Back to Journals » Clinical and Experimental Gastroenterology » Volume 15
Critical Appraisal of Filgotinib in the Treatment of Ulcerative Colitis: Current Evidence and Place in Therapy
Authors Dal Buono A , Gabbiadini R, Solitano V, Vespa E, Parigi TL , Repici A , Spinelli A, Armuzzi A
Received 4 May 2022
Accepted for publication 13 July 2022
Published 23 July 2022 Volume 2022:15 Pages 121—128
DOI https://doi.org/10.2147/CEG.S350193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Koulaouzidis
Arianna Dal Buono,1 Roberto Gabbiadini,1 Virginia Solitano,1,2 Edoardo Vespa,1,2 Tommaso Lorenzo Parigi,1,2 Alessandro Repici,2,3 Antonino Spinelli,1,2,4 Alessandro Armuzzi1,2
1IBD Center, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; 2Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; 3Endoscopy Unit, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; 4Colon and Rectal Surgery Division, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
Correspondence: Alessandro Armuzzi, IBD Center, Humanitas Research Hospital – IRCCS, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Rozzano, Milan, Italy, Tel +39(0)282245555, Fax +39(0)282242591, Email [email protected]
Background and Aims: Patients affected by moderate-to-severe Ulcerative Colitis (UC) demand a challenging management. Small molecules, administrated as oral agents, have the ambition of overcoming the limitations of the biologic agents (ie, parenteral administration, rapidity of action, primary and secondary non-responsiveness). Beyond tofacitinib, a pan-Janus kinase (JAK) inhibitor already approved for the treatment of moderate-to-severe UC, novel more selective molecules like filgotinib are being currently evaluated in randomized clinical trials. We aimed to review the current evidence on filgotinib, a JAK-1 preferential inhibitor, in the treatment of UC and its place in therapy in the current scenario.
Methods: PubMed and EMBASE were searched to identify relevant studies: those investigating the efficacy and safety of filgotinib in the treatment of UC patients were included in this narrative review.
Results: The current preliminary data have shown that filgotinib is safe and effective in inducing clinical end endoscopic response in both biologic-naïve and biologic-experienced patients with moderate-to-severe UC, also with high inflammatory burden at baseline. In the SELECTION trial, one case of pulmonary embolism occurred with filgotinib 200 mg induction, and three venous thrombosis cases were observed in the placebo maintenance/LTE; the incidence of herpes zoster was ≤ 1% in all UC treated patients. Filgotinib represents an appealing treatment option for its high selectiveness, route of administration and rapidity of action; cost-effectiveness studies and head-to-head trials are needed to better define its place in therapy.
Keywords: ulcerative colitis, filgotinib, Janus kinase inhibitors, efficacy, safety
Introduction
Ulcerative colitis (UC) is a chronic, relapsing-remitting disorder affecting the colon and rectum characterized by mucosal and submucosal inflammation.1 The pathogenesis of UC, despite not fully understood yet, is multifactorial and immune-mediated.1 The progressive behavior of UC can potentially lead to disability and long-term complications such as dysplasia and cancer development, hospitalization and irreversible bowel damage eventually requiring proctocolectomy.2,3
As recommended by the Selecting Therapeutic Targets in IBD (STRIDE II), the reduction of these late complications represents the major endpoint and the backbone of medical treatment.4
In the very last years, the progress in the knowledge of pathological mechanisms together with the advancing biotechnologies have led to an increasingly rapid advent of novel biologics and small molecules available for the treatment of moderate-to-severe UC. In particular, since the introduction of anti-tumor necrosis factor alpha biologics (anti-TNFa) (ie, infliximab, adalimumab, golimumab), anti-integrins (vedolizumab) and, more recently, of anti-interleukins (ustekinumab), the natural history of the disease has started to change. Increasing rates of corticosteroid-free remission, mucosal healing, deep remission, and an ameliorated quality of life of UC patients have become possible.5–7 Nevertheless, the treatment with biologics implicates various limitations, such as the primary non-responsiveness, a rather limited efficacy, the eventual secondary loss of response, and the risk of immunogenicity. Moreover, biologics have a parental administration, either intravenous or sub-cutaneous, which can impair their tolerance. Small molecules, administrated as oral agents, have the ambition of overcoming such limitations. Initially, tofacitinib, a Janus kinase (JAK) inhibitor, was the first small molecule drug approved by international regulatory authorities for the treatment moderate-to-severe UC.8 Indeed, the cellular transduction pathway activated by JAK, intracellular tyrosine kinases, is involved in the pathogenesis of UC,9,10 and its pharmacological inhibition has been