Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Cryptotanshinone Reverses Corticosteroid Insensitivity by Inhibition of Phosphoinositide-3-Kinase-δ in Chronic Obstructive Pulmonary Disease
Authors Xie T , Huang R, Deng D , Tang P , Fu Y, Zheng Y, Wan Y
Received 2 February 2023
Accepted for publication 30 April 2023
Published 6 May 2023 Volume 2023:18 Pages 797—809
DOI https://doi.org/10.2147/COPD.S405757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Tao Xie,1 Rong Huang,1 Daishuo Deng,1 Peipei Tang,2 Yufeng Fu,2 Yulong Zheng,1 Yufeng Wan1
1Department of Respiratory Diseases, The Affiliated Huai’an Hospital of Xuzhou Medical University, Huai’an, Jiangsu, People’s Republic of China; 2Institute of Medicinal Biotechnology, Jiangsu College of Nursing, Huai’an, Jiangsu, People’s Republic of China
Correspondence: Yulong Zheng; Yufeng Wan, Department of Respiratory Diseases, The Affiliated Huai’an Hospital of Xuzhou Medical University, Huai’an, Jiangsu, People’s Republic of China, Tel +86 137 7670 7363 ; +86 158 0523 0282, Fax +86 517 8087 1636 ; +86 517 8087 1616, Email [email protected]; [email protected]
Purpose: Corticosteroid insensitivity has become a major barrier in the treatment of chronic obstructive pulmonary disease (COPD). It is known that oxidative stress reduces the expression and activity of histone deacetylase (HDAC)-2 by activating phosphoinositide-3-kinase-δ(PI3Kδ)/Akt pathway, which is a common mechanism. The aim of this study was to investigate whether cryptotanshinone (CPT) can improve corticosteroid sensitivity and to investigate the molecular mechanisms by which this occurs.
Patients and Methods: Corticosteroid sensitivity in peripheral blood mononuclear cells (PBMCs) collected from COPD patients, or in human monocytic U937 monocytic cells exposed to cigarette smoke extract (CSE), was quantified as the dexamethasone concentration required to achieve 30% inhibition of tumor necrosis factor-α (TNFα)–induced interleukin 8 (IL-8) production in the presence or absence of cryptotanshinone. PI3K/Akt activity (measured as the relative ratio of phosphorylated Akt at Ser-473 to total Akt) and HDAC2 expression levels were determined by western blotting. HDAC activity was evaluated by a Fluo-Lys HDAC activity assay kit in U937 monocytic cells.
Results: Both PBMCs in patients with COPD and U937 cells exposed to CSE were found to be insensitive to dexamethasone, accompanied by increased phosphorylated Akt (pAkt) and decreased HDAC2 protein expression. The pretreatment of cryptotanshinone restored their sensitivity to dexamethasone, and simultaneously downregulated the level of phosphorylated Akt and upregulated the level of HDAC2 protein. Pretreatment with cryptotanshinone or IC87114 reversed the decrease in HDAC activity in CSE-stimulated U937 cells.
Conclusion: Cryptotanshinone restores corticosteroid sensitivity induced by oxidative stress via inhibition of PI3Kδ and is a potential treatment for corticosteroid-insensitive diseases such as COPD.
