Back to Journals » Cancer Management and Research » Volume 12
Curcumin Affects Gastric Cancer Cell Migration, Invasion and Cytoskeletal Remodeling Through Gli1-β-Catenin
Authors Zhang X, Zhang C, Ren Z, Zhang F , Xu J, Zhang X, Zheng H
Received 31 December 2019
Accepted for publication 8 April 2020
Published 21 May 2020 Volume 2020:12 Pages 3795—3806
DOI https://doi.org/10.2147/CMAR.S244384
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Xiao Zhang,1,* Chenli Zhang,1,* Zhiheng Ren,1 Fangfang Zhang,1 Jinyu Xu,1 Xu Zhang,1 Haixue Zheng2
1Department of Pathology, School of Basic Medicine, Lanzhou University, Lanzhou, Gansu 730000, People’s Republic of China; 2National Foot and Mouth Diseases Reference Laboratory, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, Gansu 730000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xu Zhang
Department of Pathology, School of Basic Medicine, Lanzhou University, 222 Tianshui Road, Lanzhou, Gansu 730000, People’s Republic of China
Email [email protected]
Haixue Zheng
National Foot and Mouth Diseases Reference Laboratory, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, 1 Yanchang Road, Lanzhou, Gansu 730000, People’s Republic of China
Email [email protected]
Purpose: The function of curcumin on the gastric cancer cell line, SGC-7901 is unknown. The present study aimed to observe the effects of curcumin on gastric cancer cells through the Shh and Wnt signaling pathways.
Methods: SGC-7901 cells were transfected with si-Gli1 and si-β-catenin siRNA, then cells were stimulated with curcumin and its effects on cell migration, invasion, cytoskeleton remodeling, EMT, apoptosis and cell cycle were investigated by transwell assays, immunofluorescence and flow cytometry assays. The interaction between Gli1 and β-catenin was observed by co-immunoprecipitation.
Results: We show that curcumin suppressed the expression of Shh, Gli1 and Foxm1 in the Shh signaling pathway, and the expression of β-catenin in the Wnt signaling pathway in SGC-7901 cells, both in mRNA and protein. As a result, cellular migration, invasion and cytoskeletal remodeling ability decreased. Our results revealed that when stimulated with curcumin, cells showed decreased cellular migration and invasion, while enhanced apoptosis. In addition, curcumin induced cytoskeletal remodeling and S phase cell cycle arrest. The inhibition of Shh and Wnt signaling pathway and the addition of curcumin also inhibited the epithelial–mesenchymal transition process. Furthermore, a physical interaction was observed between Gli1 of the Shh signaling and β-catenin of the Wnt signaling in these cells, but curcumin inhibited the interaction of these two proteins.
Conclusion: The present study indicated that curcumin plays an anti-tumor role through Gli1-β-catenin pathway in gastric cancer SGC-7901 cells.
Keywords: curcumin, Gli1, β-catenin, migration, invasion, cytoskeleton
Introduction
Malignant tumors have become the leading cause of death in humans.1 Gastric cancer is one of the most common types of cancer according to a ten-year tumor statistics analysis from Wuwei district, Gansu province, China.2 Most patients with gastric cancer are diagnosed at an advanced stage due to lack of early symptoms and the limitations in screening programs.3 However, lack of effective treatments for gastric cancer and the challenge of chemotherapy resistance are still great problems in gastric cancer therapy. Therefore, it is important to understand the molecular mechanisms behind gastric cancer and explore new therapeutic drugs.
Curcumin is extracted from turmeric and used widely in India and China.4 The biological effects of curcumin are primarily anti-inflammatory,5 anti-oxidative6 and anti-cancer.7 The antitumor effect of curcumin is widely studied.8,9 Curcumin exerts pharmacological effect by acting on a variety of signaling pathway molecules.10–15 It has been reported that curcumin have anti-tumor effect by modulate immune T cells,16 In addition, curcumin can also play an anti-tumor effect by regulating various microRNAs in different cancers.17
The sonic hedgehog (Shh) signaling pathway plays an important role in embryonic development, adult tissue maintenance and oncogenesis.18,19 Shh canonical signaling occurs when Shh binds to Ptch1, Smo inhibition is abolished and the Shh signal is transmitted downstream of Smo by a cytoplasmic protein complex, composed of kinin (Kif7), fusion inhibitor (Sufu) and GliFL.20 Smo signals Sufu to release the Gli activator (GliA). Gli migrates to the nucleus and activates the expression of target genes such as Foxm1, cell cycle regulators (cyclinD1) and apoptosis regulator (Bcl2).21 Studies have shown that the Shh signaling pathway plays an important key role in the progression of many cancers.22–25
The abnormal activation of Wnt signaling is associated with a variety of diseases, particularly cancer.26 In the canonical Wnt signaling pathway, Wnt proteins bind to the FZD transmembrane receptor and cellular Dsh to form a complex. The Wnt/FZD/Dsh complex prevents phosphorylation of β-catenin by inhibiting GSK-3β activity. β-catenin is further degraded by ubiquitination and accumulates in the cytoplasm, from where it translocates to the nucleus, promoting target gene transcription.26,27
Several studies have shown that Notch signaling,28 Shh signaling21 and Wnt signaling29 play important roles in tumor formation. Our laboratory has previously demonstrated that curcumin affects gastric cancer cells, via the Notch signaling pathway.30 However, whether curcumin affects gastric cancer cells via the Shh and Wnt signaling pathways remains unknown. Our data show that inhibition of the Shh and Wnt signaling pathways affects the migration and invasion of SGC-7901 gastric cancer cells. Additionally, curcumin inhibits the proliferation, migration, invasion and epithelial–mesenchymal transition (EMT) processes, and cytoskeletal remodeling in gastric cancer cells. We explored physical interactions between Gli1 of the Shh signaling pathway and β-catenin of the Wnt signaling pathway, providing novel insights for the development of molecular targets for gastric cancer.
