyclin D1 (1:1000), Bcl-2 (1:500) and Pim1 (1:500) in lesion skins was assayed by Western blot. The lesion skin samples were lysate by RIPA lysis buffer (P0013B, Beyotime) through electric grinder on ice bath. Forty milligrams of lesion skin with 160μL of precooled RIPA lysis buffer, homogenized in ice bath, centrifuged at 4°C, 950 g for 6 min. The upper suspension was collected and assessed by the BCA kit (P0012, Beyotime). Take 50μg of protein samples that will run by 12% SDS-PAGE electrophoresis gel. Subsequently, the indicated protein was moved to a pre-trimmed PVDF membrane for antibody incubation. Finally, the indicated proteins were imaged on the device (ChemiScope 6000) via the light-emitting solution.
Immunohistopathological Analysis (IHC)
The 6μm thickness section was prepared as the process of “Histopathological analysis” description. The primary antibody was incubated with the dilution ratio of 1:500 for TNF-α, p-STAT3, and 1:250 for IL-6 at 4°C (no less than 8h), respectively, which was followed by immersion with specific goat anti-mouse antibody at 24 °C for 50 min. The main procedures were as previous showed.18 The magnification of acquired images was 200× in specific device (AttoStar® 4800A).
Statistical Analysis
All data showed in this work were represented as mean ± SD. One-way ANOVA test was considered as an important statistical means with SPSS17.0 software. We performed all the experiments three times independently. In addition, only P < 0.05 was considered to be statistically significant.
Results
Skin Morphology Observation of Mice
After finishing the experiment, we observed the changes of skin lesion in each group. The acquired skin tissues of the mice in untreated group were smooth, with no observed white scales, skin lesions and thickening psoriasis. The skin tissues in the imiquimod-induced group showed a significant erythema, scales and hypertrophic psoriasis-like changes. After CUR treatment, we could see that the changes of the featured skin lesions dramatically alleviate, especially in the 100mg/kg dose of CUR-treated group (Figure 1A).
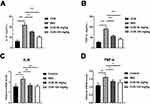
PASI Score Analysis for Psoriasis Model Mice
PASI score is an important index for evaluating the degree of inflammation of psoriasis symptoms.18 In the study, we also evaluate the psoriasis symptoms of mice through PASI score. From Figure 2A–D, we can see that in the control group, the PASI score is 0, which reflected that the skin of the control group mice was smooth, without white scales, skin lesions and thickening psoriasis. While after smear with 5% imiquimod for 6 days, the PASI score was dramatically increased, which means the skin thickening, white scales increasing, skin lesions and deep red skin. In two CUR-treated groups, the PASI score was inhibited, respectively, which significantly alleviated the symptoms of psoriasis mice (Figure 2A–D).
 |
Figure 2 PASI score of the lesion skin in psoriasis mice. (A–D) PASI score comparison between different groups, IMQ vs Cur, *P< 0.05, **P<0.01. |
Histopathological Analysis for Psoriasis Model Mice
In order to further judge the pathological changes of the mice in each group. We continue assayed the skin pathology by H&E staining. In the control group, the epidermis was flat and smooth (Figure 3A). In the model group, the epidermis extended regularly, the lower part of the epidermis became thicker and rod-like, and accompanied by hyperkeratosis and incomplete keratosis in varying degrees, decreased or disappeared granular layer, thickened spinous layer, and mild-to-moderate lymphocyte infiltration in the dermis (Figure 3A). In low dose of CUR group (50 mg/kg), there showed a significantly decreased redness and swelling but still have a small amount of white psoriasis (Figure 3A). The 100 mg/kg group of CUR group significantly alleviated the psoriasis characteristics of the model group, without significant skin swelling and redness, and the phenomenon of skin thickening (Figure 3A). The result showed that CUR could resist psoriasis and had a good inhibitory effect on psoriasis.
IHC Analysis of the Expression of TNF-α and IL-6 Regulated by CUR
Based on the above result, we continue to detect the expression levels of TNF-α and IL-6 in affected skin area (Figure 3B and C). As the result of the epidermal levels, the protein levels of TNF-α and IL-6 were largely heightened in the imiquimod-induced group alone, compared with all samples of the control group (Figure 3B and C). In the low dose of CUR-treated group, the protein levels of TNF-α and IL-6 slightly decreased, in contrast with the imiquimod-induced group alone (Figure 3B and C). While we can see that the high dose of CUR significantly reduced the protein levels of TNF-α and IL-6 in affected lesion area (Figure 3B and C).
