Back to Journals » Clinical Epidemiology » Volume 7
Cross-sectional study on comorbidities and adverse events in patients with advanced and recurrent ovarian cancer in France
Authors Le Saux O, Taylor A, Chia V, Pillas D, Kaur M, Freyer G
Received 10 April 2015
Accepted for publication 1 August 2015
Published 27 October 2015 Volume 2015:7 Pages 431—440
DOI https://doi.org/10.2147/CLEP.S86429
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Vera Ehrenstein
Olivia Le Saux,1 Aliki Taylor,2 Victoria Chia,3 Demetris Pillas,2 Moninder Kaur,2 Gilles Freyer1
1Department of Medical Oncology, Centre Hospitalier Lyon-Sud, Pierre-Bénite Cédex, France; 2Center for Observational Research, Amgen Ltd, Uxbridge, UK; 3Center for Observational Research, Amgen Inc., Thousand Oaks, CA, USA
Purpose: The purpose of this study was to evaluate the prevalence of comorbidities and adverse events (AEs), and determine the treatment patterns according to platinum-sensitivity status in patients with advanced (stage IIIB–IV) or recurrent epithelial ovarian cancer (EOC).
Methods: A cross-sectional study was carried out in France with patients over 18 years, diagnosed with advanced (stage IIIB–IV) or recurrent EOC between 2009 and 2012. A total of 23 physicians (oncologists and gynecologists) participated, contributing 127 patients. Data were abstracted by participating physicians into a case report form.
Results: Of the 127 patients included, 92 (72.4%) had advanced EOC and 35 (27.6%) had recurrent EOC. A total of 73 comorbidities were reported in 44 patients (34.6%). Vascular (10.2%), metabolic (7.1%), respiratory (5.5%), and psychiatric disorders (5.5%) were the most common types of comorbidities reported. Prevalence of AEs was 74.8%, of which 12.6% were classified as serious. The most common AEs were anemia (16.5%), hematologic events (12.6%), taste change (11.8%), and headache (7.1%). Throughout the follow-up period, twelve patient deaths were reported (six due to disease progression). Of 35 patients with recurrent disease, 16 were highly platinum sensitive (recurrence >12 months after stopping platinum-based therapy), eleven were partially platinum sensitive (recurrence 6–12 months after stopping platinum-based therapy), seven were platinum resistant (recurrence within 6 months of stopping platinum-based therapy or progression while receiving second- or later-line platinum-based therapy), and one was platinum refractory (recurrence within 6 months from the start of first-line platinum-based therapy).
Conclusion: In this cross-sectional study of advanced and metastatic ovarian cancer patients, approximately one-third of patients were diagnosed with comorbidities, and approximately three-quarters were diagnosed with AEs (12.6% with severe AEs).
Keywords: ovarian neoplasms, platinum sensitivity, drug-related side effects, comorbidity
Introduction
In European women, ovarian cancer is the fifth most commonly diagnosed cancer. The estimated number of new ovarian cancer cases in Europe in 2012 was 65,538, with 42,704 deaths in 2012.1 Approximately 15% of women present with early disease that is localized in the ovaries, and for this group, 5-year survival is over 90%. However, most women with epithelial ovarian cancer (EOC) present with FIGO (Fédération Internationale de Gynécologie et d’Obstétrique) stage III disease (with peritoneal spread beyond the pelvis, with or without nodal involvement) or stage IV disease (distant metastases). Five-year relative survival after metastatic ovarian cancer is currently estimated to be less than 30%.2 The current standard of care for patients with advanced or metastatic ovarian cancer is cytoreductive surgery, followed by systemic chemotherapy. First-line chemotherapy typically is platinum-based therapy (carboplatin) in combination with paclitaxel.3 Despite high initial response rates, most ovarian cancer patients will eventually experience disease recurrences and require further treatments. Recurrences are often classified as platinum-sensitive or platinum-resistant diseases according to the time elapsed between the completion of platinum-based treatment and the detection of relapse. Patients with platinum-free intervals greater than 6 months are considered to be platinum sensitive, while patients with platinum-free intervals less than 6 months are considered to be platinum resistant. Platinum-resistant patients typically have lower response rates to subsequent chemotherapy and shorter progression-free and overall survival.4