Keywords: corticosteroid insensitivity, chronic obstructive pulmonary disease, phosphoinositide-3-kinase-δ, histone deacetylase 2, cryptotanshinone
Introduction
COPD is a worldwide public health problem. Nowadays, COPD has become the third leading cause of death in the world.1 At present, there is no effective treatment to prevent the progress of COPD. Long-acting β2 agonist (LABA) and long-acting muscarinic antagonist (LAMA) are the main treatment methods for stable COPD at present, which can relieve clinical symptoms, but will not significantly affect the potential disease progression.2 Inhalation of toxic gases and particles, including those in cigarette smoke, is a major risk factor for COPD. Chronic obstructive pulmonary disease is a chronic inflammatory disease. This inflammation is characterized by increased numbers of alveolar macrophages, neutrophils, T lymphocytes (predominantly TC1, TH1, and TH17 cells), and innate lymphoid cells recruited from the circulation. These cells secrete a variety of proinflammatory mediators, including cytokines, chemokines, growth factors, and lipid mediators.3 In the respiratory tract, cigarette smoke and other irritants may activate alveolar macrophages and airway epithelial cells to release chemokines, and then attract circulating leukocytes to the lungs. IL-8 is one of the first chemokines to be studied in depth, and it is also a well-known neutrophil inflammatory mediator.4 IL-8 is produced by alveolar macrophages and bronchial epithelial cells.5 Many studies have confirmed that IL-8 plays an important role in neutrophilic inflammation in COPD airways.6 Previous studies have confirmed TNF-α-induced IL-8 production in PBMCs is a good marker for evaluating corticosteroid sensitivity.7
In clinical practice, inhaled corticosteroid can control asthma well, but for patients with COPD, even inhaled or oral high-dose corticosteroid have no obvious benefits.8 Corticosteroid resistance is common in COPD patients. Corticosteroid resistance seriously hinders the effective treatment of COPD. There are many molecular mechanisms of corticosteroid insensitivity in COPD. In patients with COPD, oxidative stress induced by cigarette smoke and other external toxic stimuli leads to PI3Kδ/Akt pathway activation, which reduces the expression and activity of HDAC2, and leads to corticosteroid receptor (GR) deacetylation (Lys494 and Lys495). It reduces the ability of corticosteroid to inhibit the expression of proinflammatory factors, thus causing corticosteroid insensitivity.9–12 Studies have found that compared with normal non-smoking volunteers, the expression level of PI3K and phosphorylated Akt in lung macrophages, peripheral blood monocytes, sputum cells and lung tissue samples of COPD patients are significantly increased, while the expression and activity of HDAC2 are significantly decreased.13–15 Previous in vitro and in vivo experiments have shown that inhibiting the PI3K/Akt pathway can increase the expression and activity of HDAC2, thereby effectively improving corticosteroid sensitivity in COPD patients.16–18 Therefore, searching for drugs that can effectively inhibit PI3K/Akt signal pathway may provide a new therapeutic idea for improving the corticosteroid sensitivity of COPD patients.
The molecular formula of cryptotanshinone is C19H20O3, the relative molecular weight is 296.35, and the structural formula is shown in Figure 1A. Cryptotanshinone is extracted from the root of salvia miltiorrhiza bunge (Danshen) and has numerous pharmacological effects, including anti-cancer, anti-inflammatory, immune regulatory, neuroprotective, and anti-fibrosis activities.19 Cryptotanshinone is a natural antibacterial agent, which has been proved to be effective against infections caused by a variety of pathogens. It inhibits the release of inflammatory factors by regulating intracellular signal transduction, thereby protecting cells and tissues.20 It has been previously reported that cryptotanshinone can play an anti-inflammatory role by inhibiting the PI3K/Akt pathway in mouse macrophage RAW264.7 cells induced by lipopolysaccharide (LPS).21 The effect of cryptotanshinone has also been found to alleviate radiation-induced lung injury in rats, particularly pulmonary fibrosis.22 In this study, we investigated if cryptotanshinone could restore corticosteroid sensitivity in COPD and explored the potential mechanisms involved in cryptotanshinone-mediated steroid re-sensitization.
Materials and Methods
Details of the corticosteroid sensitivity assays are provided in Supplement Materials.
Materials
U937 cells were purchased from Shanghai Zhong Qiao Xin Zhou Biotechnology (Shanghai, China). Cryptotanshinone and IC87114 (PI3K-δ inhibitor) were purchased from MCE (State of New Jersey, USA). Dexamethasone was purchased from Shiyao Yinhu Pharmaceutical Co., Ltd (Hubei, China). TNFα was purchased from Pepro Tech (State of New Jersey, USA). Human IL-8 ELISA KIT was purchased from Anhui Qiaoyi Biotechnology Co., Ltd (Anhui, China). Fluo-Lys HDAC activity assay kit was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Antibodies against phospho-Akt and Akt were purchased from Cell Signalling (Hitchin, UK). Antibodies against HDAC2 and β-actin were purchased from Abcam (Cambridge, UK).