The Levels of TNF-α and IL-6 in the Skin Can Be Regulated by CUR
Keratinocytes are the extremely imperative cells in the epidermis and act on a core role in psoriasis. Keratinocytes, stimulated by the outside world, transcribe and translate a great many inflammatory factors (eg, TNF-α, IL-l and IL-6), which induce or exacerbate psoriasis. LPS is an inflammatory stimulator that induces cell inflammation. It initiates a series of inflammatory reactions by activating TLR4 receptor binding, and ultimately activates various pathways leading to the secretion of inflammatory factors.20 To further study the suppressive effect of CUR on inflammation, we assessed the effect of CUR on epidermal TNF-α and IL-6. From Figure 4A and B, we noticed that the epidermal expression of TNF-α and IL-6 was significantly heightened in the imiquimod-induced group mice, while CUR-treated group showed a markedly reduction in the expression of epidermal TNF-α and IL-6. Meanwhile, the mRNA levels of IL-6 and TNF-α also showed the same results after CUR treatment (Figure 4C and D).
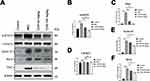
Effect of CUR on STAT3 and Its Downstream Signaling Pathway
Furthermore, based on the above potent therapeutic effect, we continue to analyze the potential mechanism of CUR on psoriasis mice (Figure 5A). We assayed the effect of CUR on the signaling of STAT3 and its downstream signaling pathway, and the result revealed that the expression changes of phosphor STAT3 and the downstream Cyclin D1, Bcl-2 and Pim1 levels were significantly promoted in the imiquimod-induced group alone, in contrast with the normal control group. Importantly, CUR-treated group largely repressed the protein levels of phosphor STAT3, Cyclin D1, Bcl-2 and Pim1 with a dose-dependent manner (Figure 5A). Gray statistics quantified the differences in each protein between different groups (Figure 5B–F). In addition, IHC also revealed that CUR can inhibit the protein levels of p-STAT3 in lesion skin (Figure 6A). We demonstrated that the impact of CUR on the psoriasis mice may be through the regulation of STAT3.
Discussion
Psoriasis is an inflammatory skin disorder prone to recurrence in clinic. Currently, we still do not fully understand the pathogenesis of psoriasis, which seriously disturbs the quality of patients. Moreover, there are no complete cure measures for psoriasis.21
Thus, finding new drugs to treat psoriasis is extremely important for a large number of patients suffering from the disorder. It has been found that psoriasis depends on the interaction between macrophages, activated dendritic cells, T cells and other immune system cells. They can activate multiple cytokines, including IL-1β, TNF-α, IFN-γ, IL-22 and IL-6, etc. Activated cytokines act as specific ligands to bind to the corresponding receptors and coordinate the pathological changes of psoriasis.22
Imiquimod is an agonist activating Toll-like (TLR)-7 and possible immune-activator for treating condyloma acuminatum, and skin basal cell carcinoma. One of its side effects is that it can induce scaly erythema in the epidermis of susceptible people. Scholars have found that the inflammatory changes induced by imiquimod are largely similar to those of psoriasis vulgaris in general lesion characteristics, pathological manifestations and immune mechanisms. Its mechanism is that imiquimod can bind with epidermal plasma-like dendritic cells and TLR-7 within macrophages and activate the production of downstream targets (e g TNF-α, IL-1β, IL-6, IL-22 and IL-23), and to imitate inflammatory changes in psoriasis.23
IL-6, as a proinflammatory cytokine, acts as a vital role in the process of inflammation. IL-6 and its receptor form specific complex, then carry on signal transduction, and then play its biological function.24 Nuclear transcription factor signal transducer and activator of transcription 3 (STAT3) play an imperative role for signal transduction. They are responsible for transporting extracellular signals to the nucleus and exerting biological effects by inducing transcription and expression of target genes. The combination of IL-6 with its specific receptor IL-6R changes the conformation of IL-6R and then binds to signal transduction protein gp130, resulting in the formation of gp130 homologous dimer.25 The dimerization of gp130 makes the coupled JAK kinases close to each other and activates through the interaction of tyrosine phosphorylation. IL-6 is the key driver of the JAK/STAT3 signaling, which is involved in psoriasis pathogenesis.26 The activated JAK kinase can also catalyze the phosphorylation of tyrosine residues of gp130. Gp130 then phosphorylated STAT3 carboxyl-terminal tyrosine residues by binding phosphorylated tyrosine residues to STAT3 and under the action of JAK kinase. Two-molecule phosphorylated STAT3 forms dimer through the interaction between arginine in SH2 domain and phosphorylated tyrosine, leaves receptor and enters nucleus, connects to a specific promoter region of aimed gene and promotes transcription and expression of corresponding gene.27
