Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Cross-sectional and longitudinal assessments of risk factors associated with hypertension and moderately increased albuminuria comorbidity in patients with type 2 diabetes: a 9-year open cohort study
Authors Hadi Alijanvand M, Aminorroaya A , Kazemi I, Aminorroaya Yamini S , Janghorbani M , Amini M, Mansourian M
Received 20 October 2018
Accepted for publication 21 May 2019
Published 15 July 2019 Volume 2019:12 Pages 1123—1139
DOI https://doi.org/10.2147/DMSO.S189726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Moluk Hadi Alijanvand,1–3 Ashraf Aminorroaya,2 Iraj Kazemi,4 Sima Aminorroaya Yamini,5 Mohsen Janghorbani,1,2 Masoud Amini,2 Marjan Mansourian1,2
1Department of Epidemiology and Biostatistics, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran; 2Isfahan Endocrine and Metabolism Research Centre, Isfahan University of Medical Sciences, Isfahan, Iran; 3Student Research Center, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran; 4Department of Statistics, College of Science, University of Isfahan, Isfahan, Iran; 5Department of Engineering and Mathematics, Sheffiled Hallam University, Sheffield, UK
Background: Moderately increased albuminuria (MIA) is strongly associated with hypertension (HTN) in patients with type 2 diabetic mellitus (T2DM). However, the association between risk factors and coexisting HTN and MIA remains unassessed.
Objectives: This study aimed to determine both cross-sectional and longitudinal associations of risk factors with HTN and MIA comorbidity in patients with T2DM.
Methods: A total of 1,600 patients with T2DM were examined at baseline and longitudinal data were obtained from 1,337 T2DM patients with at least 2 follow-up visits to assess the presence of HTN alone (yes/no), MIA alone (yes/no) and the coexistence of both (yes/no) in a 9-year open cohort study between 2004 and 2013. Bivariate mixed-effects logistic regression with a Bayesian approach was employed to evaluate associations of risk factors with HTN and MIA comorbidity in the longitudinal assessment.
Results: After adjustment for age and BMI, patients with uncontrolled plasma glucose, as a combined index of the glucose profile, were more likely to have HTN [odds ratio (OR): 1.73 with 95% Bayesian credible intervals (BCI) 1.29–2.20] and MIA [OR: 1.34 (95% BCI 1.13–1.62)]. The risks of having HTN and MIA were increased by a one-year raise in diabetes duration [with 0.89 (95% BCI 0.84–0.96) and 0.81 (95% BCI 0.73–0.92) ORs, respectively] and a one-unit increase in non-high-density lipoprotein-cholesterol (Non-HDL-C) [with 1.30 (95% BCI 1.23–1.34) and 1.24 (95% BCI 1.14–1.33) ORs, respectively].
Conclusions: T2DM patients with HTN, MIA, and the coexistence of both had uncontrolled plasma glucose, significantly higher Non-HDL-C, and shorter diabetes duration than the other T2DM patients. Duration of diabetes and uncontrolled plasma glucose index showed the stronger effects on HTN and MIA comorbidity than on each condition separately.
Keywords: hypertension, moderately increased albuminuria, microalbuminuria, type 2 diabetes, comorbidity, risk factor
Introduction
Globally, the prevalence of type 2 diabetes mellitus (T2DM) continues to increase.1,2 More than 50% of patients with diabetes mellitus have also hypertension (HTN)3 with three times higher rate than the general population.4 The risk of cardiovascular disease (CVD) in the T2DM patients with HTN is four-times higher than the normotensive non-diabetic people.5 Abnormal levels of urinary albumin, a marker of kidney disease, occur in 30–40% of patients with T2DM, and increase the mortality rate caused by CVD worldwide.6 Furthermore, hypertensive patients with albuminuria have almost a four-fold increased risk of ischemic heart disease than hypertensive patients without albuminuria.7 The association of HTN with moderately increased albuminuria (formerly called microalbuminuria) has been reported frequently.8,9 However, there are limited numbers of cross-sectional studies, which evaluated the association of HTN with moderately increased albuminuria (MIA) in patients with T2DM.10 Although the risk factors associated with only HTN or only MIA in patients with T2DM have been reported previously,11,12 the association of risk factors with MIA and HTN comorbidity combined have yet to be determined.
The main objective of the present study was to identify and compare the risk factors associated with HTN, MIA, and the coexistence of both in patients with T2DM using the mixed-effects models. These approaches incorporate repeated HTN, MIA, and the coexistence of both prevalence data across visits which involve the same T2DM patients.
Methods
Setting and data collection
This study was focused on high-risk individuals with T2DM to identify the cross-sectional and longitudinal associations of risk factors with HTN and MIA comorbidity combined. Furthermore, within the longitudinal assessment, the repeated measurements of potential risk factors are considered as time-dependent covariates and repeated measurement of outcome variables took into account the participants’ changes during the follow-up period.