Materials and Methods
Cell Culture and Reagent
The human gastric cancer cell line, SGC-7901 was obtained from the Laboratory of Pathology, School of Basic Medical, Lanzhou University (Lanzhou, China),31 and the cells were authenticated by STR. Cells were cultured in RPIM-1640 (HyClone, UT, USA) supplemented with 10% fetal bovine serum (FBS; Kibbutz Beit Haemek, Israel) and 1% penicillin/streptomycin (Sigma-Aldrich, MO, USA) in a humidified atmosphere of 5% CO2 at 37°C. Curcumin and a CCK-8 kit were purchased from Beijing Solarbio Science & Technology (Beijing, China).
Primary antibodies included: Anti-Shh (Abcam, Cambridge, UK), anti-Gli1 antibody (Abcam), anti-Foxm1 antibody (Abcam), anti-β-catenin antibody (Cell Signaling Technology, MA, USA), anti-E-Cadherin antibody (Cell Signaling Technology), anti-vimentin antibody (Cell Signaling Technology), anti-F-actin antibody (Abcam) and anti-β-actin antibody (Thermo Fisher Scientific, MA, USA). Secondary antibodies included: HRP-labeled goat anti-rabbit IgG (Abcam) and HRP-labeled goat anti-mouse IgG (Abcam). All the primary antibodies were diluted to 1:1000. The secondary antibodies were diluted to 1:5000.
Cell Transfection
Small interfering RNAs (siRNA) for transfection assays were synthesized by Gene Pharma (Shanghai, China). The knockdown of Gli1 and β-catenin was performed by the transfection of si-Gli1 and si-β-catenin into SGC-7901 cells. NC siRNA was used as a negative control. SiRNA transfection was performed using Lipofectamine 2000® (Thermo Fisher Scientific) according to the manufacturer’s protocol. The following siRNA primers were used;
Negative control sense: 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ, antisense 5ʹ-ACGUGACACGUUCGGAGAATT-3ʹ,
Gli1 siRNA Sense: 5ʹ-CCAGGAAUUUGACUCCCAATT-3ʹ and Antisense: 5ʹ-UUGGGAGUCAAAUUCCUGGCT-3ʹ;
β-catenin siRNA: 5ʹ-GGACCUAUACUUACGAAAATT-3ʹ, antisense 5ʹ-UUUUCGUAAGUAUAGGUCCTC-3ʹ.
Cell Proliferation Assay (CCK-8)
SGC-7901 cells were seeded into 96 well plates (Corning, NY, USA) (5000 cells/well), and divided into a control group and curcumin treatment groups (10, 20, 40, and 80 µM). After incubation at 5% CO2 at 37°C for 48 h, 10 µL CCK-8 solution was added to each well and further incubated for 2 h. Absorbance values were measured at 490nm using a microplate reader (Flash Spectrum Biotechnology, Shanghai, China) and the cell survival rate was calculated using the formula: [Cell Viability (%) = (A experiment-A blank)/(A control-A blank) x100].
RNA Extraction and Reverse Transcription-QuantitativePCR (RT-qPCR)
Total RNA was extracted from cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific) following manufacturer’s instructions. cDNA was synthesized using M-MLV reverse transcriptase (Promega Corporation, WI, USA) and random hexamer primers (Takara, Otsu, Japan). The Mx3005P qPCR System (Agilent Technologies, CA, USA) and SYBR Premix Ex Taq reagents (Takara, Dalian, China) were used for qPCR, according to manufacturer’s instructions. Theromocyling conditions consisted of 95°C for 2 min (hold stage); 95°C for 10 sec, 60°C for 34 sec (40 cycles, PCR stage); 95°C for 15 sec, 60°C for 1 min, 95°C for 1 sec (melt curve stage). Relative mRNA expression levels were normalized to GAPDH using the comparative cycle threshold 2−ΔΔCT method.32 All primer sequences are shown in Table 1.