Study Participants
PBMCs were obtained from 12 patients with COPD and 12 healthy volunteers (HVs). The characteristics of the subjects are summarized in Table 1. All participants were recruited from September 2022 to December 2022 at the respiratory department and physical examination center at the Affiliated Huai’an Hospital of Xuzhou Medical University. This study was conducted in accordance with the tenets of the Declaration of Helsinki, and written informed consent was obtained from all participants. This study was conducted under the approval of the Ethics Committee of the Affiliated Huai’an Hospital of Xuzhou Medical University (shown in Supplemental Figure 1).
 |
Table 1 Clinical Features of Study Participants |
Isolation and Stimulation of PBMCs
PBMCs were separated with ficoll density gradient centrifugation (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) from each COPD patient and healthy volunteer. PBMCs (8×105 cells/well) were stimulated with TNFα (1ng/mL), and with dexamethasone (10−11–10−6 M, 1hours) or with or without cryptotanshinone (2μM, 4hours). Supernatants were removed 18 hours later and analyzed for IL8 by enzyme-linked immunosorbent assay (Anhui Qiaoyi Biotechnology Co., Ltd, Anhui, China).
Cigarette Smoke Extract
CSE was prepared as described previously.23 One full-strength Marlboro cigarette with a filter removed (Phillip Morris, Richmond, VA) was combusted through a modified 60-ml syringe into 10 ml of RPMI1640 medium. The optical density was measured at a wavelength of 320 λ, and the medium was diluted to achieve a value of 0.15 to provide a concentration that stimulated the cells without inducing cell death.
qPCR
Total RNA was extracted from U937 cells using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. After determining the RNA quality and concentration, complementary DNA (cDNA) was synthesized using PrimeScript™RT kit and gDNA Eraser reverse transcription kit (Takara, Japan). qPCR was performed to validate gene expression using 2× SYBR Green PCR Master Mix (Sangon Biotech, China) on an Mx3000P QPCR System (Stratagene, USA) with the following thermal cycling conditions: 95°C for 3 min, followed by 40 cycles at 95°C for 12s and 62°C for 40s. The primer pairs used are shown in Table 2.
 |
Table 2 The Primer Pairs are Listed as Following |
Western Blot Analysis
Total proteins were extracted from the U937 cells and PBMCs using a protein lysate containing DL-dithiothreitol (DDT) and sodium dodecyl sulfate (SDS). Protein samples were separated using 10% SDS-polyacrylamide gel electrophoresis/Western blot (Invitrogen, Paisley, UK). Specific proteins were probed with primary antibodies and suitable horseradish peroxidase-conjugated secondary antibodies. The band intensity was measured using a Gel-Pro Analyzer. Band densities of phospho-Akt and HDAC2 were normalized to total Akt and β-actin, respectively.
HDAC Activity Assays
U937 cells were seeded at 3×106 cells/ml, pretreated with 2 μM CPT or 1μM IC87114 for 4h before being exposed to CSE-infused media for 10 min. Nuclear extracts were prepared as described previously,10 and HDAC activity was measured in 10μl samples from nuclear extracts and normalized to protein concentrations.
Statistical Analysis
All data shown are expressed as means ± SEM. Analysis of variance was performed by Kruskal–Wallis analysis and when significant, Mann–Whitney U-test using GraphPad Prism (GraphPad Software, San Diego, CA). Wilcoxon matched-pairs test and Student’s t-test were also used to determine significance when applicable. P values less than 0.05 were considered to be significant.
Results
Effects of CSE and CPT on the Growth of U937 Cells
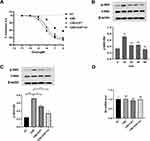
To determine whether exposure of U937 cells to CSE and CPT were cytotoxic, cellular growth assays were performed in the concentration range of CSE and CPT. Treatment of U937 cells with a 0.125% or 0.5% CSE solution and 8μM CPT did not reduce cell proliferation over a 48h time period. A 2% CSE solution, however, slowed U937 cell growth when compared to untreated control samples (Figure 1B and C). A 0.5% concentration of CSE was therefore used in subsequent experiments to avoid any cytotoxic effects. No decrease in proliferation and no significant morphological changes were observed by microscopy in U937 cells or PBMCs treated with 1μM, 2μM, 4μM, and 8μM CPT. When U937 cells were exposed to CSE (0.5%) and TNF-α (10ng/mL), the release of IL-8 was significantly reduced by CPT at 2μM concentration (Figure 1D).