The important target gene products of STAT3 include cyclin D1, Bcl-2 and Pim1.28 Previous reports have confirmed that promoting expression of Bcl-2 is a key cause of the occurrence and development of multiple tumors. Antagonistic effects between regulated apoptosis-related proteins in the BCL-2 family determined the extent of cellular response to apoptotic stimuli. Bad, as a member of promoting apoptosis in the Bcl-2 family, may suppress the effect of apoptotic inhibition of Bcl-2 via the interaction between proteins. Pim-1 kinase could directly promote the phosphorylation of Bad and repress its combination with Bcl-2, thereby eliminating the inhibition of Bad on Bcl-2.29 The relationship between Cyclin D1 and the proliferation of keratinocytes in psoriasis has been reported. The main factors involved in cell cycle regulation are Cyclin, CDKs and CDKIs.30 Cyclin D1 is a positive regulator of cell cycle G1/S phase transition and a key protein of cell proliferation signal in G1 phase. Cyclin D1 binds to CDK4/6 and leads cells to G1 phase. Cyclin D1 overexpression shortens the G1/S phase transition time of cell cycle and promotes cell proliferation. The expression of Cyclin D1 in psoriasis vulgaris was significantly higher than that in normal subjects, suggesting that Cyclin D1 is associated with the excessive proliferation of keratinocytes in psoriasis vulgaris.31
In our study, we first analyzed the impact of CUR in imiquimod-induced psoriasis-like lesions of mice. As shown in Figure 6B, CUR exhibited a wonderful therapy effect on psoriasis mice despite its simple chemical structure, which evidently suppressed the PASI scores, inhibited the lesional levels of TNF-α and IL-6, remarkably alleviate the symptoms in the psoriasis. Furthermore, we continue to clarify the potential mechanisms of CUR on psoriasis mice. We revealed that CUR group mice could evidently reduce the protein levels of phosphor STAT3, cyclin D1, Bcl-2 and Pim1. It can also reduce the protein levels of TNF-α and IL-6 in affected lesion area.
Although this study lacked direct evidence that CUR exerted its psoriasis-relieving effect via the IL-6/STAT 3 pathway, it demonstrated that the therapeutic effect of CUR could be tightly associated with this pathway. Taken together, the whole study implied that CUR may be an effective drug for the therapeutics of treating psoriasis in the future. Moreover, further studies will be needed to explore the specific mechanisms by which curcumin exerts its therapeutic effects.
Ethics Approval and Consent to Participate
All experiments related animals in the manuscript were reviewed and approved by the Institutional Animal Care and Use Committee on the Ethics of Animal Experiments of Fudan University (Protocol Number: YZ201709).
Acknowledgments
We are very grateful to the kindly animal care and the technical method guide by the workers in the animal facility of Fudan University.
Funding
The study was supported by the grant of Natural Science Foundation of Shanghai (YKHHFX01190408), High-level Backbone Physicians Training Program of Minhang District (2020MZYS18) and Minhang District Natural Science Research project (2022MHZ076).
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nature Rev. 2016;2:16082. doi:10.1038/nrdp.2016.82
2. Loft ND, Andersen CH, Halling-Overgaard AS, Thyssen JP, Skov L, Egeberg A. Validation of psoriasis diagnoses in the Danish national patient register. Acta Derm Venereol. 2019;99(11):1037–1038. doi:10.2340/00015555-3278
3. Shrivastava VK, Londhe ND, Sonawane RS, Suri JS. First review on psoriasis severity risk stratification: an engineering perspective. Comput Biol Med. 2015;63:52–63. doi:10.1016/j.compbiomed.2015.05.005
4. Oji V, Luger TA. The skin in psoriasis: assessment and challenges. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S14–S19.
5. Yi F, Zheng X, Fang F, Zhang J, Zhou B, Chen X. ALA-PDT alleviates the psoriasis by inhibiting JAK signalling pathway. Exp Dermatol. 2019;28(11):1227–1236. doi:10.1111/exd.14017
6. Miao X, Xiang Y, Mao W, Chen Y, Li Q, Fan B. TRIM27 promotes IL-6-induced proliferation and inflammation factor production by activating STAT3 signaling in HaCaT cells. Am J Physiol Cell Physiol. 2020;318(2):C272–C281. doi:10.1152/ajpcell.00314.2019
7. Hou M, Xing H, Cai Y, et al. Short-term effect and safety of a new generation of monoclonal antibodies targeting interleukin-23p19 for treatment of psoriasis: a systematic review and meta-analysis. Eur J Dermatol. 2019;29(3):302–314. doi:10.1684/ejd.2019.3553
8. Nogueira M, Torres T. Guselkumab for the treatment of psoriasis - evidence to date. Drugs Context. 2019;8:212594. doi:10.7573/dic.212594
9. Mikhaylov D, Hashim PW, Nektalova T, Goldenberg G. Systemic psoriasis therapies and comorbid disease in patients with psoriasis: a review of potential risks and benefits. J Clin Aesthet Dermatol. 2019;12(6):46–54.