 |
Table 1 qPCR Primers Used in This Study |
Western Blotting and Co-immunoprecipitation (Co-IP)
After transfection and curcumin treatment, cell protein was extracted and protein concentration was measured using BCA protein assay kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s protocol. Protein lysates were loaded and separated on 10% SDS-polyacrylamide gels, then transferred 2 h in cold transfer buffer. Membranes were blocked in 5% fat-free milk for 2 h at room temperature and then incubated with primary antibodies and secondary antibodies as described previously.33 Protein bands were visualized using enhanced chemiluminescence detection reagent (Thermo Fisher Scientific) and Image Lab™ software 4.1 (Bio-Rad Laboratories, CA, USA). The protein bands were analyzed as a percentage of β-actin levels.
For Co-IP assays, cells were cultured in 10 cm dishes (Corning) and divided into untreated (mock) and curcumin-treated groups for 48 h. The cells were lysed in RIPA lysis buffer (Beijing Solarbio Science & Technology) on ice for 30 min, and precleared with protein-G agarose beads (Sigma) for 4 h at 4°C. Lysates were then incubated overnight at 4°C with anit-Gli1 or anti-β-catenin primary antibodies, on a rotating wheel. The next day, antibody-antigen complexes were analyzed by Western blotting as described.
Cell Migration and Invasion Assays
SGC-7901 cell migration and invasion assays were performed in 24 well transwell chambers (Corning) containing polycarbonate filters with 8-µm pores, with or without Matrigel (BD Biosciences, NJ, USA). Matrigel was mixed with serum-free RIPM-1640 (1:8 ratio) in upper chambers, and incubated at 37°C for 2 h. SGC-7901 cells were suspended in serum-free RPMI-1640 medium at a density of 2x105 cells. Approximately 100 uL of this serum-free cell suspension was added to the upper chamber, and 800 µL 20% serum-containing medium was added to the lower chamber. The cells were incubated at 37°C, 5%CO2 for 48 h and later fixed in 4% paraformaldehyde for 30 min. They were then stained in 0.1% crystal violet for 15 min. After this period, surface crystal violet was removed using cotton swabs, and cells that had passed through the membrane were counted and photographed under a microscope (magnification, x100). The experiment was repeated three times and five images were taken for each sample. Cell numbers that had crossed the membrane were counted for statistical analysis.
Immunofluorescence Assay (IFA)
After transfection and curcumin treatment for 48 h, SGC-7901 cells were plated into Nunc glass-bottom dishes (Thermo Fisher Scientific). Cells were washed in PBS and fixed in 4% paraformaldehyde for 30 min at room temperature. The cell cytoskeleton was stained with fluorescein isothiocyanate (FITC)-phalloidin (Sigma) for 40 min and nuclei were stained with DAPI (Sigma) at room temperature for 5 min, in the dark. Finally, cells were analyzed and imaged on a fluorescent microscope (magnification, x63).28
Cell Cycle and Apoptosis Assays Using Flow Cytometry
For the cell cycle assay, SGC-7901 cells were transfected with Gli1 or β-catenin siRNA and treated with curcumin for 48 h. Cells were then digested in 0.25% EDTA-free trypsin and washed in PBS, before fixing in 70% alcohol at 4°C overnight. Cells were then incubated in an RNAse-free buffer containing propidium iodide (PI) (Beyotime Biotechnology) and quantified by flow cytometry (BD FACSverse™). All data were processed in Modfit LT™ software.
For the cell apoptosis assay, SGC-7901 cells were treated as above and digested in 0.25% EDTA-free trypsin. Cells were then stained with an FITC Annexin V Apoptosis Detection Kit (BD Biosciences) in the dark and quantified using flow cytometry.
Statistical Analysis
Each assay was repeated at least three times. Measured values are represented as Mean ± SD, from three independent experiments. Student’s t-tests, of GraphPad Prism software 7 (GraphPad Software, CA, USA), were used to compare groups. Data were considered significant when ∗P < 0.05, and highly significant when ∗∗P < 0.01.
Results
Curcumin Inhibits the Proliferation of SGC-7901 Cells and Shh and Wnt Signaling Pathways
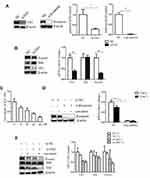
To verify the effects of Shh and Wnt signaling on the biological behavior of gastric cancer cell line SGC-7901, we knocked down the key transcription factors Gli1 in the Shh signaling pathway and β-catenin in the Wnt signaling pathway. SGC-7901 cells were transfected with siRNA, targeting Gli1 and β-catenin expression. At 48 h post-transfection (hpt), cells were collected to analyze the expression of associated factor by Western blotting and qPCR. The results indicated that Gli1 and β-catenin expression was successfully knocked down by Gli1 siRNA and β-catenin siRNA, respectively (Figure 1A). In addition, the knockdown of Gli1 decreased Foxm1 expression in the Shh signaling pathway (Figure 1B).