EOC may occur at any age; however, it is more common in women over the age of 50 years. Older women have poorer survival after EOC diagnosis: the 5-year survival rate of advanced EOC is less than 50% for patients aged less than 65 years, but approximately 30% for patients aged 65 years or more.5 Many older patients have at least one underlying comorbidity which has been shown to be an important predictor of prognosis in patients with cancer.6,7 It is possible that patients with comorbidities may experience a delay in diagnosis of cancer, resulting in a more advanced stage at diagnosis. For women with EOC, the presence of one or more comorbidities may substantially influence the diagnostic workup, alter treatment efficacy, and affect survival.8 Approximately 10%–15% of EOC patients with advanced disease will have long-term remission of their cancer, and many patients will be treated with several sequential treatment cycles. These treatments often have cumulative and irreversible adverse effects which can negatively impact patients’ quality of life and also limit future treatment options.9
There is limited published data on the types and rates of adverse events (AEs) and comorbidities in patients with EOC. There is also a shortage of published data which describes patient treatment regimens according to platinum-sensitivity regimes in the observational setting. The primary objective of this study was to evaluate the prevalence of comorbidities and AEs in women with advanced (stage IIIB–IV) or recurrent EOC. The secondary objectives of this study were to evaluate platinum status and to determine treatment regimens according to platinum-sensitivity status. We carried out a review of medical records in France in order to obtain clinical data to address these objectives.
Material and methods
This was a cross-sectional medical records review of patients diagnosed with advanced or recurrent EOC.
Study population
The study population comprised 127 female patients with advanced or recurrent EOC, and they were the total number of eligible patients included based on the medical records of 23 participating physicians (gynecologists, oncologists, or both) in France. Five hundred forty-six physicians working in a hospital or oncology center, recruited from the complete and representative lists of French Oncologists and Gynecologists provided by the OneKey™ (Cegedim Relationship Management, Boulogne-Billancourt, France) database (an international database including more than 5 million health care professionals) were randomly selected. The 546 physicians were contacted randomly by telephone and screened to be sure that they were able to provide patients who met the patient inclusion criteria within the specified time frame. In order to be eligible for inclusion, physicians must have treated patients with advanced (stage IIIB–IV) or recurrent EOC and be able to collect and record clinical data regarding diagnosis, AEs, comorbidities, treatment, and relapse of EOC. Such data should be complete and include records of dates for events collected. Physician recruitment continued for 2 months. Twenty-three physicians were eligible and accepted to participate in this study.
Eligible patients had to meet the following inclusion criteria: 1) age over 18 years; 2) present with advanced cancer (defined as stage IIIB–IV) or recurrent EOC; 3) have initial diagnosis of EOC in the year 2009 or later. For inclusion of patients into the study, a diagnosis of stage must have been made either cytologically, with carcinoembyronic antigen 125 elevation, or histologically. For advanced EOC, patients must have been treated with a platinum type drug. For recurrent EOC, patients must have previously or currently (at the time of last chemotherapy) been treated with a platinum type drug and for whom platinum status was known.
Data collection
Each participating physician was requested to abstract data from his/her patient’s medical records and to include two to five patients on average. This request was to ensure that the contribution of the numbers of patients was similar across physicians. Physicians were requested to select consecutive patients who were treated most recently and met the inclusion criteria in order that the most recent data could be included into the study. Data were retrospectively collected from initial EOC diagnosis until a maximum of 12 months after the end of last chemotherapy period to enable collection of sufficient detail on treatment-related AEs and comorbidities and to capture all relevant treatments. Data were collected using electronic case report forms (eCRFs) or paper CRFs, according to the choice of the participating physicians (see Tables S1–S3). A Data Validation Plan was written, and queries, either automatic for eCRFs or manual for paper CRFs, were created for incorrect or invalid data identified. If the day was missing in the date values, it was replaced by the 15th day of the month. Other missing data were not replaced.