The present study was conducted with data from the Isfahan Diabetes Open Cohort Study (IDOCS), an open cohort study in central Iran to determine various potential risk factors for CVD in the patients with T2DM. Details of the design and setting of the IDOCS have been described elsewhere.11,12 In the IDOCS study, the participants were followed-up for detection of major CVD events, including myocardial infarction, unstable angina pectoris, stroke, and sudden cardiac death.
Study participants
The present study was performed as a sub-study of IDOCS. Therefore, we retrieved information of 1608 T2DM patients without any history of major CVD recorded in the database of the Isfahan Endocrine and Metabolism Research Center (IEMRC), between 2004 and 2013. Physical examination and laboratory measurements were conducted at baseline, and repeated for cases without any major CVD events. All participants received follow-up tests, according to the Standard of Medical Care in Diabetes, to update information on anthropometric and demographic characteristics, and to record diagnosed MIA and HTN.12,13 Otherwise, the tests were repeated annually.
According to the inclusion criteria (Figure 1), T2DM patients with severely increased albuminuria (formerly called macroalbuminuria) or kidney transplantation14 were excluded at baseline (8 out of 1,608 of patients). Albumin excertion (24 hrs urine albumin concentrations) above 300 mg was considered as severely increased albuminuria based on the guideline of the European Society of Cardiology.15
Definition and assessment of outcomes, determinants, and confounders
The blood pressure was taken twice on the right arm of patients in a seated position, after 15 and 20 mins of rest, using a standard mercury sphygmomanometer (ALPK2, Japan). The mean of two measurements was recorded as the blood pressure value. The hypertensive cases were identified according to the European Society of Cardiology and European Society of Hypertension criteria.16 Patients were defined as hypertensive if they were already being treated with antihypertensive or had the systolic blood pressure ≥140 mmHg, and/or diastolic blood pressure ≥90 mmHg. The blood pressure values were coded as a binary variable by one in hypertensive patients and zero in normotensive patients.
In order to measure urinary albumin level accurately, patients were trained to collect urine samples. Urine samples with specific gravity of more than 1.015 were examined for MIA when there was no evidence of infection and/or hematuria in urinalysis. Urinary albumin and creatinine were measured by the kits, obtained from the Pars Azmoon Company, using BT3000, Zinsser Analytic, Germany. Urinary creatinine was measured by the Jaffe method to assure that 24 hrs urine was collected correctly.
The European Society of Cardiology criteria was used to diagnose MIA, and defined by a 24-hrs urine albumin concentration between 30 mg and 300 mg.15 In cases that albumin concentration exceeded 30 mg, a second 24-hrs urine sample was collected and examined for MIA. Therefore, we coded urinary albumin level as a binary response variable by one, in patients with MIA, and zero in patients with normoalbuminuria (24 hrs urine albumin concentrations of <30 mg).
Information on anthropometric indices, duration of diabetes, blood pressure, lipid profile, and plasma glucose were collected at baseline and through follow-up intervals. All clinical examination and laboratory measurements at baseline and at follow-up visits were performed, using the same standardized protocol. Anthropometric assessment, including weight and height, were conducted while participants were barefoot and lightly clothed. Patients’ weight was measured to the nearest 0.1 kg, using the Seca scale (Hamburg, Germany). Height was measured while patients were in a standing position with their shoulders in the normal position, using the Seca stadiometer. The BMI (kg/m2) was calculated as the weight (kg) divided by height (m) squared. Duration of diabetes was obtained by the interval between the age of the patient and their age in which the diabetes was diagnosed.
Blood samples were taken after 12 hrs overnight fasting to measure biochemical characteristics. FPG was analyzed by the glucose oxidase method (Clinical Chemistry Analyzer Liasys, Roma, Italy). HbA1c was measured, using a DSS machine and the ion-exchange chromatography method. Total cholesterol and HDL-C were measured. LDL-C and Non-HDL-C were calculated by the Friedewald’s equation17 and subtracting HDL-C from the total-cholesterol, respectively. All biochemical characteristics were measured in the central laboratory of the Isfahan Endocrine and Metabolism Research Center using the enzyme-linked method.