Curcumin has been shown to inhibit the proliferation of cancer cells.34,35 In the study, SGC-7901 cells were plated in 96 well plates and stimulated with 10, 20, 40 and 80 µM curcumin for 48 h. The effects of curcumin on cell proliferation were observed by CCK-8 assay. Our results showed that the growth-inhibiting effect of curcumin on SGC-7901 cells was dose-dependent (Figure 1C). The medial IC50 was 32 µM curcumin for these cells. Therefore, a 30 µM curcumin dose and 48 h incubation range were used in subsequent experiments.
We also detected the expression of Gli1, Foxm1, and β-catenin in cells that were transfected with Gli1 or β-catenin siRNA, and treated with curcumin. The curcumin significantly reduced the expression of Shh, Gli1, Foxm1, and β-catenin at the protein and mRNA levels (Figure 1D and E). Taken together, these results suggest that curcumin inhibited the proliferation of SGC-7901 cells and reduced the expression of Shh, Gli1 and Foxm1 in the Shh signaling pathway and β-catenin in Wnt signaling pathway.
Inhibition of Shh and Wnt Signaling Pathways and Curcumin Stimulation Affect the Migration and Invasion of SGC-7901 Cells
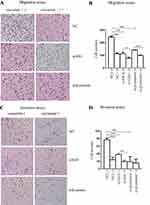
To implicate inhibition of Shh and Wnt signaling, as well as the effects of curcumin on the migration and invasion of SGC-7901 cells, SGC-7901 cells were transfected with Gli1 or β-catenin siRNAs and treated with curcumin for 48 h. They were then transferred to transwell chambers (without Matrigel for the migration assay and with Matrigel for the invasion assay) to observe cell numbers crossing the 8-µm pores membranes. As shown (Figure 2A and B), transmembrane cells were reduced for Gli1 or β-catenin siRNA-treated cells in the migration assay when compared to NC siRNA-treated cells. Transmembrane cell numbers for curcumin-treated cells were significantly decreased when compared with untreated cells.
For the invasion assay, transmembrane cell numbers were significantly reduced for Gli1 or β-catenin siRNA-transfected cells when compared with NC siRNA-transfected cells. Similar results were also observed for the curcumin-treated group when compared with the non-treated group (Figure 2C and D). Collectively, the decreased expression of Gli1 in the Shh signaling pathway and the decreased expression of β-catenin in the Wnt signaling pathway, and curcumin treatment, all inhibited SGC-7901 migration and invasion.
Inhibition of Shh and Wnt Signaling Pathways and Curcumin Stimulation Treatment Regulate Apoptosis and Cell Cycle Arrest of SGC-7901 Cells
To explore the effects of Shh and Wnt signaling and curcumin on SGC-7901 apoptosis and cell cycle, cells were plated into six-well plates and transfected with Gli1 siRNA or β-catenin siRNA and treated with curcumin for 48 h. The cells were stained with FITC Annexin V/PI and quantified by flow cytometry. No significant changes in cellular apoptosis rates were observed in Gli1 or β-catenin siRNA-treated cells, when compared to NC siRNA-treated cells. However, apoptosis rates were significantly increased in curcumin-treated cells when compared to the untreated group (Figure 3A and C).
Then, we performed cell cycle assays to investigate apoptotic mechanisms induced by curcumin. SGC-7901 cells were transfected with Gli1 or β-catenin siRNA and treated with curcumin for 48 h. Cells were then stained with PI and quantified by flow cytometry. There was a reduction of Gli1 induced cell cycle arrest at the G0/G1 phase, while the reduction of β-catenin induced cell cycle arrest at the S phase. Cell cycle arrest at the S stage increased significantly in curcumin-treated cells when compared with the non-treated group (Figure 3B and D). Collectively, these data indicated that curcumin-induced apoptosis and cell cycle arrest at the S stage in SGC-7901 cells. The reduction in Gli1 and β-catenin expression-induced cell cycle arrest, but had no significant effects on apoptosis.
Inhibition of Shh and Wnt Signaling Pathways and Curcumin Stimulation Affect the EMT Process and Cytoskeletal Remodeling in SGC-7901 Cells
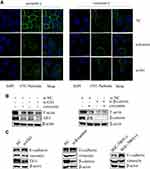
The impact of curcumin on the gastric cancer cell cytoskeleton is unknown. IFA was performed to detect the impact of Shh and Wnt signaling and curcumin on the cytoskeleton of SGC-7901 cells. Cells were transfected with Gli1 or β-catenin siRNA and treated with curcumin for 48 h. Cells were then observed under a confocal microscope. Pseudopods and skeleton fibers on the cell membrane surface were significantly reduced in curcumin-treated cells when compared with untreated cells. Similar transfections were performed as described above, showing that skeleton fibers in Gli1 or β-catenin siRNA-transfected cells were slightly decreased when compared to NC siRNA-transfected cells (Figure 4A). To further confirm this effect, SGC-7901 cells were transfected with Gli1 siRNA or β-catenin siRNA and treated with curcumin for 48 h. The cells were then analyzed for F-actin expression by Western blotting. F-actin was decreased in curcumin-treated cells when compared with non-treated cells. Similar results were observed for Gli1 or β-catenin siRNA-transfected cells when compared to NC siRNA-transfected cells (Figure 4B).