Comorbidities and AEs
Comorbidities and AEs, including severe adverse events (SAEs), were collected throughout the follow-up period and coded with MedDRA (Version 12.0; International Federation of Pharmaceutical Manufacturers and Associations, Geneva, Switzerland). The list of comorbidities and AEs are described in the Supplementary material. Definitions for the purposes of this study are:
- Comorbidities are defined as medical conditions that exist at the time of diagnosis of the index disease
- An AE is defined as any untoward medical occurrence in a patient administered a pharmaceutical product, and which does not necessarily have to have a causal relationship with this treatment
- An SAE is any AE, as defined using the criteria above as one that interrupts the patient’s normal daily activities and generally requires systemic drug therapy or other treatment.
Platinum-sensitivity status
Platinum-sensitivity status comprised a secondary study outcome and was evaluated based on the data reported in the patient records. Patients were categorized into the following categories based on their platinum-sensitivity status: 1) highly platinum sensitive (recurrence within more than 12 months after stopping platinum-based therapy); 2) platinum partially sensitive (recurrence within 6–12 months of stopping platinum-based therapy); 3) platinum resistance (recurrence within 6 months of stopping platinum-based therapy or progression while receiving second- or later-line platinum-based therapy); 4) platinum refractory (recurrence within 6 months of the start of first-line platinum-based therapy). Physicians were given instructions based on the definition of platinum-sensitivity status to allocate patients into these four groups.
Statistical methods
Considering that proportions of AEs vary from 0.10 to 0.90, the two-sided 95% confidence intervals of the proportions, calculated on 150 patients, will have a width of 0.16 at most, that is considered satisfactory. The expected number of participating physicians was 40, to enable a sample of patients of 150 to be included in the study (between two and five patients on average per physician). To reach the target of 40 physicians, we randomly selected 546 physicians from the OneKey™ database.
The analysis was descriptive, and results were reported in terms of prevalence, proportions (including cumulative proportions and continuous data), number of missing values, mean, standard deviation, median, and range for continuous data, and in terms of frequency, number of missing values, and percentages (the denominator being the number of nonmissing values) for categorical data. Chi-square tests were used to assess whether differences between specific groups of patients were statistically significant. For these analyses, statistical significance was set at P<0.05 level.
Ethical approval
Ethical approvals were obtained from CCTIRS (Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé) – French Advisory Committee on information Processing in Health Research. Patient consent was not required, as this study involved the retrospective analysis of the patients’ data. All data collected were anonymized, so no patient identifiable data were used.
Results
Descriptive data
Of the 23 oncologists or gynecologists who participated in the study, the majority of the participating physicians were oncologists (n=13), and approximately one-third of participating physicians were gynecologists (n=7); two physicians were both oncologists and gynecologists, and the specialty of one was missing. Medical records were retrospectively evaluated to assess comorbidities, AEs, recurrences, and types of treatments from January 2013 to May 2013. Of the 127 patients, 92 (72.4%) had presented for the first time with advanced EOC and 35 (27.6%) had recurrent EOC and 106 patients had at least 6 months of follow-up. The median age of included patients was 61.5 years (range: 20–84). The median age of patients with advanced and recurrent EOC was 60.8 (range: 20–84) and 63.3 years (range: 29–81), respectively. All patients with advanced FIGO stage disease were treated with chemotherapy. Of the 35 patients with recurrent EOC in this study, one patient had stage IC at initial presentation, one patient had stage II at initial presentation, 26 patients had stage III at initial presentation, and seven had stage IV EOC at initial presentation. The baseline characteristics of the patient population are shown in Table 1.
Comorbidities
A total of 73 comorbidities were reported in 44 patients (34.6%). Vascular (10.2%, 13 patients), metabolic (7.1%, nine patients), respiratory (5.5%, seven patients), and psychiatric disorders (5.5%, seven patients) were the most common. Patients with recurrent EOC had fewer comorbidities compared to patients with advanced EOC (ten patients, 28.6% versus 34 patients, 37%, respectively); however, this difference was not statistically significant (P=0.41). Comorbidities reported were mostly low grade, with only one grade 3 reported (depression). In patients with comorbid conditions, 23 were ≤65 years of age (30.7% of all ≤65-year-olds) compared to 21 who were >65 years of age (41.2% of all ≤65-year-olds) (P=0.26). Fifteen patients had more than one comorbidity, nine patients had two comorbidities, and six patients had more than two comorbidities. One patient had eight comorbidities reported. The prevalence and severity of most common comorbidities is reported in Tables 2 and 3, respectively. Chi-square tests suggested that no differences existed in terms of comorbidity (P=0.65) occurrence between the two center types (hospital based and oncology center).