CPT and IC87114 Restored CSE-Induced Corticosteroid Sensitivity in U937 Cells
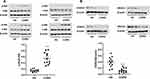
After stimulation by CSE, the log (Dex-IC30) and log (Dex-EC50) values were increased from −8.34±0.05 and −8.23±0.04 to −7.21±0.11 (p < 0.001) and −7.57±0.12 (p < 0.01), respectively. CSE also decreased the Emax value from 66.5±2.0% to 42.2±2.2% (p < 0.01) (Figure 2A and Table 3). This confirms that CSE caused corticosteroid insensitivity as previously reported. CPT treatment (2 μM, 4h) prior to treatment with CSE significantly decreased the log (Dex-IC30) and log (Dex-EC50) to −7.98±0.03 and −7.97±0.03 (from −7.21±0.11 and −7.57±0.12), (p < 0.001, p < 0.05) respectively, and increased the Emax to 60.1±1.0% (from 42.2±2.2%) (p < 0.01), proving that CPT was able to completely reverse corticosteroid insensitivity induced by CSE (Figure 2A and Table 3). Similarly, IC87114 (1μM) significantly restored corticosteroid sensitivity (Figure 2A and Table 3).
 |
Table 3 Effects of Cryptotanshinone and IC87114 on Corticosteroid Sensitivity in U937 Cells |
CPT and IC87114 Prevented CSE-Induced Phosphorylation of Akt in U937 Cells
Similar to the results found in previous studies,24 PI3K/Akt activity, measured as the relative ratio of phosphorylated Akt at Ser-473 to total Akt, was quickly (10 min) and transiently activated after CSE stimulation (p < 0.01) (Figure 2B). Ser-473Akt phosphorylation was increased by CSE (p < 0.001) (Figure 2C). Preincubation with CPT (2 μM, 4h) significantly prevented CSE-Induced phosphorylation of Akt (p < 0.05). IC87114 (1 μM, 4h) also produced a inhibition of CSE-induced pAkt (p < 0.001) (Figure 2C). Notably, the Akt mRNA levels and total Akt protein levels do not change after CSE exposure or pretreatment with CPT or IC87114, but pAkt levels are significantly affected (Figure 2C and D).
CPT and IC87114 Increased HDAC2 mRNA, Protein Expression and HDAC Activity in U937 Cells
U937 cells exposed to CSE for 18 hours showed a significant reduction in HDAC2 mRNA and protein expression. Addition of CPT (2 μM, 4h) or IC87114 (1 μM, 4h) blocked the HDAC2 decrease (Figure 3A and B). After exposure to CSE for 10 min, the total HDAC activity of U937 cells was significantly decreased (p < 0.001). Pretreatment with CPT (2 μM, 4h) or IC87114 (1 μM, 4h) restored HDAC activity after CSE exposure (p < 0.05; Figure 3C).
Corticosteroid Insensitivity in PBMCs from Patients with COPD
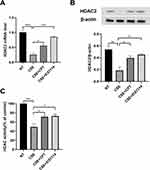
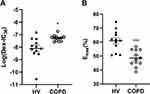
The log (Dex-IC30) value in Healthy volunteers was −8.11±0.27, and the log (Dex-IC30) in COPD was significantly higher (−7.28±0.07), indicating that the PBMCs from the patients with COPD were 6.76 fold less steroid-sensitive as compared with those from the Healthy volunteers (Figure 4A, p < 0.05). Furthermore, the Emax value in patients with COPD was also significantly lower than that in the Healthy volunteers (48.7±1.7% vs 61.0±2.2%, respectively; p < 0.001) (Figure 4B). These results demonstrate that PBMCs from patients with COPD are less corticosteroid sensitive than PBMCs from Healthy volunteers. PI3K/Akt activity, which was assessed by Akt phosphorylation, was significantly increased in COPD PBMCs compared with Healthy volunteers PBMCs (p < 0.0001) (Figure 5A). HDAC2 protein expression was significantly decreased in COPD PBMCs compared with Healthy volunteers PBMCs (p < 0.0001) (Figure 5B).
CPT Treatment Improves Corticosteroid Sensitivity in PBMCs from Patients with COPD
Pretreatment of COPD PBMCs with CPT restored dexamethasone anti-inflammatory effects on TNFα-induced IL-8 production with a significant decrease in log (Dex-IC30) value from −7.28±0.07 to −7.68±0.08 (Figure 6A, ***P < 0.001). CPT also facilitated dexamethasone anti-inflammatory actions in PBMCs from Healthy volunteers (Figure 6A, **P < 0.01). On the other hand, Emax values were not improved by CPT (Figure 6B, *P > 0.05). This clearly showed that CPT improved only corticosteroid sensitivity in PBMCs of COPD, not maximal inhibition. Pre-treatment with CPT (2 μM) decreased PI3K/Akt activity in PBMCs from patients with COPD (Figure 7A, *** P < 0.001), and also increased HDAC2 protein expression (Figure 7B, **** P < 0.0001).