The EMT process plays an important role in the development of malignant tumors. We examined expression changes in the molecules E-cadherin and vimentin, which are associated with EMT. To investigate the effects of Shh and Wnt signaling and curcumin on EMT in SGC-7901 cells, cells were transfected with siRNAs targeting Gli1 or β-catenin expression and treated with curcumin for 48 h. Cells were then collected to analyze the expression of proteins associated with EMT by Western blotting. Our results showed that expression of E-cadherin significantly increased, while vimentin expression decreased in Gli1 or β-catenin siRNA-transfected cells, when compared with NC siRNA-transfected cells. Similar results were observed for the curcumin-treated group when compared with the non-treated group (Figure 4C).
Taken together, these data indicate that inhibition of Shh and Wnt signaling pathways and curcumin stimulation affected EMT processes and cytoskeleton remodeling in SGC-7901 cells.
Gli1 Interacts with β-Catenin
To investigate the potential interaction between Shh and Wnt signaling pathways, a Co-IP assay was performed. SGC-7901 cells were cultured in 10 cm dishes and divided into a control group (mock) and curcumin treatment group for 48 h. After this period, cells were lysed and lysates were immunoprecipitated with anti-Gli1 antibody, and analyzed by Western blotting. As shown (Figure 5), Gli1 pulled down β-catenin. A reverse immunoprecipitation experiment was also performed using anti-β-catenin antibodies to pull down Gli1. Taken together, these results indicate that Gli1 in the Shh signaling interacted with β-catenin in the Wnt signaling.
Discussion
Currently, the main treatment strategies for gastric cancer are surgery and chemotherapy.36 Some patients are resistant to chemotherapy and their prognosis remains poor, especially if diagnosed with lymph node metastases. In recent years, natural herbal extracts have also been found to elicit anti-tumor effects.37,38 A previous study reported that curcumin had anti-tumor properties, but mechanisms at the cellular level were not defined. Curcumin inhibited the proliferation and migration of malignant glioma through Shh signaling,39 which was consistent with our results that curcumin inhibited the migration of SGC-7901 cells through Shh signaling pathway. In this study, we showed that curcumin inhibited the proliferation, migration, invasion and cytoskeletal remodeling of SGC-7901 cells through Shh and Wnt signaling pathways. It has been reported that curcumin suppresses the proliferation, cell cycle arrest and induction of apoptosis in mantle cell lymphoma, through the suppression of NF-κB-regulated gene products.40 We have shown that curcumin-induced apoptosis in SGC-7901 cells and cell cycle arrest at the S phase. Studies have found that curcumin can exert anti-tumor effects on colorectal cancer cells by activating the apoptosis pathway, These targets include enzymes, transcription factors (β-catenin, NF-κB), ROS, Bcl-2 family members (Bak, Bcl-2, Bax, and Bcl-xL), BH3 proteins (Bim, Bad, and Bid), protease enzymes (caspase3, caspase8), death receptors, and other important signaling pathways (p53, PI3K/AKT, JNK, and ER stress).41 We also found that curcumin affected SGC-7901 cells by inhibiting cytoskeletal remodeling and EMT progression, further delineating curcumin-mediated molecular mechanisms in gastric cancer cells.
Some studies have shown that Shh signaling plays key roles in tumor progression.22,42 Activation of the Shh pathway is common in advanced gastric adenocarcinoma, and elevated Gli1 and Ptch expression levels are associated with poor tumor differentiation and prognoses.43 Overexpression of sonic hedgehog is a driving factor and prognostic index of gastric cancer development.44,45 Interestingly, studies have shown that high levels of Shh protein in human benign bladder urothelium were detected; however, there was little Shh in the primary cancer cells of all invasive carcinomas.25 These findings suggest that Shh expression is different in different types of tumors. Here, we observed the biological effects of Shh signaling in SGC-7901 cells. Inhibition of Shh signaling decreased migration, invasion and inhibited cytoskeletal remodeling and EMT progression of SGC-7901 cells. In addition, some studies have shown that Shh overexpression in tumor cells plays a role in perineural invasion and tumor metastasis.46 These observations suggest that Shh signaling is associated with malignant behaviors of tumor cells.