demonstrated as an effective treatment for UC.8 Filgotinib is an oral JAK1 preferential inhibitor, that has been evaluated in several randomized controlled trials (RCTs) for both rheumatological disorders (ie, psoriatic arthritis, ankylosing spondylitis)11–13 and IBD, and finally approved in patients affected by rheumatoid arthritis and moderately to severely active UC. In 2016 the randomized, double-blind, placebo-controlled Phase 2 study, the FITZROY study provided the first evidences on the efficacy and safety of filgotinib for the treatment of active moderate-to-severe Crohn’s disease (CD).14 Specifically, in the treatment arm the clinical remission, defined as Crohn’s Disease Activity Index (CDAI) less than 150 at week 10, was achieved by a significantly greater proportion of CD patients as compared with placebo (47% vs 23%, respectively, p=0.0077).14 Furthermore, filgotinib was superior to placebo as concerns improvements in quality of life, endoscopic response, remission, and deep remission in CD patients.14
In this clinical scenario of a constantly growing therapeutic armamentarium, due to the lack of studies of direct comparisons, drug positioning remains a clinical challenge for dedicated physicians with a focus on IBD.
In this review, we aim to examine and summarize the current evidence and the latest advances on filgotinib as a therapeutic agent for UC.
Pharmacology
Small molecules are organic compounds characterized by a low molecular weight (<900 Da); they are mostly administered orally, and can pass through cell membranes reaching intracellular targets and regulating cellular transduction.15 JAK inhibitors (JAKi) belong to this pharmacological class, being non-immunogenetic, rapidly absorbed and with short half-lives of a few hours.16
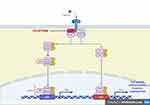
As concerns the rationale of inhibiting the JAK-STAT pathway, these cellular kinases are crucial in mediating the signaling of inflammatory cytokine.9,10 JAK proteins, intracellular cytoplasmic tyrosine kinases, are divided into four subtypes: JAK1, JAK2, JAK3 and TYK2;17 every JAK isoform exhibits binding preferences for different cytokines' receptors.17 They are constitutively associated with intracellular domains of type I and/or type II cytokine receptors, once the extracellular ligand binds the receptor, JAKs activate in pairs via phosphorylation and downstream activate STAT transcription factors18,19 (Figure 1). Finally, cellular growth, differentiation, migration, and survival, especially of the T lymphocytes, are regulated by this pathway.18,19 Since every JAK subtype is associated with different cytokine receptors, its inhibition simultaneously modulates multiple effects.
Filgotinib is an orally, once daily administered JAKi, selectively blocking JAK1 and, in a lower degree, JAK2 with a greater potency as compared with JAK3 and TYK2.20 A 28 to 30-fold higher selectivity in inhibiting JAK1 over JAK2 has been observed in human whole-blood assays.20 The absorption of filgotinib is rapid and reaches the median peak plasma concentration 2 to 3 hours after administration.21
Importantly, the concentrations of filgotinib are not influenced by food or proton pump inhibitors' coadministration.20–22 With respect to its metabolism, that occurs independently from CYP450, filgotinib is metabolized by carboxylesterases with the production of an active metabolite. The mean half-life of filgotinib is 7 hours, with a terminal elimination half-life of its active metabolite of 21 to 27 hours, after administration in single dose.21 The contribution to the clinical activity is given both by the parent molecule and the active metabolite, and the maximum pharmacodynamic effects are achieved for the dosage of 200 mg filgotinib daily.21 Filgotinib and its active metabolite are mainly eliminated in the urine (80–85%), but a small amount is eliminated throughout the intestine in the feces (15%).18 The pharmacokinetics of filgotinib is affected by renal impairment only in advanced stages (estimated creatinine clearance [CrCl] from 30 to 60 mL/min, or CrCl from 15 to 30 m/min): in these patients a lower dose (ie, 100 mg/day) is recommended.18,21
Furthermore, no clinically significant differences were observed in patients treated with filgotinib depending on age, gender, race or bodyweight.18,21 Lastly, filgotinib and its active metabolite do not interact with uridine 5’-diphospho-glucuronosyltransferases, do not inhibit key drug transporters, and showed a lack of relevant pharmacokinetic interactions with drugs that are substrates of CYP3A4 (ie, midazolam, levonorgestrel/ethinyl estradiol) exhibiting a low liability for drug–drug interactions.23,24
Strictly linked to its mechanism of action, the most common adverse events (AEs) associated with filgotinib include upper respiratory infections, nasopharyngitis, urinary tract infections, nausea, headache, and occasional diarrhea. These reported AEs are generally not serious. Safety data of special interest (ie, incidence of herpes zoster and cardiovascular events) are discussed in details below.