Discussion
Corticosteroid resistance is common in patients with COPD, and several studies have shown that both resident cells in lung (alveolar macrophages) and circulating cells (peripheral-blood mononuclear cells) in patients with COPD are not sensitive to corticosteroid.25,26 In this study, we have explored the role of monocytes/macrophages in corticosteroid insensitivity using COPD PBMCs, CSE-exposed human U937 monocytic cells and confirmed the effectiveness of cryptotanshinone in regaining corticosteroid sensitivity in COPD accompanied by inhibiting PI3K/Akt activity and restoring HDAC2 levels in mononuclear cells. This is the first report to show corticosteroid re-sensitizing capability of cryptotanshinone in modulating PI3K/Akt activity and HDAC2 in the context of COPD.
Oxidative stress caused by cigarette smoke and environmental pollutants plays an important role in the pathogenesis of COPD and the induction of corticosteroid insensitivity.27,28 Cigarette smoking is the main and most common risk factor for COPD. Smoking more than 20 packs per year can triple the prevalence of COPD in China.29 Therefore, in order to more accurately simulate the process of corticosteroid resistance in COPD patients in vitro, we used CSE instead of H2O230 as an exogenous oxidative stimulator to stimulate U937 cells.
A major problem in the therapy of COPD is its poor response to corticosteroid. Previous research has shown that inflammatory mediators such as IL-8 and TNF-α are released in COPD patients who smoke.31 IL-8 is the first identified chemokine to play a role in the recruitment of inflammatory cells in COPD.4 The induction level of IL8 is robust and the IL8 production was not often interfered with test agents. It has first been identified as a LPS-stimulated monocyte-secreted factor that stimulated neutrophil exocytosis (granule release) and oxidative burst.32 However, LPS was not used as a stimulus to induce IL8 production in our study. Because previous studies have confirmed that LPS-induced cytokine production is corticosteroid sensitive and the levels of cytokines are very variable, possibly due to different expressions of TLR4 in patients.33 It has been found that TNF-α-induced IL8 in PBMCs is better readout than other cytokines and stimulation.7,11,24 We therefore established a TNF-α-induced IL8 system to evaluate the effect of drugs on corticosteroid sensitivity.
It has been reported that downregulation of HDAC2 activity and expression is the main molecular mechanism causing corticosteroid resistance in COPD patients. Oxidative stress activates PI3Kδ, which phosphorylates the Akt, resulting in the phosphorylation and inactivation of HDAC2. In addition, oxidative and nitrative stress generate peroxynitrite, which nitrates (NO) tyrosine residues (Tyr) on HDAC2 to inhibit its activity. These modifications of HDAC2 result in its ubiquitination (Ub), targeting the enzyme for degradation by the proteasome and leading to reduced expression and steroid resistance.8 Inhibition of the PI3K/Akt pathway has been shown to restore corticosteroid sensitivity in steroid-resistant experimental models and in PBMCs from COPD patients through restoration of the HDAC2 level and activity.15,17,34 Mammals have four class I PI3K catalytic subunits, including p110α, p110β, p110γ and p110δ.35 Activation of PI3Kδ subtype was found to increase pAkt in COPD lung tissues and cells.7 Another study found that PI3Kδ(-/-) null mice were protected from cigarette smoke-induced corticosteroid resistance and down-regulation of HDAC2 activity.36 IC87114, a selective PI3Kδ inhibitor, has been shown to improve the corticosteroid reactivity of CSE exposed U937 cells and smoking-exposed mice by inhibiting the activity of PI3Kδ to up-regulate the activity and expression of HDAC2, which is consistent with our findings (Figures 2A, C and 3A–C), cryptotanshinone also has a similar effect to that of IC87114. Our results showed that none of the treatments affected Akt gene and protein levels in U937 cells, suggesting that the treatments affected the post-translational modification of Akt protein (phosphorylation) rather than the expression of Akt mRNA and protein (Figure 2C and D). This is consistent with previous research.17