Statistical analysis
Results were reported as mean (SE) for the quantitative continuous variables and percentages for the categorical variables. At baseline, the mean age and BMI of four groups were compared, including patients with neither HTN nor MIA as control group, with only MIA, with only HTN, and with the coexistence of both, using one-way analysis of variance (ANOVA) and Tukey’s post-hoc test. The age- and BMI-adjusted means of the selected baseline characteristics including diabetes duration, glucose, and lipid profile were calculated and compared between four groups, based on one-way ANCOVA and Bonferroni correction post-hoc test. The numbers of patients with HTN, MIA, and the coexistence of both were changing during 9 years of follow-up; therefore, we presented the prevalence of these conditions, annually in Table 1, and used the mean of prevalence (overall prevalence) over the duration of the study.
 |
Table 1 Number (percent) and status of type 2 diabetic patients that newly entered into the present open cohort in each year |
In the longitudinal assessment, double univariate binary logit components were obtained from separate logistic regressions for blood pressure variable alone (dichotomized into “HTN” versus “normotension”) and urinary albumin level variable alone (dichotomized into “MIA” versus “normoalbumin”). We used the bivariate longitudinal logistic model to consider the inherent association between two above-mentioned binary outcomes. The dropouts (unbalanced longitudinal data) are shown in Figure 2. In order to determine the longitudinal associations between predictors and outcomes, considering the dropout data, mixed-effects logistic regression was performed as an appropriate method with enough precision.18 In addition to dropouts, there was missing data in the covariates (described in Figure 1). We use all the available follow-up data (Figure 2) in the open cohort database to maximize the power and generalizability of the longitudinal results. We adopted the Bayesian approach, as an advanced statistical approach to estimate parameters while of the missing covariates handled in the above-mentioned models. The Bayesian data analysis was conducted, using Open BUGS version 3.2.3.19 The results of the applied models were demonstrated as odds ratios (ORs) and related 95% Bayesian credible intervals (BCI). Deviance information criterion (DIC) was used to select the best model; a lower value of DIC, indicating better model fitness. Based on all available data and the literature, the potential confounders were also identified; the confounders were then selected based on the significant correlation (or association) of the potential confounding variables with both the outcomes and the risk factors of interest.
We realized that there is a statistically significant correlation between lipid and glucose profile using Pearson’s coefficient with P<0.01 (Table S1). Therefore, to deal with the multicollinearity and to include somehow all lipid and glucose profiles in the fitted longitudinal models, Non-HDL-C was presented as lipid profile. Non-HDL-C includes various fractions of lipoproteins: low-density lipoprotein, intermediate-density lipoprotein, and very low-density lipoprotein. We also proposed the uncontrolled plasma glucose index as a combined index of the glucose profile that was coded as a binary variable. According to the glycemic control recommendation in non-pregnant diabetic patients,20 we created a local definition for the uncontrolled plasma glucose index; the FPG of ≥7.2 mmol/L (130 mg/dL) and/or 2 hrs PPG of ≥10 mmol/L (180 mg/dL) and/or HbA1C of ≥7% were coded 1 and 0 otherwise. On the other hand, age and BMI were significantly correlated with gender, uncontrolled plasma glucose, diabetes duration, Non-HDL-C, and the outcomes with (P<0.001). Therefore, in our implemented longitudinal models, gender and time-dependent variables including diabetes duration, uncontrolled plasma glucose index, and non-HDL-C were considered as the determinants, and age and BMI were counted as the confounders. Age and BMI adjusted-odds ratios were presented to determine the association of the gender, diabetes duration, uncontrolled plasma glucose index, and increasing Non-HDL-C with the adjusted-odds of having HTN and MIA, during follow-up visits.
The bivariate logistic regression with random subject-effects of longitudinal binary responses as blood pressure and urinary albumin level variables is provided (Equation (1) and Equation (2)). Where i =1, 2, …, 1,337 is the number of subject, t=1,2,3, …, 9 is the time point of subject i and 1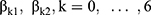 are fixed-effect terms;
are fixed-effect terms; 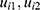 present subject-specific random intercepts for i-th subject.
present subject-specific random intercepts for i-th subject.
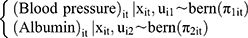
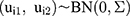
where
2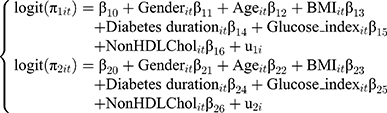
A model which uses HbA1C instead of the uncontrolled plasma glucose index was further examined to assess whether the association of glucose profile (based on the HbA1C as the gold standard of glycemic control), lipid profile, and diabetes duration with having HTN and MIA was appropriate. Finally, the above-mentioned model that included uncontrolled plasma glucose index was selected based on the model’s selection criteria.
A p-value of <0.05 was considered statistically significant for all tests, which were two-tailed. The 95% Bayesian credible intervals do not include the one value for considering significant effects of risk factors in the univariate and bivariate Bayesian models.
Results
Results of the descriptive analysis
The mean of prevalence over subsequent years of the open cohort study (overall baseline prevalence) for HTN, MIA, and the coexistence of both in patients with T2DM is 10.3% (95% confidence interval [CI] 8.8–11.8), 34.4% (95% CI 32.7–36.7), and 21.7% (95% CI 19.6–23.7), respectively (Table 1). Overall, the prevalence of HTN alone is lower than the prevalence of MIA and the prevalence of MIA and HTN combined. Moreover, Table 1 indicates that the prevalence of MIA and HTN decreases over time.