Table 1 resumes the pharmacokinetic and pharmacodynamic features of filgotinib.
 |
Table 1 Pharmacokinetic and Pharmacodynamic Features of Filgotinib |
Efficacy
The phase 2b/3, double-blind, randomised, placebo-controlled trial (the SELECTION trial), including two induction studies and one maintenance study, assessed the efficacy and safety of filgotinib in patients with moderately to severely active UC.25 The treatment arms for both biologic-naive (induction study A) and biologic-experienced (induction study B) patients, randomly assigned, were filgotinib 200 mg, filgotinib 100 mg, and placebo.25 The primary endpoint of this trial was clinical remission at week 10, defined as Mayo endoscopic subscore ≤1, rectal bleeding subscore = 0, and ≥1-point decrease in stool frequency subscore from baseline to achieve a subscore ≤1.25
Indeed, significantly higher rates of clinical remission at week 10 was achieved by both biologic-naive and biologic-experienced patients given filgotinib 200 mg compared with placebo (26.1% vs 15.3%, 95% CI 2.1–19.5; p=0·0157 and 11.5% vs 4.2%, 95% CI 1.6–12.8; p=0·0103, respectively).25 As concerns the maintenance study phase, 37.2% of patients in the filgotinib 200 mg group remained in clinical remission at week 58, compared with 11.2% of patients in the placebo group (95% CI 16.0–35.9; p<0·0001).25 Clinical remission did not significantly differ between filgotinib 100 mg and placebo at week 10, but was significant at week 58 (23.8% vs 13.5%, 95% CI 0.0–20.7; p=0·0420).25 Remarkably, the efficacy of filgotinib 200 mg on clinical remission compared with placebo at week 58 was consistent across all the subgroups of patients (ie, biologic-naïve, biologic-experienced, anti-TNFa failure, vedolizumab failure, and both TNF antagonist and vedolizumab) both at week 10 and 58.25 The investigated secondary endpoints in the induction studies were Mayo Clinic Score remission (a total of 2 or less and no single subscore higher than 1), endoscopic remission (defined as Mayo endoscopic subscore of 0), histologic remission (based on Geboes Scale), at week 10 and at week 58, whereas in the maintenance study, 6-months corticosteroid-free clinical remission and sustained clinical remission at week 58 were also assessed.25 In details, endoscopic remission at week 10 was achieved by 12.2% of biologic-naive patients given filgotinib 200 mg vs 3.6% of patients in the placebo group (95% CI 2.9–14.3; p=0.0047) and by 3.4% of biologic-experienced patients given filgotinib 200 mg vs 2.1% of patients in the placebo group (95% CI 2.5–5.1; p=0.42).25 Furthermore, histologic remission at week 10 was achieved in a significantly higher proportion of biologic-naive patients (35.1% vs 16.1%, 95% CI 9.9–28.2; p<0·0001) and of biologic-experienced patients given filgotinib 200 mg (19.8 vs 8.5%, 95% CI 4.2–18·6; p=0·0019) as compared to patients in the placebo group.25 As concerns longer term endpoints, it was observed that at week 58 a significantly greater amount of patients receiving filgotinib 200 mg achieved all the secondary endpoints than those who received placebo.25
Recently, data of the interim analysis of the ongoing long-term extension (LTE) SELECTIONLTE trial have shown that filgotinib was effective in maintaining long-term improvements in UC with respect to symptoms’ control and health-related quality of life (HRQoL).26
Moreover, in the sub-analysis of the e SELECTION and SELECTIONLTE studies it has been reported that re-treating with filgotinib 200 mg patients with a disease worsening was beneficial: at LTE week 12, over 75% of patients had a clinical response and over 40% of patients were in clinical remission (based on partial Mayo score).27
Finally, the recently presented post-hoc analysis from the SELECTION trial, evaluating clinical, biological, health-related quality of life (HRQoL) remission and endoscopic improvements as a combined endpoint in UC patients treated with filgotinib 200 mg compared with placebo, reported that this combined endpoint was achieved by 17.6% vs 4.41% of the patients (p<0.001) by week 10 in the biologic-naïve induction cohort, and by 22.1% vs 7.14% of the patients (p=0.002) by week 58 in the maintenance cohort.28 During maintenance a lower proportion of minimal clinically important difference (MCID) decline, assessed thorough EQ5D utility, and visual analogue scale, was experienced by those patients achieving the combined endpoint.28
