Back to Journals » International Journal of Nanomedicine » Volume 13 » T-NANO 2014 Abstracts
Curcumin Ag nanoconjugates for improved therapeutic effects in cancer
Authors Shah D, Savaliya R, Patel P, Kansara K, Pandya A, Dhawan A, Singh S
Received 14 October 2016
Accepted for publication 21 November 2016
Published 15 March 2018 Volume 2018:13(T-NANO 2014 Abstracts) Pages 75—77
DOI https://doi.org/10.2147/IJN.S124696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Darshini Shah, Reema Savaliya, Pal Patel, Krupa Kansara, Alok Pandya, Alok Dhawan, Sanjay Singh
Institute of Life Sciences, School of Science and Technology, Ahmedabad University, Ahmedabad, Gujarat, India
Abstract: Curcumin has a broad spectrum of pharmacological activities, one of them is anticancer activity that is mediated through multiple mechanisms. The major disadvantage associated with the use of curcumin is its low bioavailability due to its poor aqueous solubility. Nanoformulations of curcumin provide an effective solution for this problem. In this study, we have synthesized curcumin Ag nanoconjugates and evaluated their anticancer potential.
Keywords: curcumin, anticancer, nanoconjugate, AgNPs
Introduction
The major challenge faced by modern scientific and medical research is the need to develop new anticancer treatments with greater efficacy and fewer side effects.1 Natural products have been widely used by our ancestors to combat illness, and that trend has been growing over the past few decades. Current medicines have been revolutionary in curing diseases, but their harmful side effects have led to the use of natural products. Curcumin is one of the many natural products containing such beneficiary properties. Curcumin is a polyphenol derived from Curcuma longa plant, commonly known as turmeric. Curcumin has been used extensively in Ayurvedic medicine for centuries, as it is nontoxic and has a variety of therapeutic properties including antioxidant, analgesic, anti-inflammatory, and antiseptic activities.2 Recently, curcumin has been shown to possess anticancer activities through its effect on a variety of biological pathways. It has not been clinically used yet, due to its sparing solubility in water and low bioavailability. Various studies have reported that the bioavailability of curcumin could be increased by conjugating it with metal nanoparticles (NPs). Hence, in this study, we have made an attempt to synthesize AgNPs functionalized by curcumin and analyze its anticancer properties. The effects of curcumin-reduced AgNPs (C-AgNPs), L-tyrosine-reduced AgNPs (T-AgNPs), and free curcumin on the viability of human epidermoid carcinoma cell line (A431 cells) was compared.
Materials and methods
Curcumin AgNPs synthesis
C-AgNPs were synthesized using the method described by Bettini et al with slight modifications.3 Briefly, AgNO3 (10−3 M) was mixed with 10−5 M curcumin solution. The pH was adjusted to 9 using 1 M NaOH, and the solution was allowed to boil until the color changed to orangish yellow.
Synthesis of T-AgNPs
T-AgNPs were synthesized using the method described by Selvakannan et al with slight modifications.4 Briefly, AgNO3 (10−3 M) solution was mixed with 10−3 M aqueous solution of L-tyrosine in 1:1 ratio. The pH was adjusted to 10 using 1 M NaOH, and this solution was allowed to boil until the color changed to yellow.
Characterization of AgNPs
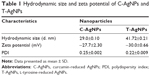
C-AgNPs and T-AgNPs were dialyzed for 24 h by 12 kDa dialysis bag against the copious amount of deionized water. This removed the unreduced ions of Ag and other reaction products, which might have formed during the NPs synthesis. Dialysis also decreased the pH (~7) of NPs suspension, which was alkaline (>9) as required by NPs synthesis. Hydrodynamic size and zeta potential of C-AgNPs and T-AgNPs were determined using Zetasizer Nano-ZS, and absorbance spectrum was determined using UV–visible spectrophotometer (Synergy HT multi-mode microplate reader).
Cell viability assessment
The effects of curcumin, C-AgNPs, and T-AgNPs was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay on A431 cell line.5 A431 cells were commercially purchased from the National Center for Cell Sciences, Pune, India.
Results and discussion
The hydrodynamic size and zeta potential of C-AgNPs and T-AgNPs were found to be 29.0±0.10, −27±2.30 and 41.72±0.21, −30.0±0.66, respectively (Table 1). The synthesized AgNP solutions were stable for more than a month. High zeta potential value further supports the high stability of AgNP solutions.
The UV–visible absorbance (Figure 1) spectrum of C-AgNPs and T-AgNPs along with their respective controls was recorded as shown in Figure 1. The maximum absorbance of C-AgNPs was found to be 420 nm, and it coincides with that of T-AgNPs, indicating that AgNPs have been successfully synthesized. Other solutions such as curcumin, AgNO3–NaOH, curcumin–AgNO3, and curcumin–NaOH after extensive boiling showed absorbance at 425 nm, 530 nm, 425 nm, and 460 nm. However, as clearly evident in Figure 1, the absorbance intensity of these solutions was found to be very weak and did not match with the absorbance of AgNPs.
  | Figure 1 UV–visible spectrum of C-AgNPs and T-AgNPs along with controls. |
Human skin carcinoma cells (A431) were exposed to different concentrations of free curcumin, T-AgNPs, and C-AgNPs for 24 h, and cell viability was determined using MTT assay. Here, curcumin concentration corresponds to the amount of curcumin required to synthesize 40 and 60 μg/mL of C-AgNPs (Figure 2). Free curcumin showed only marginal decrease in cell viability. However, T-AgNPs showed a concentration-dependent decrease in cell viability ranging from 20% (at 40 μg/mL) to 37% (at 60 μg/mL). However, exposure to C-AgNPs leads to significant reduction in cell viability ranging from 44% to 98.5% at 40 and 60 μg/mL concentrations, respectively (Figure 2). This observation suggests that the synergistic anticancer effect of curcumin and AgNPs leads to cell death in A431 cells.
  | Figure 2 Cell viability assessment of curcumin, T-AgNPs, and C-AgNPs on A431 cells. |
Conclusion
C-AgNPs and T-AgNPs are found to be toxic to the skin carcinoma (A431) cells than free curcumin at higher concentration (60 μg/mL). Promising anticancer activity of C-AgNPs suggests its potential to be used as a chemotherapeutic agent for cancer treatment.
Acknowledgments
The authors gratefully acknowledge the Centre for Nanotechnology Research and Applications (CENTRA) by The Gujarat Institute for Chemical Technology (GICT). RS would like to thank the University Grant Commission (UGC), New Delhi, for the award of Junior Research Fellowship. The manuscript contains ILS-manuscript No 045.
Disclosure
The authors report no conflicts of interest in this work.
References
Salem M, Rohani S, Gillies ER. Curcumin, a promising anti-cancer therapeutic: a review of its chemical properties, bioactivity and approaches to cancer cell delivery. RSC Advances. 2014;4(21):10815–10829. | ||
Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10(12):1–19. | ||
Bettini S, Pagano R, Valli L, Giancane G. Drastic nickel ion removal from aqueous solution by curcumin-capped Ag nanoparticles. Nanoscale. 2014;6(17):101–113. | ||
Selvakannan PR, Swami A, Srisathiyanarayanan D, et al. Synthesis of aqueous Au core-Ag shell nanoparticles using tyrosine as a pH-dependent reducing agent and assembling phase-transferred silver nanoparticles at the air-water interface. Langmuir. 2004;20(18):7825–7836. | ||
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferate and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.