Results of the cross-sectional assessment
No statistically significant difference is observed in most baseline characteristics between attendees at the follow-up visits and non-attendees including systolic blood pressure, diastolic blood pressure, 24 hrs urine albumin concentrations, age, diabetes duration, height, weight, body mass index (BMI), glycosylated hemoglobin (HbA1c) level, fasting plasma glucose (FPG), 2 hrs postprandial plasma glucose (PPG) levels, triglyceride, total cholesterol, low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), and non-high-density lipoprotein-cholesterol (Non-HDL-C) concentrations (Table S2).
 |
Table 2 Baseline characteristics in the different groups of type 2 diabetic patients |
The baseline characteristics of the normotensive normoalbuminuric T2DM patients and T2DM patients with HTN, MIA, and the coexistence of both are shown in Table 2. The gender was balanced between the groups. However, significant differences were observed in mean age, age-adjusted means of BMI, age- and BMI-adjusted means of FPG, HbA1C, 2 hrs PPG, total-cholesterol, LDL-C, Non-HDL-C, across different study groups. According to the post-hoc comparisons of Bonferroni-corrected test, adjusted by the baseline age and BMI, the age- and BMI-adjusted mean differences of glucose and lipid profile are statistically significant between the group of patients with the MIA and HTN comorbidity, and the group with neither MIA nor HTN (P<0.001, Table S3 and Figure S1).
Among 1,600 participants at the baseline (1,071 men and 529 women), 263 subjects were also excluded from longitudinal assessment because they did not attend the follow-up examination. Therefore, 1,337 patients (912 men and 425 women) with a mean (standard error [SE]) baseline age of 45.0 (0.24) years old (range 30–81 years old) who participated in repeated measurements with available information on outcomes and covariates were included in the longitudinal assessments. Patients were followed up until detecting major CVD events (n=9), severely increased albuminuria (n=35), dropping out (n=275), or reaching the end of the study (see Figure 1), all of whom had at least one subsequent review, during the follow-up duration (median of 4 years and a range of 1–7 years). The detailed description of the participants’ numbers of visits and follow-up duration of visits are presented in Figure 2 for T2DM patients.
Results of the longitudinal analysis
The results of the selected univariate and bivariate longitudinal models (Figure 3 and Table S4) determine the association of the risk factors with MIA and HTN comorbidity (for bivariate strategy), in comparison with the longitudinal association of the risk factors with MIA, and HTN disorders separately (for univariate strategies) during follow-ups. In the bivariate model, a joint significant positive association between HTN and MIA was estimated to be 0.47 (95% CI: 0.36–0.57) during the follow-up period. The bivariate model fits better to the collected data than the separate analysis of each outcome variable, resulted in a smaller DIC (Table S4). The associations of potential risk factors with HTN and MIA in the T2DM patients were determined after measurement and adjustment for age and BMI. The results of the un-selected univariate and bivariate longitudinal models, which include Hba1c instead of glucose index, are also presented in Table S5.
The results of the selected models indicated that the odds of HTN, MIA, and the coexistence of both were significantly higher in the patients with uncontrolled plasma glucose, higher Non-HDL-C, and shorter diabetes duration than the normotensive and normoalbuminuric T2DM patients, after age- and BMI-adjustment. Furthermore, female patients are more likely to have MIA alone and MIA with HTN comorbidity than male patients (Table S4 and Figure 3).
During follow-up, diabetes duration (age- and BMI-adjusted ORs [95% BCIs] of HTN: 0.95 [0.91, 0.98] and MIA: 0.87 [0.80, 0.96] in univariate model versus age- and BMI-adjusted ORs [95% BCIs] of HTN: 0.89 [0.84–0.96] and MIA: 0.81 [0.73–0.92] in bivariate model) showed considerably stronger effects on HTN and MIA in bivariate model than in univariate model (P<0.05). Uncontrolled plasma glucose index (age- and BMI-adjusted ORs [95% BCIs] of HTN: 1.62 [1.23, 1.86] and MIA:1.25 [1.02, 1.56]: in univariate model versus age- and BMI-adjusted ORs [95% BCIs] of HTN: 1.73 [1.29–2.20] and MIA: 1.34 [1.13–1.62] in bivariate model) shows significant stronger effects on coexisting hypertension and moderately increased albuminuria than on each condition separately in Table S4 and Figure 3 (P<0.05).