It has been previously reported that HDAC activity decreased in U937 cells after 15 minutes of H2O2 (200 μM) stimulation, while HDAC activity decreased after 2 hours of CSE (100%) stimulation, with no change in HDAC2 protein expression during the same period.37 It has also been reported that HDAC2 activity in BEAS-2B cells decreased after 4 hours of H2O2 (100 μM) stimulation, but HDAC2 expression decreased only at 24 hours or longer, suggesting that a post-translational modification of HDAC is involved in attenuating HDAC activity.38 However, in our study, PI3Kδ activity and HDAC2 activity decreased 10 min after CSE (0.5%) stimulation of U937 cells (Figure 2B and C), while HDAC2 mRNA and protein expression did not change significantly until 18h after CSE (0.5%) stimulation (Figure 3A and B). This indicated that the change of HDAC2 protein expression was lagging behind the change of activity, and that the post-translational modification of HDAC2 caused the change of activity first and then the change of expression. At the same time, it also indicated that different cell types (U937 cells or BEAS-2B cells), different sources of oxidative stress (CSE or H2O2) and different stimulus intensities (100 μM H2O2 or 200 μM H2O2; 0.5%CSE or 100% CSE) would affect the time point of significant changes in PI3Kδ activity and HDAC2 activity and protein expression.
Different from previous studies,37 our study found that CPT and IC87114 not only improved the corticosteroid sensitivity of CSE-stimulated U937 cells but also significantly increased the Emax value (Figure 2A and Table 3), suggesting that the increase in Emax was PI3K-dependent. The reason for our consideration is that there were certain differences in experimental conditions between the previous study and our current study, such as inconsistent concentrations of CSE and different inhibition degree of PI3Kδ subtype by drugs. LY294002 used in previous study is a non-selective PI3K inhibitor, while IC87114 is a selective PI3Kδ inhibitor. However, in PBMCs of patients with COPD, although pretreatment with CPT significantly reduced the IC30 value, it did not change the Emax value (Figure 6A and B), indicating that the ability of CPT to improve corticosteroid sensitivity in COPD is partial.
Several concerns are noticed in this study. First, we cannot prove whether CPT directly affects HDAC2 or indirectly affects HDAC2 by inhibiting PI3K/Akt. To the best of our knowledge, there is no known evidence that PI3K/Akt can directly affect HDAC2 expression. However, there may be some signaling molecules that indirectly regulate the effect of PI3K/Akt on HDAC2. In monocytes, GSK3β inactivation reduced corticosteroid suppression of proinflammatory responses by inhibition of the enzymatic activity of HDAC2.39 Glycyrrhizic acid inhibited inflammatory factors by mediation of the PI3K/Akt/GSK3β pathway.40 Thus, GSK3β may provide a link between the PI3K/Akt pathway and HDAC2. However, whether other signaling molecules play an important role in the relationship between PI3K/Akt and HDAC2 requires further investigation. Second, cryptotanshinone may also be able to improve corticosteroid sensitivity through other targets such as MyD88/p38 MAPK/NF-κB, which also needs further investigation.41 Third, although previous studies have confirmed that there is no significant difference in the sensitivity of peripheral blood mononuclear cells to corticosteroid between smoking volunteers and non-smoking healthy volunteers.7,11 However, we still need to set up a group of smoking volunteers to rule out the effect of smoking as a risk factor on corticosteroid sensitivity in healthy people.
Conclusion
In conclusion, our results suggest that cryptotanshinone can reverse corticosteroid insensitivity by inhibiting PI3K signaling and restoring HDAC2 activity and expression under oxidative stress. Therefore, cryptotanshinone may be a potentially useful treatment for resensitizing corticosteroid in COPD patients.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supporting information.
Ethics Statement
This study was conducted in accordance with the tenets of the Declaration of Helsinki, and written informed consent was obtained from all participants. This study was conducted under the approval of the Ethics Committee of the Affiliated Huai’an Hospital of Xuzhou Medical University (shown in Supplemental Figure 1).
Acknowledgments
We are very grateful to Dr. Song Chen for his guidance and revision of the article.