10. Nagata M, Kamata M, Ohtsuki M, Sato S, Tada Y. Scalp psoriasis in a haemodialysis patient successfully treated with a half-dose of apremilast. Eur J Dermatol. 2019;29(3):341–342. doi:10.1684/ejd.2019.3588
11. Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The Essential Medicinal Chemistry of Curcumin. J Med Chem. 2017;60(5):1620–1637. doi:10.1021/acs.jmedchem.6b00975
12. Lestari ML, Indrayanto G. Curcumin. Profiles Drug Subst Excip Relat Methodol. 2014;39:113–204.
13. Tsuda T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018;9(2):705–714. doi:10.1039/C7FO01242J
14. Bachmeier BE, Melchart D. Therapeutic effects of curcumin-from traditional past to present and future clinical applications. Int J Mol Sci. 2019;20(15):3757. doi:10.3390/ijms20153757
15. Gong Z, Zhou J, Li H, et al. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Mol Nutr Food Res. 2015;59(11):2132–2142. doi:10.1002/mnfr.201500316
16. Baek JO, Byamba D, Wu WH, Kim TG, Lee MG. Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch Dermatol Res. 2012;304(9):699–706. doi:10.1007/s00403-012-1272-y
17. Wu JK, Siller G, Strutton G. Psoriasis induced by topical imiquimod. Australas J Dermatol. 2004;45(1):47–50. doi:10.1111/j.1440-0960.2004.00030.x
18. Shao F, Tan T, Tan Y, Sun Y, Wu X, Xu Q. Andrographolide alleviates imiquimod-induced psoriasis in mice via inducing autophagic proteolysis of MyD88. Biochem Pharmacol. 2016;115:94–103. doi:10.1016/j.bcp.2016.06.001
19. Cai Z, Zeng Y, Shi X, Zhang X, Zhu H, Wang W. Benvitimod inhibits MCM6-meditated proliferation of keratinocytes by regulating the JAK/STAT3 pathway. J Dermatol Sci. 2023;109(2):71–79. doi:10.1016/j.jdermsci.2023.01.010
20. Hollywood KA, Winder CL, Dunn WB, et al. Exploring the mode of action of dithranol therapy for psoriasis: a metabolomic analysis using HaCaT cells. Mol Biosyst. 2015;11(8):2198–2209. doi:10.1039/C4MB00739E
21. Morar II, Tabăran FA, Mocan T, et al. Immunohistochemical study of psoriatic plaques and perilesional skin in psoriasis vulgaris patients: a pilot study. Exp Ther Med. 2019;18(2):888–894. doi:10.3892/etm.2019.7596
22. Pușcaș AD, Cătană A, Pușcaș C, et al. Psoriasis: association of interleukin-17 gene polymorphisms with severity and response to treatment. Exp Ther Med. 2019;18(2):875–880. doi:10.3892/etm.2019.7624
23. Bocheńska K, Smolińska E, Moskot M, Jakóbkiewicz-Banecka J, Gabig-Cimińska M. Models in the research process of psoriasis. Int J Mol Sci. 2017;18(12). doi:10.3390/ijms18122514
24. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi:10.1101/cshperspect.a016295
25. Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci. 2012;122(4):143–159. doi:10.1042/CS20110340
26. Horsten U, Schmitz-Van de Leur H, Müllberg J, Heinrich PC, Rose-John S. The membrane distal half of gp130 is responsible for the formation of a ternary complex with IL-6 and the IL-6 receptor. FEBS Lett. 1995;360(1):43–46. doi:10.1016/0014-5793(95)00053-C
27. Moshapa FT, Riches-Suman K, Palmer TM. Therapeutic targeting of the proinflammatory IL-6-JAK/STAT signalling pathways responsible for vascular restenosis in type 2 diabetes mellitus. Cardiol Res Pract. 2019;2019:9846312. doi:10.1155/2019/9846312
28. Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016;31:1–15. doi:10.1016/j.cytogfr.2016.05.001
29. Jan R, Chaudhry GE. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharma Bull. 2019;9(2):205–218. doi:10.15171/apb.2019.024
30. Song JY, Song L, Herrera AF, et al. Cyclin D1 expression in peripheral T-cell lymphomas. Modern Pathol. 2016;29(11):1306–1312. doi:10.1038/modpathol.2016.136
31. Kim SA, Ryu YW, Kwon JI, Choe MS, Jung JW, Cho JW. Differential expression of cyclin D1, Ki‑67, pRb, and p53 in psoriatic skin lesions and normal skin. Mol Med Rep. 2018;17(1):735–742. doi:10.3892/mmr.2017.8015
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.