Discussion
The current study demonstrated a high baseline prevalence of MIA and its coexistence with HTN, among Iranian patients with T2DM; the prevalence of HTN and MIA comorbidity was much higher than the prevalence of HTN alone in the baseline assessment. A large number of T2DM patients are at high risk for ischemic heart disease due to HTN and MIA comorbidity.7 Moreover, decreasing trend of the prevalence of MIA and/or HTN indicates that diabetes care in Iran has improved since the beginning of our study.21 In parallel, the Tehran Lipid and Glucose Study cohort reported that the use of antihypertensive, antihyperglycemic, and antihyperlipidemic medications was increased substantially over a follow-up period of more than a decade (1999–2011) among Iranian patients with type 2 diabetes.21 Hence, in the current follow-up study of 1,337 T2DM patients, after age- and BMI-adjustment during follow-up, the effects of glucose, lipid profile, and diabetes duration on having HTN and MIA separately and the coexistence of both were also estimated using relevant statistical methods.
After adjustment for age and BMI, the risk of having MIA in women was significantly higher than men, similar to the previous study, disregarding the MIA and HTN comorbidity in T2DM patients after adjusting for age.22 Although estrogen has protective effects on CVD, our data might be influenced by the use of oral contraceptive in the pre-menopausal women or post-menopausal setting and hormone replacement therapy after menopause, which were shown to be associated with an increased prevalence of MIA.23 However, our findings indicated that male patients show a slightly higher risk of having HTN, when HTN occurred separately or simultaneously with MIA in age- and BMI-adjusted analysis; the association of the gender with HTN became insignificant. This finding is consistent with previous report where the age was adjusted.11 The higher risk of HTN in male patients with type 2 diabetes might originate from poor blood pressure control in this group of patients.3
At the follow-up, T2DM patients with shorter diabetes duration were at higher risk of having MIA and/or HTN, where HTN and MIA occurred separately or simultaneously after age- and BMI-adjustment. This is in agreement with the previous cross-sectional (without adjustment for confounders) and longitudinal studies (after base adjustment for randomized treatments) conducted on the T2DM patients;24,25 however, the association of the diabetes duration with the HTN and MIA comorbidity was not considered. The longer duration of diabetes was reported as a strong risk factor for MIA and HTN disorders separately,12 without considering diabetes duration as a time-dependent variable and the longitudinal analysis. Although we anticipated higher accuracy in our findings, this discrepancy might also originate from divergent distribution of participants’ diabetes duration or difficulties in recording the duration of diabetes for T2DM patients in different studies; hyperglycemia usually starts 4–7 years before the clinical diagnosis of diabetes.26 Moreover, in our study, the effect of diabetes duration on the risk of HTN and MIA comorbidity is noticeably stronger than that of HTN or MIA disorders separately. The results obtained from the effects of shorter diabetes duration on HTN and/or MIA suggest that genetic factors might play a role in the pathogenesis of diabetes complications, including hypertension, moderately increased albuminuria, and the coexistence of both conditions at an early stage of the disease.
At baseline, the age- and BMI-adjusted means of FPG, 2h-PP, and HbA1c were significantly higher for T2DM groups with high blood pressure, MIA, and combined conditions than the normotensive normoalbuminuric T2DM patients. This was in agreement with parallel results for glucose profile of T2DM patients with only MIA or with only HTN in the previous studies.11,12 A systematic review and a meta-analysis study27 showed that 2 h-PPG and FPG with HbA1c contributes significantly to overall glycemic control. Furthermore, monitoring of 2 h-PPG will be more helpful to prevent long-term diabetes complications and achieve optimal glycemic control than FPG alone in the absence of HbA1c, especially in developing countries.27 On the other hand, FPG and 2 h-PPG were recommended as short-term indicators, in addition to the long-term measure of HbA1c, to improve the quality of glycemic control.28 Therefore, it was suggested that these three indices should be considered simultaneously as a useful glycemic control index for predicting different diabetes complications. According to this recommendation and to deal with the observed multicollinearity in glucose profile, we combined the cut-off points for 2 h-PPG, FPG, and HbA1c variables to generate a new uncontrolled plasma glucose index, based on the glycemic control recommendation in non-pregnant diabetic patients.20
We assessed and compared the glycemic status with uncontrolled plasma glucose index as a combined index of the glucose profile and HbA1c among patients with T2DM. The results obtained from the use of HbA1c instead of uncontrolled plasma glucose index indicated that the associations of the indicator of glycemic control based on Hba1c with the risk of having HTN and/or MIA weakened and are insignificant; however, the associations of other determinants and confounders with the HTN and MIA remained significant. In parallel, partial raw data of the current study were used to estimate the incidence and risk factors of HTN in T2DM patients11 similar to the current study where the continuous form of HbA1c was included, no significant effect of HbA1c was detected as an indicator of glycemic control, even after the data were adjusted for other variables such as cigarette smoking and therapeutic regimen besides to age and BMI. In the present study, univariate and bivariate models included uncontrolled plasma glucose index as a combined index of the glucose profile, instead of using HbA1c. This model was selected based on the selection