Funding
This work was supported by grants from the Natural Science Foundation of Huaian, Jiangsu, China (No. HAB202115 and No. HAB202073) and Natural Science Research Projects in Jiangsu Universities, Jiangsu, China (21KJD310002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guidetti D, Spallazzi M, Baldereschi M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–1222. doi:10.1016/S0140-6736(20)30925-9
2. Celli BR, Singh D, Vogelmeier C, Agusti A. New perspectives on chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2022;17:2127–2136. doi:10.2147/COPD.S365771
3. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. doi:10.1016/j.jaci.2016.05.011
4. Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi:10.1164/ajrccm.153.2.8564092
5. Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;41:631–638. doi:10.1165/rcmb.2009-0220TR
6. Henrot P, Prevel R, Berger P, Dupin I. Chemokines in COPD: from implication to therapeutic use. Int J Mol Sci. 2019;21:20. doi:10.3390/ijms21010020
7. To Y, Ito K, Kizawa Y, et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:897–904. doi:10.1164/rccm.200906-0937OC
8. Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131:636–645. doi:10.1016/j.jaci.2012.12.1564
9. Hakim A, Adcock IM, Usmani OS. Corticosteroid resistance and novel anti-inflammatory therapies in chronic obstructive pulmonary disease: current evidence and future direction. Drugs. 2012;72:1299–1312. doi:10.2165/11634350-000000000-00000
10. Ito K, Lim S, Caramori G, et al. A molecular mechanism of action of theophylline: induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci U S A. 2002;99:8921–8926. doi:10.1073/pnas.132556899
11. Rossios C, To Y, Osoata G, Ito M, Barnes PJ, Ito K. Corticosteroid insensitivity is reversed by formoterol via phosphoinositide-3-kinase inhibition. Br J Pharmacol. 2012;167:775–786. doi:10.1111/j.1476-5381.2012.01864.x
12. Lewis BW, Ford ML, Rogers LK, Britt RD. Oxidative stress promotes corticosteroid insensitivity in Asthma and COPD. Antioxidants. 2021;10. doi:10.3390/antiox10091335
13. Lai T, Tian B, Cao C, et al. HDAC2 Suppresses IL17A-mediated airway remodeling in human and experimental modeling of COPD. Chest. 2018;153:863–875. doi:10.1016/j.chest.2017.10.031
14. Marwick JA, Caramori G, Casolari P, et al. A role for phosphoinositol 3-kinase delta in the impairment of glucocorticoid responsiveness in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2010;125:1146–1153. doi:10.1016/j.jaci.2010.02.003
15. Liao W, Lim AYH, Tan WSD, Abisheganaden J, Wong WSF. Restoration of HDAC2 and Nrf2 by andrographolide overcomes corticosteroid resistance in chronic obstructive pulmonary disease. Br J Pharmacol. 2020;177:3662–3673. doi:10.1111/bph.15080
16. Weng J-Z, Wang Y, Sun T-Y. Cathelicidin LL-37 restoring glucocorticoid function in smoking and lipopolysaccharide-induced airway inflammation in rats. Chin Med J. 2019;132:569–576. doi:10.1097/CM9.0000000000000107
17. Sun X-J, Li Z-H, Zhang Y, et al. Combination of erythromycin and dexamethasone improves corticosteroid sensitivity induced by CSE through inhibiting PI3K-δ/Akt pathway and increasing GR expression. Am J Physiol Lung Cell Mol Physiol. 2015;309:L139–L146. doi:10.1152/ajplung.00292.2014
18. Sun X, Chen L, He Z. PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr Drug Metab. 2019;20:301–304. doi:10.2174/1389200220666190227224748
19. Li H, Gao C, Liu C, et al. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Bio Pharmacot. 2021;137:111332. doi:10.1016/j.biopha.2021.111332
20. Cha J-D, Lee J-H, Choi KM, Choi S-M, Park JH. Synergistic effect between cryptotanshinone and antibiotics against clinic methicillin and vancomycin-resistant staphylococcus aureus. Evid Based Complement Alternat Med. 2014;2014:450572. doi:10.1155/2014/450572
21. Li -X-X, Zheng X, Liu Z, et al. Cryptotanshinone from Salvia miltiorrhiza Bunge (Danshen) inhibited inflammatory responses via TLR4/MyD88 signaling pathway. Chin Med. 2020;15:20. doi:10.1186/s13020-020-00303-3