Among the several sub-analyses available, the assessment of Work Productivity and Activity Impairment (WPAI) during the SELECTION trial demonstrated that a significant improvement of patients’ general quality of life and work-related outcomes was achieved in the treatment arms with filgotinib 200 mg in comparison with placebo both in the induction and maintenance phases.29
Finally, filgotinib has been confirmed to be efficacious in inducing and maintaining clinical remission regardless of age, including UC patients aged >60 years.30
Safety
As concerns the safety profile of JAKi on IBD and other immune-mediated diseases patients, according to a recent meta-analysis that included also seven studies on filgotinib, the estimated global incidence rate of serious infection was 3.36 per 100 patient-years, and the estimated incidence of herpes zoster infection was 2.11 per 100 patient-years (>42.000 patients included in the analysis).31 Furthermore, the authors reported an incidence rate of non-melanoma skin cancer (NMSC) of 0.51 per 100 patient-years (one study on filgotinib included, overall 26.334 patients exposed to JAKi), and an incidence rate of deep vein thrombosis and pulmonary embolism of 0.31 per 100 patient-years years (three studies on filgotinib included, overall 24.128 patients exposed to JAKi).31
Regarding the safety of filgotinib in UC, in the induction studies of the SELECTION trial, treatment-emergent adverse events were observed in similar proportion in the placebo, in the filgotinib 100 mg, and in the filgotinib 200 mg arms:25 serious adverse events were reported in 4.7%, 5.0% and 4.3% of the patients, respectively.25 In deeper details, no deaths occurred during the induction studies with filgotinib in UC patients and the main adverse events of interest were infections (82/562, 14.6% in filgotinib 100 mg; 92/507, 18.1% in filgotinib 200 mg).25 Among these, 6 patients in the 100 mg treatment arm and 3 patients in the 200 mg treatment arms experienced a serious infection.25 One case of pulmonary embolism occurred with filgotinib 200 mg induction and three venous thrombosis cases were observed in the placebo maintenance/LTE; the incidence of herpes zoster was ≤1% in all UC treated patients.21 Adverse events, mostly mild or moderate in severity, in the maintenance study were comparably reported for both treated and untreated patients.25
Of note, in the LTE study safety events were comparable to those above reported and no new safety signals have been added.26 Similarly, upon restarting therapy with filgotinib, safety was equivalent to aforementioned data and no new safety signals were classified.27
Nevertheless, attention should be paid to FDA warning regarding the increased risk of major adverse cardiovascular events, malignancy, thrombosis and mortality with all JAKi, including filgotinib. FDA recommends that clinicians find a balance between the benefits and risks for individual patients before starting or continuing treatment with JAKi, particularly among patients at higher risk of venous and arterial thrombosis (eg, current or past smokers, those who develop a malignancy, those with cardiovascular risk factors), as does EMA recommending additional monitoring in patients treated with filgotinib.32,33
Incidences of malignancies, non-melanoma skin cancer and major adverse cardiovascular events have been found to be slightly increased in patients aged ≥60 years compared with other age groups.30
Adult males with potential reproductive interest are another population who warrant attention due to findings observed in the male reproductive system of both dogs and rats’ models, including dose-dependent impairment of spermatogenesis with reported cessation of sperm production, loss of spermatids, and seminiferous atrophy.34,35 However, data on animals are reassuring since the histopathological findings were fully or partially reversible. Furthermore, the ongoing trials on rheumatoid arthritis and IBD male patients addressing testicular safety (NCT03926195 and NCT03201445) have confirmed the safety of filgotinib in the recent ad interim analysis.