criteria described in methods. In fact, the uncontrolled plasma glucose index as a combined index of the glucose profile provided us with a more reliable risk assessment for HTN, MIA, and the coexistence of both conditions. Hence, we showed the longitudinal association of uncontrolled plasma glucose level with HTN, MIA, and the coexistence of both, based on uncontrolled plasma glucose index as a combined index of the glucose profile, after age- and BMI-adjustment. This supported the results of a recent study, showing an increased prevalence of HTN in T2DM patients with poor glycemic control (HbA1C of ≥6.5%), without adjustment for confounder effects.29 Moreover, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, the Action in Diabetes and Vascular Disease (ADVANCE) study, and the Veterans Affairs Diabetes Trial (VADT) showed the importance of glycemic control (HbA1C of <6.5%) on MIA, after therapy adjustments.30 However, discordance existed between the research evidence and clinical policy statements about the intensity of glycemic control required to reduce micro- and macro-vascular complications; because population studies were different in terms of glycemic targets, used anti-hyperglycemic agents, comorbidities, and duration of diabetes mellitus.31,32 T2DM patients with uncontrolled plasma glucose have a different phenotype (who predominately have a loss of beta cells) to those who have controlled plasma, such that microvascular damage is experienced.33 Moreover, reduced arterial elasticity, due to long-term exposure of the vascular wall to elevated glucose level, leads to HTN by increasing peripheral vascular resistance.34
In the current study, uncontrolled plasma glucose further showed the stronger effect on MIA and HTN comorbidity than on each disorder separately. The relationship between uncontrolled plasma glucose and the HTN and MIA comorbidity was not investigated previously.
The Non-HDL-C was used as representative of lipid profile in the longitudinal assessment. Non HDL cholesterol provides a single index of apolipoprotein B-containing lipoproteins.35 This parameter includes very low-density lipoproteins remnants and potentially atherogenic particles which are important participants in the development of CVD disease.36 Our results showed higher age and BMI-adjusted odds for HTN and MIA in patients with higher Non-HDL-C, in agreement with the results of previous studies without considering the coexistence of both and without adjustment for confounder effects;36,37 however, the longitudinal association of non-HDL-C with HTN and MIA comorbidity were not considered in those reports.
It should be noted that the comparison of our findings with the previous literature is difficult, especially when it comes to comparing the effect of risk factors on MIA and HTN comorbidity with the effect of risk factors on MIA, and HTN disorders separately, because previous cross-sectional or longitudinal studies on the associations of risk factors with HTN and MIA in patients with T2DM have not tended to focus on HTN and MIA comorbidity.
Study strengths
The strengths of the current study include the use of samples, consisting of both men and women, using standard criteria for information on potential determinants of HTN and MIA. All measurements performed by trained staff; and the same methods were used to obtain both the baseline and the follow-up laboratory data. There was no difference between the potential risk factors of HTN and/or MIA in participants who were examined once, non-attendees and those with the follow-up.
Furthermore, we used univariate/bivariate mixed-effects (with random subject effects) logistic regression. This statistical model is an appropriate and efficient method for the analysis of natural history longitudinal data with dropout. Although it has been common to use traditional univariate/bivariate fixed-effect logistic regression for the cross-sectional analysis, and standard univariate/bivariate Cox models for time to incident detection of the outcomes, these common approaches do not make use of all the available repeated measures data.18 Bayesian inference was practically feasible for univariate/bivariate mixed-effects logistic regression, as well as for model-based handling missing covariates at random with higher statistical power than complete case (eliminate cases with missingness) method.38
Study limitations
Several limitations need to be considered in interpreting our results. First, we were unable to include several possible confounding variables that were known as risk factors for HTN and MIA, such as the type of treatment (diet, pills, and insulin), smoking behavior, alcohol and salt consumption (sodium-intake), sleeping disorder, socioeconomic status, and genetic factors. Previous studies11,12 on Iranian population showed no statistically significant association between smoking, the type of treatment and HTN and MIA, considering other covariates and disregarding the coexistence of both disorders. The second limitation is the observational design of our cohort study, which makes it impossible to assess the exposure that is not made by the researcher (there is no intervention); it is difficult to assess the causal-effects of risk factors. Furthermore, the probability of exposure can be influenced by extraneous factors, or confounders, which can also affect the outcome of the study. Therefore, the causal-effects of the risk factors are also influenced by unmeasured confounding factors. However, our modifications of the statistical models (specifically the random-subject effects in the univariate/bivariate mixed-effects logistic regressions) allowed us to somehow overcome this issue in the statistical analysis. Hence, the random subject effects often interpreted as latent characteristics that are specific to the subject represent the collective impact of factors relevant to the outcome variables but not included in the model.39 Nonetheless, we do not claim the inclusion of the random subject effects as ‘adjustment’ for unmeasured confounding variables.