Free β-catenin, located in the cytoplasm, is a key participant in the Wnt signaling pathway.47 It has been reported that the Wnt signaling pathway is activated in ovarian cancer and may become a new target for the treatment of drug-resistant ovarian cancer.48 Increasingly, evidence has shown that excessive β-catenin accumulation is associated with tumor invasion and proliferation.49,50 It has been reported that aberrant membranous β-catenin expression was significantly correlated with poor survival in patients with craniopharyngioma.51 We demonstrated that inhibition of β-catenin in Wnt signaling decreased migration, invasion and inhibited cytoskeletal remodeling and EMT progression in SGC-7901 cells. Taken together, these data suggest that Wnt signaling is associated with tumor progression, and may be a target for tumor therapy.
Cancer occurrence is usually associated with signaling pathway activation, these signaling pathways constitute a complex network regulating the proliferation, migration and invasion of cancer cells.52 Zhang et al, observed that crosstalk between Shh-Gli1 and PI3k-Akt signaling pathways in the cellular EMT of ovarian cancer.53 There was an interacting network of the Hippo, Wnt/β-catenin and Notch signaling pathways in hepatocellular carcinoma, which controls the tumor development.54 Identifying relationships between these signaling pathways is highly significant in understanding the mechanisms behind tumor progression. In liver cancer, the negative regulation of AMPK by Gli1 has also been reported.55 Additionally, β-catenin knockdown inhibits the expression of STAT3 and AKT in pituitary adenoma cells.56 Here, we confirmed that Gli1 interacted with β-catenin in SGC-7901 cells, and the interaction between these molecules is inhibited by curcumin. Similarly, the interaction between Gli1 and β-catenin can also be observed in medulloblastoma, it was further found that stable β-catenin increased the interaction, leading to Gli1 degradation and inhibiting Shh signaling.57 In our study, the mechanism by which curcumin inhibits the interaction between Gli1 and β-catenin in gastric cancer cells is unknown, and is the focus of our future work.
In conclusion, we have shown that Shh and Wnt signaling pathways are important in migration, invasion, apoptosis and cytoskeletal remodeling in gastric cancer SGC-7901 cells. We identified a physical interaction between Gli1 and β-catenin, and discovered that curcumin inhibits this interaction. The data from this study lay the foundation in identifying target molecules for the exploration of mechanisms in gastric cancer tumors in the future.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript. The present study was supported by grants from the Gansu Science Foundation (grant nos. 1606RJdA313 and 18JR3RA293) and the Lanzhou Science and Technology planning project (grant nos. 2018-3-44).
Author Contributions
Xiao Zhang, Chenli Zhang, Zhiheng Ren, and Jinyu Xu performed the experiments. Xiao Zhang and Fangfang Zhang contributed to the data analysis and wrote the manuscript. Xu Zhang and Haixue Zheng contributed to the study design and concept, and contributed to the experiment materials. All authors read and approved the final manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Li CY, Ye YC, Liang GY, et al. Cancer incidence and mortality survey in Wuwei, Gansu Province, Northwestern China from 2003 to 2012: a retrospective population-based study. Chin Med J. 2016;129:636–644. doi:10.4103/0366-6999.177969
3. Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol. 2014;20(38):13767–13774. doi:10.3748/wjg.v20.i38.13767
4. Shishodia S. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056(1):206–217. doi:10.1196/annals.1352.010
5. Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16(6):4567–4598. doi:10.3390/molecules16064567
6. Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother. 2017;87:223–229. doi:10.1016/j.biopha.2016.12.105
7. Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi:10.1186/1476-4598-10-12
8. Giordano A, G T. Curcumin and cancer. Nutrients. 2019;11:2376. doi:10.3390/nu1110237610
9. Mirzaei H, Naseri G, Rezaee R, et al. Curcumin: a new candidate for melanoma therapy? Int J Cancer. 2016;139(8):1683–1695. doi:10.1002/ijc.30224