Finally, safety data on the use of filgotinib in UC patients undergoing or scheduled for surgery with respect to suspension timing and possible post-operative complications are missing.
Conclusion and Future Perspectives
This review illustrates the current evidence on efficacy and safety of filgotinib in the treatment of UC. Filgotinib is a second-generation JAKi, orally administered with a rapid mechanism of action. What emerges from our review is that, despite the lack of long-term data, the evidence on safety and efficacy of filgotinib is extremely encouraging.25–27 In particular, as observed in the SELECTION trial, both clinical remission, endoscopic remission, and histologic remission were achieved in the treatment arm with filgotinib 200 mg.25 These endpoints are particularly valuable in the treat-to-target era, where their achievement is increasingly being linked to the reduction of long-term complications. With respect to safety, the findings in UC patients were consistent to those observed for rheumatoid arthritis, and herpes zoster infections and serious infections occurred at low rates in all treatment groups,25 differently from safety data reported for pan-JAKi.
A concern on an increased risk for venous thromboembolism for all agents in the JAK class exists, however, the available evidence on filgotinib is reassuring and risk minimization measures in clinical practice are manageable. Regarding testicular toxicity, ongoing trials will evaluate reproductive safety in adult males.
Treatment scenarios for moderate-to-severe UC are rapidly evolving toward more selective and safer drugs, with possibly higher rapidity of action, and filgotinib appears as an optimal candidate for providing fast clinical benefits, improving the quality of life of our patients with a safer profile as compared to several biologic agents. Filgotinib might additionally find place in the treatment of hospitalized patients with acute severe UC in analogy with tofacitinib, that, according to recent retrospective data, has been demonstrated to be an effective induction option in this life-threatening condition.36
The precise disease phenotype that might upmost respond to filgotinib is yet to be defined, surely a concomitant arthropathy and/or the failure to anti-TNF appear to be possible clinical criteria as well as steroid-dependency or in alternative to azathioprine. Wider data are needed to define the use of filgotinib in case of recent oncological history and in case of fragile or elderly patients. In addition, the shorter half-life of filgotinib in comparison to biologic agents might represent an advantage when a rapid drug elimination is needed (ie, for a scheduled surgery).
A further open issue regards the sequence of therapies. The most recent data derived from a Bayesian network meta-analysis in patients with moderate-to-severe UC have shown the superiority of upadacitinib in terms of both efficacy and safety compared with other therapies including filgotinib.37 However, filgotinib followed upadacitinib in almost all sub-analyses.37 Specifically designed head-to-head trials comparing the different molecules will clarify this matter.
Additional relevant aspects to consider are the costs of a long-term therapy with filgotinib. Indeed, in the treatment of rheumatoid arthritis, for each patient the average price per year is valued at £10.508 based on the list price.38 As concerns UC, the exact costs for the annual treatment are still unknown, and cost-effectiveness studies are needed to support the prescription of filgotinib eventually above the different available biologic and non-biologic agents, keeping in mind that small molecules could also reduce the costs related to the scheduled visits for drug infusions and the necessity for a specialized nurse.
The current preliminary data have shown that filgotinib is safe and effective in inducing clinical and endoscopic response and remission in both biologic-naïve and biologic-experienced patients with moderate-to-severe UC, also with high inflammatory burden at baseline.25–27 As far as we are concerned, filgotinib represents an appealing treatment option for its high selectiveness, route of administration and rapidity of action, pending its approval by regulatory authorities and availability on the market.
Author Contributions
Guarantor of the article: Alessandro Armuzzi.
Author contributions: All authors made a significant contribution to the work reported, whether that is in the conception, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This paper was not funded.
Disclosure
A Armuzzi has received consulting and/or advisory board fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, Takeda, lecture and/or speaker bureau fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mitsubishi Tanabe, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, Tigenix, and research grants from MSD, Pfizer, Takeda and Biogen. A Spinelli has served as a speaker, consultant or advisory board member for Ethicon, Takeda, Pfizer, Sofar, Oasis. A Repici received consultancy fee from Medtronic. A Dal Buono, R Gabbiadini, V Solitano, E Vespa and T Parigi declare no conflict of interests. The authors report no other conflicts of interest in this work.