  | Table 2 Prevalence of comorbidities by system organ class |
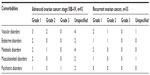  | Table 3 Prevalence of comorbidities by system organ class by grade |
Adverse events
The overall prevalence of AEs was 74.8% among all patients, with 95 patients experiencing at least one AE. The most common AEs were anemia (16.5%, 21 patients), hematologic events (12.6%, 16 patients), taste change (11.8%, 15 patients), and headache (7.1%, 9 patients). Sixteen patients experienced SAEs (12.6%). In patients experiencing AEs, 56 were ≤65 years of age (which makes 74.6% of all ≤65-year-olds) compared to 39 who were >65 years of age (which makes 76.5% of all ≤65-year-olds) (P=0.82). AEs were more frequent in the recurrent EOC patients compared with advanced EOC patients (30/35 [85.7%] versus 65/92 [70.7%], respectively), although the result is of marginal nonsignificance (P=0.08). SAEs were more frequent in recurrent EOC patients compared with advanced EOC patients (9/35 [25.7%] versus 7/92 [7.6%]), a difference which was statistically significant (P=0.006). AEs were more frequent in the first-line setting (71 out of 127 patients, 55.9%). Twenty out of 45 patients experienced AEs in the second-line setting (44.4%), three out of twelve patients experienced AEs in the third-line setting (25%), and one out of four patients experienced AEs in the fourth-line setting (25%). Chi-square tests suggested that no differences existed in terms of AE (P=0.55) occurrence between the two center types. Of the 95 patients with AEs reported, 63 patients had more than one AE reported, while 40 patients had two AEs reported, two patients had seven AEs reported. AEs are listed in Table 4. Two patients experienced grade 4 gastrointestinal perforation. One patient experienced grade 5 electrolyte disorders in the recurrent setting. The majority of AEs reported were grade 1–3, and a few grade 4 and 5 events were reported (in recurrent EOC patients): severity of AEs is listed in Table 5. Of 127 patients, twelve patient deaths were reported (six due to disease progression).
  | Table 4 Prevalence of adverse events by system organ class |
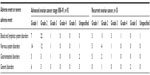  | Table 5 Severity of adverse events |
Platinum sensitivity
Table 6 reports treatment patterns and platinum sensitivity. Of a total of 35 patients in this group, 16 patients were highly sensitive to platinum (45.7%), eleven were platinum sensitive (31.4%), seven were platinum resistant (20.0%), and one patient was platinum refractory (2.9%). The majority of the 35 patients in all the platinum groups were in the below 70 years age group, except the platinum resistant patient group, where five of seven patients were in the ≥70 years age group. In the first-line setting, all patients had received platinum-based chemotherapy. One patient received a platinum monotherapy regimen. For second-, third-, and fourth-line chemotherapy, 79.4%, 30%, and 75% of patients, respectively, received platinum-based treatment.
  | Table 6 Treatment patterns and platinum sensitivity |
Discussion
In this French hospital-based chart review, 127 patients with advanced or recurrent EOC were included. The overall prevalence of comorbidities was 34.6%, and the prevalence of AEs was 74.8% in this study. The most common comorbidities were vascular, metabolic, and nutrition disorders. Recently, four consecutive positive randomized trials adding bevacizumab to chemotherapy in the treatment of both front-line (GOG 218)10 and (ICON7)11 and recurrent setting platinum resistant (AURELIA Trial)12 or platinum sensitive (OCEANS Trial)13 have been reported. In this French cohort study, we report, 10.2% and 1.6% of patients with vascular and cardiac preexisting comorbidities, respectively. This highlights the necessity of carefully considering the benefit–risk balance while prescribing bevacizumab, as its use has been linked to an increased risk for arterial thromboembolic events.