22. Jiang Y, You F, Zhu J, Zheng C, Yan R, Zeng J. Cryptotanshinone ameliorates radiation-induced lung injury in rats. Evid Based Complement Alternat Med. 2019;2019:1908416. doi:10.1155/2019/1908416
23. Walters MJ, Paul-Clark MJ, McMaster SK, Ito K, Adcock IM, Mitchell JA. Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: implications for steroid resistance. Mol Pharmacol. 2005;68:1343–1353. doi:10.1124/mol.105.012591
24. Mitani A, Ito K, Vuppusetty C, Barnes PJ, Mercado N. Restoration of corticosteroid sensitivity in chronic obstructive pulmonary disease by inhibition of mammalian target of rapamycin. Am J Respir Crit Care Med. 2016;193:143–153. doi:10.1164/rccm.201503-0593OC
25. Andelid K, Öst K, Andersson A, et al. Lung macrophages drive mucus production and steroid-resistant inflammation in chronic bronchitis. Respir Res. 2021;22:172. doi:10.1186/s12931-021-01762-4
26. Bin Y-F, Ma N, Lu Y-X, et al. Erythromycin reverses cigarette smoke extract-induced corticosteroid insensitivity by inhibition of the JNK/c-Jun pathway. Free Radic Biol Med. 2020;152:494–503. doi:10.1016/j.freeradbiomed.2019.11.020
27. Pinart M, Hussain F, Shirali S, et al. Role of mitogen-activated protein kinase phosphatase-1 in corticosteroid insensitivity of chronic oxidant lung injury. Eur J Pharmacol. 2014;744:108–114. doi:10.1016/j.ejphar.2014.10.003
28. Albano GD, Gagliardo RP, Montalbano AM, Profita M. Overview of the mechanisms of oxidative stress: impact in inflammation of the airway diseases. Antioxidants. 2022;11. doi:10.3390/antiox11112237
29. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–1717. doi:10.1016/S0140-6736(18)30841-9
30. Heijink I, van Oosterhout A, Kliphuis N, et al. Oxidant-induced corticosteroid unresponsiveness in human bronchial epithelial cells. Thorax. 2014;69. doi:10.1136/thoraxjnl-2013-203520
31. Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;2008:122.
32. Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167:1547–1559. doi:10.1084/jem.167.5.1547
33. Kobayashi Y, Wada H, Rossios C, et al. A novel macrolide/fluoroketolide, solithromycin (CEM-101), reverses corticosteroid insensitivity via phosphoinositide 3-kinase pathway inhibition. Br J Pharmacol. 2013;169:1024–1034. doi:10.1111/bph.12187
34. Sun X-J, Li Z-H, Zhang Y, et al. Theophylline and dexamethasone in combination reduce inflammation and prevent the decrease in HDAC2 expression seen in monocytes exposed to cigarette smoke extract. Exp Ther Med. 2020;19:3425–3431. doi:10.3892/etm.2020.8584
35. Burke JE. Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol Cell. 2018;71:653–673. doi:10.1016/j.molcel.2018.08.005
36. Marwick JA, Caramori G, Stevenson CS, et al. Inhibition of PI3Kdelta restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med. 2009;179:542–548. doi:10.1164/rccm.200810-1570OC
37. Mercado N, To Y, Ito K, Barnes PJ. Nortriptyline reverses corticosteroid insensitivity by inhibition of phosphoinositide-3-kinase-δ. J Pharmacol Exp Ther. 2011;337:465–470. doi:10.1124/jpet.110.175950
38. Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315:240–245. doi:10.1016/j.bbrc.2004.01.046
39. Ngkelo A, Hoffmann RF, Durham AL, et al. Glycogen synthase kinase-3β modulation of glucocorticoid responsiveness in COPD. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1112–L1123. doi:10.1152/ajplung.00077.2015
40. Ortiz JL, Milara J, Lluch J, De Diego A, Sanz C, Cortijo J. Phosphodiesterase-4 inhibition improves corticosteroid insensitivity in pulmonary endothelial cells under oxidative stress. Allergy. 2013;68:64–73. doi:10.1111/all.12055
41. Li Z, Cheng Q, Yu L, et al. Dan-Lou tablets reduces inflammatory response via suppression of the MyD88/p38 MAPK/NF-κB signaling pathway in RAW 264.7 macrophages induced by ox-LDL. J Ethnopharmacol. 2022;298:115600. doi:10.1016/j.jep.2022.115600
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.