Conclusion
The high prevalence rates of moderately increased albuminuria and its coexistence with hypertension at baseline have been shown in T2DM patients. The age- and BMI-adjusted odds of having hypertension, moderately increased albuminuria, and the coexistence of both conditions were significantly increased by increasing non-high-density lipoprotein cholesterol, shorter diabetes duration, and uncontrolled plasma glucose. Furthermore, after adjustment for age and BMI during follow-up visits, diabetes duration and uncontrolled plasma glucose showed significant stronger effects on coexisting hypertension and moderately increased albuminuria than on each separate condition. Our findings encourage comprehensive and motivated bad lipid and glucose profile-loss programmers at an early stage of the diagnosis of the type 2 diabetes to prevent hypertension, moderately increased albuminuria, and particularly the coexistence of both.
Ethics approval and informed consent
The present study conforms to the principles outlined in the Declaration of Helsinki. The protocol of the IDOPS was also approved by the Ethics Committee of the Isfahan University of Medical Sciences (IUMS) and Research Ethics Committee of Isfahan Endocrine and Metabolism Research Center (IEMRC) (No. 394909). Informed consent was obtained from all individual participants included in the study.
Data availability
The data used to support the findings of the present study are available from the corresponding authors upon request.
Acknowledgments
The authors would like to thank all participants. We are thankful to Mrs Maryam Zare and Mr Majid Abyar who assisted authors in using the recorded data of Isfahan Endocrine and Metabolism Research Center. We would also like to thank the Student Research Center, School of Health, Endocrine and Metabolism Research Centre, Isfahan University of Medical Sciences, Isfahan, Iran. This research was funded by the Student Research Center, School of Health, Endocrine and Metabolism Research Centre, Isfahan University of Medical Sciences, Isfahan, Iran (No. 394909).
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in regard to this work.
References
1. Collaboration NRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet. 2016;387(10027):1513–1530. doi:10.1016/S0140-6736(16)00618-8
2. Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6–12. doi:10.1089/pop.2015.0181
3. Lastra G, Syed S, Kurukulasuriya LR, Manrique C, Sowers JR. Type 2 diabetes mellitus and hypertension: an update. Endocrinol Metab Clin. 2014;43(1):103–122. doi:10.1016/j.ecl.2013.09.005
4. Ko S-H, Kwon H-S, Kim DJ, et al. Higher prevalence and awareness, but lower control rate of hypertension in patients with diabetes than general population: the fifth korean national health and nutrition examination survey in 2011. Diabetes Metab J. 2014;38(1):51–57. doi:10.4093/dmj.2014.38.1.51
5. Hu G, Jousilahti P, Tuomilehto J. Joint effects of history of hypertension at baseline and type 2 diabetes at baseline and during follow-up on the risk of coronary heart disease. Eur Heart J. 2007;28(24):3059–3066. doi:10.1093/eurheartj/ehm501
6. Mohan MM. Prevalence and risk factors of microalbuminuria in type 2 diabetes mellitus. Ijam. 2017;2(4):283–386.
7. Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis. 2014;7:13.
8. Alharf A, Cleland S, Webster J, McInnes G, Padmanabhan S. Microalbuminuria in subjects with hypertension attending specialist blood pressure clinics. J Hum Hypertens. 2016;30(9):527–533. doi:10.1038/jhh.2015.116
9. Pascual JM, Rodilla E, Costa JA, Garcia-Escrich M, Gonzalez C, Redon J. Prognostic value of microalbuminuria during antihypertensive treatment in essential hypertensionnovelty and significance. Hypertension. 2014;64(6):1228–1234. doi:10.1161/HYPERTENSIONAHA.114.04273
10. Ali A, Taj A, Amin MJ, Iqbal F, Iqbal Z. Correlation between microalbuminuria and hypertension in type 2 diabetic patients. Pak J Med Sci. 2014;30(3):511. doi:10.12669/pjms.306.5684
11. Janghorbani M, Amini M. Hypertension in type 2 diabetes mellitus in Isfahan, Iran: incidence and risk factors. Diabetes Res Clin Pract. 2005;70(1):71–80. doi:10.1016/j.diabres.2005.02.017
12. Amini M, Safaei H, Aminorroaya A. The incidence of microalbuminuria and its associated risk factors in type 2 diabetic patients in Isfahan, Iran. Rev Diabet Stud. 2007;4(4):242. doi:10.1900/RDS.2007.4.242
13. Association AD. Standards of medical care in diabetes—2015 abridged for primary care providers. Clin Diabet. 2015;33(2):97. doi:10.2337/diaclin.33.2.97
14. Amer H, Fidler M, Myslak M, et al. Proteinuria after kidney transplantation, relationship to allograft histology and survival. Am J Transplant. 2007;7(12):2748–2756. doi:10.1111/j.1600-6143.2007.02006.x
15. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis. 2012;223(1):1–68. doi:10.1016/j.atherosclerosis.2012.05.007
16. Mancia G, De Backer G, Dominiczak A, et al. ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25(9):1751. doi:10.1097/HJH.0b013e3282f0580f
17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
18. Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annu Rev Clin Psychol. 2010;6:79–107. doi:10.1146/annurev.clinpsy.032408.153550
19. Spiegelhalter D, Thomas A, Best N, Lunn D. OpenBUGS User Manual, Version 3.0. 2. Cambridge: MRC Biostatistics Unit; 2007.