Malnutrition and depression have previously been reported to be independent poor prognostic factors in EOC.14,15 In this study, nutrition and metabolic disorders are the most common comorbidities reported, and depression was the worst comorbidity reported (grade 3). The frequency of these comorbidities highlights the need for a multidisciplinary approach when managing EOC patients, to give them the best chance of successful treatment.
Almost three-quarters of the patients in this study experienced at least one AE. Given the fact that AEs are frequent, reporting them is particularly important to assess the benefit–risk balance of a therapy. In this context, the Consort group recommended to report patient reported outcomes as primary or secondary outcomes in randomized controlled trials to improve clinical decision-making.16,17 The AEs reported in this cross-sectional study were mostly anemia and hematological events. As anemia was considered an AE after initiation of treatment, it is most likely to be due to chemotherapy. Stålberg et al18 reported that hematologic complications such as thrombocytopenia and anemia were important prognostic factors in EOC. One out of eight patients (12.5%) in this study had SAEs reported, with a predominance in patients with recurrent EOC (25.7%) compared to advanced EOC patients (7.6%). The frequency of SAEs in this palliative context is a serious issue, as one of the objectives of palliative chemotherapy is to improve patients’ quality of life, decrease symptoms in relation to disease, and limit treatment-related toxicities. SAEs can be directly related to or unrelated to medical treatment (including chemotherapy) or to the natural history of disease including disease progression.
With regard to sensitivity to platinum-based chemotherapy, older patients in this study had lower rates of platinum sensitivity at 6 months; however, due to the small number of patients in this category, it is difficult to draw firm conclusions. In contrast, Eisenhauer et al19 reported similar rates of platinum sensitivity at 6 months: 61% in patients ≥65 years compared to 65% in patients <65 years, respectively, in a cohort study of EOC patients. However, elderly patients may experience different clinical management compared to younger patients and may develop excessive treatment-related toxicity, leading to dose limitations and treatment termination. It is possible that the higher rate of platinum-resistant diseases in older patients might be explained by suboptimal use of platinum-based chemotherapy, leading to a decreased efficacy.
Eight patients of 35 were in the platinum-resistant or refractory group in this study, and three of these eight patients received a platinum-based chemotherapy in the second-line setting, in a combination regimen. This is not recommended clinical practice, as referenced by the National Comprehensive Cancer Network guidelines for treatment of EOC, and adherence to these guidelines is known to be correlated with improved survival.20 However, considering the small number of patients in this group, this finding must be interpreted with caution.
Limitations
One of the main limitations in this study is that the total number of patients included in the final report is relatively small (n=127), making interpretation of subgroup analysis difficult. It may also be difficult to generalize the results to other similar populations due to the potential for selection bias. It is possible that the physicians who participated in this study were not adequately representative of oncologists or gynecologists who treat EOC in France due to the relatively small number included. Patient selection was not random, as physicians were requested to select the most up to date and consecutive patients. Physicians may have selected patients on active follow-up, whose medical records were easier to access. As allocation of patients into platinum status subgroups was completed by physicians, it is possible there was a degree of misclassification bias, as we were not able to check platinum status for all patients, with follow-up being less than 12 months for some patients. In addition, as we were only able to include 35 patients in the platinum sensitivity analysis section, the data in this section may not be representative of the true distributions of platinum sensitivity groups or other similar ovarian cancer populations. There is potential for information bias when assessing medical notes for records of AEs or comorbidities. Some AEs or comorbidities will have to be treated at primary care centers or outpatient clinics outside of the hospital based gynecology or oncology setting, and therefore, these will be under reported or missing in the hospital medical notes. Moreover, only the more SAEs may have been reported or recorded in the hospital setting. It is also more likely that SAEs that resulted in death (such as pulmonary embolism) were captured more often that those not resulting in death, because they were listed as the cause of death. Thus, the frequency of comorbidities and SAEs and AEs may be underreported in this study. This underreporting is more likely to happen in retrospective chart reviews such as this study, which may not rigorously capture as many reported clinical AEs as are recorded in randomized controlled trials, where ongoing clinical monitoring of events and independent source document verification is performed.