10. Yu Z, Wan Y, Liu Y, Yang J, Li L, Zhang W. Curcumin induced apoptosis via PI3K/Akt-signalling pathways in SKOV3cells. Pharm Biol. 2016;54(10):2026–2032. doi:10.3109/13880209.2016.1139601
11. Petiti J, Rosso V, Lo Iacono M, et al. Curcumin induces apoptosis in JAK2-mutated cells by the inhibition of JAK2/STAT and mTORC1 pathways. J Cell Mol Med. 2019;23(6):4349–4357. doi:10.1111/jcmm.14326
12. Zhu P, Yang M, He H, et al. Curcumin attenuates hypoxia/reoxygenation-induced cardiomyocyte injury by downregulating notch signaling. Mol Med Rep. 2019;20(2):1541–1550. doi:10.3892/mmr.2019.10371
13. Liang Z, Wu R, Xie W, et al. Curcumin reverses tobacco smoke-induced epithelial-mesenchymal transition by suppressing the MAPK pathway in the lungs of mice. Mol Med Rep. 2018;17(1):2019–2025. doi:10.3892/mmr.2017.8028
14. Zhang Z, Chen H, Xu C, et al. Curcumin inhibits tumor epithelial-mesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol Rep. 2016;35(5):2615–2623. doi:10.3892/or.2016.4669
15. Liu Y, Wang X, Zeng S, et al. The natural polyphenol curcumin induces apoptosis by suppressing STAT3 signaling in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2018;37(1):303. doi:10.1186/s13046-018-0959-0
16. Shafabakhsh R, Pourhanifeh MH, Mirzaei HR, Sahebkar A, Asemi Z, Mirzaei H. Targeting regulatory T cells by curcumin: a potential for cancer immunotherapy. Pharmacol Res. 2019;147:104353. doi:10.1016/j.phrs.2019.104353
17. Hamed M, Masoudifar A, Sahebkar A, et al. MicroRNA: a novel target of curcumin in cancer therapy. J Cell Physiol. 2018;233(4):3004–3015. doi:10.1002/jcp.26055
18. Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi:10.1101/gad.1693608
19. Bangs F, Anderson KV. Primary cilia and mammalian hedgehog signaling. Cold Spring Harb Perspect Biol. 2017;9(5):a028175. doi:10.1101/cshperspect.a028175
20. Carballo GB, Honorato JR, de Lopes GP. A highlight on sonic hedgehog pathway. Cell Commun Signaling. 2018;16(1):11. doi:10.1186/s12964-018-0220-7
21. Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the hedgehog signaling pathway in cancer: a comprehensive review. Bosn J Basic Med Sci. 2018;18(1):8–20. doi:10.17305/bjbms.2018.2756
22. Giroux Leprieur E, Vieira T, Antoine M, et al. Sonic hedgehog pathway activation is associated with resistance to platinum-based chemotherapy in advanced non-small-cell lung carcinoma. Clin Lung Cancer. 2016;17(4):301–308. doi:10.1016/j.cllc.2015.12.007
23. Chen C, Breslin MB, Lan MS. Sonic hedgehog signaling pathway promotes INSM1 transcription factor in neuroendocrine lung cancer. Cell Signal. 2018;46:83–91. doi:10.1016/j.cellsig.2018.02.014
24. Zeng C, Chen T, Zhang Y, Chen Q. Hedgehog signaling pathway regulates ovarian cancer invasion and migration via adhesion molecule CD24. J Cancer. 2017;8(5):786–792. doi:10.7150/jca.17712
25. Shin K, Lim A, Zhao C, et al. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell. 2014;26(4):521–533. doi:10.1016/j.ccell.2014.09.001
26. Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–149. doi:10.1016/j.critrevonc.2015.12.005
27. Yu J, Virshup DM. Updating the Wnt pathways. Biosci Rep. 2014;34(5):593–607. doi:10.1042/BSR20140119
28. Ren ZH, Zhang CL, Ma LN, et al. Lysophosphatidic acid induces the migration and invasion of SGC-7901 gastric cancer cells through the LPA2 and notch signaling pathways. Int J Mol Med. 2019;44(1):67–78. doi:10.3892/ijmm.2019.4186
29. Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi:10.1016/j.ctrv.2017.11.002
30. Ma LN, Zhang CL, Ren ZH, et al. Expression of LPA2 regulates the migration, invasion, proliferation, and apoptosis of gastric cancer SGC-7901 cells. Sci Technol Eng. 2018;18(19):7.
31. Liu XH, Sun K, Chen H, et al. Curcumin inhibits proliferation of gastric cancer cells by impairing ATP-sensitive potassium channel opening. World J Surg Oncol. 2014;12:389. doi:10.1186/1477-7819-12-389
32. Zhu ZX, Li PF, Yang F, et al. Peste des petits ruminants virus nucleocapsid protein inhibits interferon-β production by interacting with IRF3 to block its activation. J Virol. 2019;93(16):e00362–e00419. doi:10.1128/JVI.00362-19
33. Zhu ZX, Wang GQ, Yang F, et al. Foot-and-mouth disease virus viroporin 2B antagonizes RIG-I-mediated antiviral effects by inhibition of its protein expression. J Virol. 2016;90(24):11106–11121. doi:10.1128/JVI.01310-16.