20. Fonseca V. Diabetes: Improving Patient Care. New York: Oxford University Press; 2009.
21. Jahangiri-Noudeh Y, Akbarpour S, Lotfaliany M, et al. Trends in cardiovascular disease risk factors in people with and without diabetes mellitus: a Middle Eastern cohort study. PLoS One. 2014;9(12):e112639. doi:10.1371/journal.pone.0112639
22. Mattix HJ, Hsu C-Y, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13(4):1034–1039.
23. Monster TB, Janssen WM, de Jong PE. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med. 2001;161(16):2000–2005.
24. Zoungas S, Woodward M, Li Q, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–2474. doi:10.1007/s00125-014-3369-7
25. Bała MM, Płaczkiewicz-Jankowska E, Topór-Mądry R, et al. Characteristics of patients with type 2 diabetes of short duration in Poland. Pol Arch Med Wewn. 2009;119:533–540.
26. Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care. 1992;15(7):815–819. doi:10.2337/diacare.15.7.815
27. Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health. 2015;73(1):43. doi:10.1186/s13690-015-0088-6
28. Kohnert K-D, Heinke P, Vogt L, Salzsieder E. Utility of different glycemic control metrics for optimizing management of diabetes. World J Diabetes. 2015;6(1):17. doi:10.4239/wjd.v6.i1.17
29. Fa’iza Abdullaha TMH, Norc MBM, MAa MA, Ismaild IZ. Prevalence of hypertension and glycaemic control in adult type-2 diabetes patients: a preliminary retrospective cohort study in Kuantan, Pahang, Malaysia. Int Med J Malaysia. 2017;16:1.
30. Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials. Circulation. 2009;119(2):351–357. doi:10.1161/CIRCULATIONAHA.108.191305
31. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus. Circ Cardiovasc Qual Outcomes. 2016. CIRCOUTCOMES. 116.002901. doi:10.1161/CIRCOUTCOMES.116.002901
32. Zoungas S, Chalmers J, Ninomiya T, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55(3):636–643. doi:10.1007/s00125-011-2404-1
33. Russo GT, Giorda CB, Cercone S, Nicolucci A, Cucinotta D, Group BS. Factors associated with beta-cell dysfunction in type 2 diabetes: the BETADECLINE study. PLoS One. 2014;9(10):e109702. doi:10.1371/journal.pone.0109702
34. McVeigh GE, Bratteli CW, Morgan DJ, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension. 1999;33(6):1392–1398.
35. Aziz KM, Al-Qahtani M. Association between non-HDL and HDL cholesterol with microalbuminuria in patients with diabetes. J Diabetol. 2013;1:4.
36. Contreras F, Lares M, Castro J, et al. Determination of non-HDL cholesterol in diabetic and hypertensive patients. Am J Ther. 2010;17(3):337–340. doi:10.1097/MJT.0b013e3181c1233c
37. Popoola OA, Folaranmi OM. Hypertriglyceridemia and non-HDL cholesterol in the development of diabetic nephropathy. Eur J Pharm Med Res. 2015;2:2.
38. Kim S, Sugar CA, Belin TR. Evaluating model‐based imputation methods for missing covariates in regression models with interactions. Stat Med. 2015;34(11):1876–1888. doi:10.1002/sim.6435
39. Kahlert J, Gribsholt SB, Gammelager H, Dekkers OM, Luta G. Control of confounding in the analysis phase–an overview for clinicians. Clin Epidemiol. 2017;9:195. doi:10.2147/CLEP.S129886
Supplementary materials
 |
Table S1 Correlation between fasting plasma glucose, 2 hrs postprandial plasma glucose, and glycosylated hemoglobin at baseline |
 |
Table S2 Baseline characteristics in T2DM patients with or without follow-up |
 |
Table S3 Results of multiple pairwise comparisons between the groups at baseline |
 |
Table S4 Result of Bayesian univariate and bivariate logistic regression with random subject effect, with and without considering moderately increased albuminuria and hypertension comorbidity |
 |
Table S5 Result of Bayesian univariate and bivariate logistic regression with random subject effect, with and without considering moderately increased albuminuria and hypertension comorbidity |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