Conclusion
In conclusion, in this French cross-sectional study, one-third of EOC patients had comorbidities: the most common comorbidities reported were vascular, metabolic, and nutrition disorders. Almost 75% of the patients with advanced or recurrent EOC experienced AEs. Approximately, one in four patients in the recurrent EOC setting experienced SAEs. Further, similar studies are needed in this research field which is a matter of importance in relation to treatment of EOC patients.
Acknowledgments
The authors express their gratitude to all the physicians who accepted to participate in the study and provided data. Recruitment, data management, statistical analysis, and medical writing were provided by Cegedim Strategic Data, Boulogne-Billancourt, France. This study was funded by Amgen Ltd.
Disclosure
Olivia Le Saux has no conflicts of interest in this work; Gilles Freyer is a consultant for Amgen France; Victoria Chia is employed by Amgen Inc. and owns Amgen stock; Demetris Pillas is employed by Amgen Ltd. At the time of writing, Aliki Taylor and Moninder Kaur were employed by Amgen Ltd, and Aliki Taylor owned Amgen stock. The authors report no other conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Ervik M, et al. Globocan 2012 v 1.0. Cancer Incidence and Mortality Worldwide: IARC Cancerbase number 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013. Available from: http://globocan.iarc.fr. Accessed May 2015. | |
National Cancer Institute. SEER (Surveillance, Epidemiology and End Results Program). SEER Cancer Fact Sheets: Cancer of the Ovary. Available from: http://seer.cancer.gov/statfacts/html/ovary.html. Accessed May 2015. | |
du Bois A, Quinn M, Thigpen T, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIGOCCC 2004). Ann Oncol. 2005;16(Suppl 8):viii7–viii12. | |
Cannistra SA. Cancer of the Ovary. New Engl J Med. 2004;351: 2519–2529. | |
Lowe KA, Chia VM, Taylor A, et al. An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol. 2013;130: 107–114. | |
Janssen-Heijnen ML, Houterman S, Lemmens VE, Louwman MW, Coebergh JW. Age and co-morbidity in cancer patients: a population-based approach. Cancer Treat Res. 2005;124:89–107. | |
Gulbech Ording A, Garne JP, Witt Nyström PM, Frøslev T, Toft Sørensen H, Lash TL. comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis – a Danish nationwide matched cohort study. PLoS One. 2013;8(10):e76013. | |
Tetsche MS, Dethlefsen C, Pedersen L, Sorensen HT, Norgaard M. The impact of comorbidity and stage on ovarian cancer mortality: a nationwide Danish cohort study. BMC Cancer. 2008;8:31. | |
Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist. 2002;7(Suppl 5):20–28. | |
Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. | |
Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. | |
Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. | |
Aghajanian C, Blank S, Goff B, et al. OCEANS: a randomized, double-blind, placebo-controlled phase iii trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. | |
Torres ML, Hartmann LC, Cliby WA, et al. Nutritional status, CT body composition measures and survival in ovarian cancer. Gynecol Oncol. 2013;129:548–553. | |
Gupta D, Lis CG, Vashi PG, Lammersfeld CA. Impact of improved nutritional status on survival in ovarian cancer. Support Care Cancer. 2010;18:373–381. | |
Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD; CONSORT PRO Group. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309: 814–822. | |
Friedlander ML, King MT. Patient-reported outcomes in ovarian cancer clinical trials. Ann Oncol. 2013;24(Suppl 10):x64–x68. | |
Stålberg K, Svensson T, Lönn S, Kieler H. The influence of comorbidity on mortality in ovarian cancer patients. Gynecol Oncol. 2014;133: 298–303. | |
Eisenhauer EL, Tew WP, Levine DA, et al. Response and outcomes in elderly patients with stages IIIC–IV ovarian cancer receiving platinum-taxane chemotherapy. Gynecol Oncol. 2007;106:381–387. | |
Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121:1226–1234. |
Supplementary materials

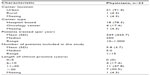  | Table S1 Participating physicians’ characteristics |
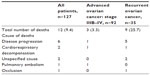  | Table S2 Number of deaths and cause of deaths |
  | Table S3 Platinum sensitivity by age and FIGO stage |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