34. Zhu JY, Yang X, Chen Y, et al. Curcumin suppresses lung cancer stem cells via inhibiting Wnt/β-catenin and sonic hedgehog pathways. Phytother Res. 2017;31(4):680–688. doi:10.1002/ptr.5791
35. Zeng Y, Shen Z, Gu W, Wu M. Inhibition of hepatocellular carcinoma tumorigenesis by curcumin may be associated with CDKN1A and CTGF. Gene. 2018;651:183–193. doi:10.1016/j.gene.2018.01.083
36. Charalampakis N, Economopoulou P, Kotsantis I, et al. Medical management of gastric cancer: a 2017 update. Cancer Med. 2018;7(1):123–133. doi:10.1002/cam4.1274
37. Zang MD, Hu L, Fan ZY, et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the notch signaling pathway. J Transl Med. 2017;15(1):52. doi:10.1186/s12967-017-1151-6
38. Zhou YJ, Guo YJ, Yang XL, Ou ZL. Anti-cervical cancer role of matrine, oxymatrine and sophora flavescens alkaloid gels and its mechanism. J Cancer. 2018;9(8):1357–1364. doi:10.7150/jca.22427
39. Du WZ, Feng Y, Wang XF, et al. Curcumin suppresses malignant glioma cells growth and induces apoptosis by inhibition of SHH/GLI1 signaling pathway in vitro and vivo. CNS Neurosci Ther. 2013;19(12):926–936. doi:10.1111/cns.12163
40. Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-κB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70(5):700–713. doi:10.1016/j.bcp.2005.04.043
41. Ismail NI, Othman I, Abas F, H Lajis, N, Naidu R. Mechanism of apoptosis induced by curcumin in colorectal cancer. Int J Mol Sci. 2019;20(10):2454. doi:10.3390/ijms20102454
42. Islam SS, Mokhtari RB, Noman AS, et al. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer. Mol Carcinog. 2016;55(5):537–551. doi:10.1002/mc.22300
43. Ma X, Chen K, Huang S, et al. Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas. Carcinogenesis. 2005;26(10):1698–1705. doi:10.1093/carcin/bgi130
44. Kim JY, Ko GH, Lee YJ, et al. Prognostic value of sonic hedgehog protein expression in gastric cancer. Jpn J Clin Oncol. 2012;42(11):1054–1059. doi:10.1093/jjco/hys137
45. Chen JH, Zhao ET, Chen SL, et al. CD44, sonic hedgehog, and Gli1 expression are prognostic biomarkers in gastric cancer patients after radical resection. Gastroenterol Res Pract. 2016:1013045. doi:10.1155/2016/1013045
46. Li XQ, Wang Z, Ma QY, et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin Cancer Res. 2014;20(16):4326–4338. doi:10.1158/1078-0432.ccr-13-3426
47. Gonzalez-Moles MA, Ruiz-Avila I, Gil-Montoya JA, et al. β-catenin in oral cancer: an update on current knowledge. Oral Oncol. 2014;50(9):818–824. doi:10.1016/j.oraloncology.2014.06.005
48. Barghout SH, Zepeda N, Xu Z, Steed H, Lee CH, Fu Y. Elevated β-catenin activity contributes to carboplatin resistance in A2780cp ovarian cancer cells. Biochem Biophys Res Commun. 2015;468(1–2):173–178. doi:10.1016/j.bbrc.2015.10.138
49. Salaroli R, Ronchi A, Buttarelli FR, et al. Wnt activation affects proliferation, invasiveness and radiosensitivity in medulloblastoma. J Neurooncol. 2015;121(1):119–127. doi:10.1007/s11060-014-1621–0
50. Mokkapati S, Niopek K, Huang L, et al. beta-catenin activation in a novel liver progenitor cell type is sufficient to cause hepatocellular carcinoma and hepatoblastoma. Cancer Res. 2014;74(16):4515–4525. doi:10.1158/0008-5472.CAN-13-3275
51. Li Z, Xu J, Huang S, et al. Aberrant membranous expression of β-catenin predicts poor prognosis in patients with craniopharyngioma. Ann Diagn Pathol. 2015;19(6):403–408. doi:10.1016/j.anndiagpath.2015.10.002
52. Giakoustidis A, Giakoustidis D, Mudan S, Sklavos A, Williams R. Molecular signalling in hepatocellular carcinoma: role of and crosstalk among Wnt/β-catenin, sonic hedgehog, notch and dickkopf-1. Can J Gastroenterol Hepatol. 2015;29(4). doi:10.1155/2015/172356
53. Zhang K, Caiping S, Qing Z, Xiaojing W. Sonic hedgehog-Gli1 signals promote epithelial-mesenchymal transition in ovarian cancer by mediating PI3K/AKT pathway. Med Oncol. 2015;32(1):368. doi:10.1007/s12032-014-0368-y
54. Kim W, Khan SK, Yang YZ. Interacting network of hippo, Wnt/β-catenin and notch signaling represses liver tumor formation. BMB Rep. 2017;50(1):1–2. doi:10.5483/BMBRep.2017.50.1.196
55. Xu Q, Liu X, Zheng X, et al. The transcriptional activity of Gli1 is negatively regulated by AMPK through hedgehog partial agonism in hepatocellular carcinoma. Int J Mol Med. 2014;34(3):733–741. doi:10.3892/ijmm.2014.1847
56. Zhao C, Zhang M, Liu W, Wang C, Zhang Q, Li W. β-catenin knockdown inhibits pituitary adenoma cell proliferation and invasion via interfering with AKT and gelatinases expression. Int J Oncol. 2015;46(4):1643–1650. doi:10.3892/ijo.2015.2862
57. Zinke J, Schneider FT, Harter PN, et al. β-catenin-Gli1 interaction regulates proliferation and tumor growth in medulloblastoma. Mol Cancer. 2015;14(1):17. doi:10.1186/s12943-015-0294–4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.