References
1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi:10.1016/S0140-6736(16)32126-2
2. Zhao M, Gönczi L, Lakatos PL, Burisch J. The burden of inflammatory bowel disease in Europe in 2020. J Crohns Colitis. 2021;15(9):1573–1587. doi:10.1093/ecco-jcc/jjab029
3. Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10(6):639–645. doi:10.1016/j.cgh.2012.01.010
4. Turner D, Ricciuto A, Lewis A, et al. International Organization for the Study of IBD. STRIDE-II: an Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi:10.1053/j.gastro.2020.12.031
5. Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45(10):1291–1302. doi:10.1111/apt.14030
6. Battat R, Duijvestein M, Guizzetti L, et al. Histologic healing rates of medical therapies for ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials. am j gastroenterol. 2019;114(5):733–745. doi:10.14309/ajg.0000000000000111
7. Paschos P, Katsoula A, Salanti G, Giouleme O, Athanasiadou E, Tsapas A. Systematic review with network meta-analysis: the impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment Pharmacol Ther. 2018;48(11–12):1174–1185. doi:10.1111/apt.15005
8. Dai C, Jiang M, Sun MJ. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;377(5):496.
9. Boland BS, Sandborn WJ, Chang JT. Update on Janus kinase antagonists in inflammatory bowel disease. Gastroenterol Clin North Am. 2014;43(3):603–617. doi:10.1016/j.gtc.2014.05.011
10. Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57(12):5023–5038. doi:10.1021/jm401490p
11. Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10162):2367–2377. doi:10.1016/S0140-6736(18)32483-8
12. van der Heijde D, Baraliakos X, Gensler LS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10162):2378–2387. doi:10.1016/S0140-6736(18)32463-2
13. Genovese MC, Kalunian K, Gottenberg JE, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA. 2019;322(4):315–325. doi:10.1001/jama.2019.9055
14. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389(10066):266–275. doi:10.1016/S0140-6736(16)32537-5
15. Shivaji UN, Nardone OM, Cannatelli R, Smith SC, Ghosh S, Iacucci M. Small molecule oral targeted therapies in ulcerative colitis. Lancet Gastroenterol Hepatol. 2020;5(9):850–861. doi:10.1016/S2468-1253(19)30414-5
16. Gilardi D, Gabbiadini R, Allocca M, et al. PK, PD, and interactions: the new scenario with JAK inhibitors and S1P receptor modulators, two classes of small molecule drugs, in IBD. Expert Rev Gastroenterol Hepatol. 2020;14(9):797–806. doi:10.1080/17474124.2020.1785868
17. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(5):521–546. doi:10.1007/s40265-017-0701-9
18. Lefevre PLC, Vande Casteele N. Clinical pharmacology of janus kinase inhibitors in inflammatory bowel disease. J Crohns Colitis. 2020;14(Supplement_2):S725–S736. doi:10.1093/ecco-jcc/jjaa014
19. Danese S, Argollo M, Le Berre C, Peyrin-Biroulet L. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut. 2019;68(10):1893–1899. doi:10.1136/gutjnl-2019-318448
20. Van Rompaey L, Galien R, van der Aar EM, Clement-Lacroix P, Nelles L, Smets B, et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol. 2013;191(7):3568–3577. doi:10.4049/jimmunol.1201348
21. Namour F, Diderichsen PM, Cox E, et al. Pharmacokinetics and Pharmacokinetic/Pharmacodynamic Modeling of Filgotinib (GLPG0634), a Selective JAK1 Inhibitor, in Support of Phase IIB Dose Selection. Clin Pharmacokinet. 2015;54(8):859–874. doi:10.1007/s40262-015-0240-z
22. Anderson K, Zheng H, Kotecha M, et al. The relative bioavailability and effects of food and acid-reducing agents on filgotinib tablets in healthy subjects. Clin Pharmacol Drug Dev. 2019;8(5):585–594. doi:10.1002/cpdd.659
23. Namour F, Desrivot J, Van der Aa A, Harrison P, Tasset C, Van’t Klooster G. Clinical confirmation that the selective JAK1 inhibitor filgotinib (GLPG0634) has a low liability for drug-drug interactions. Drug Metab Lett. 2016;10(1):38–48. doi:10.2174/1872312810666151223103353
24. Begley R, Anderson K, Watkins TR, et al. Lack of drug-drug interaction between filgotinib, a selective JAK1 inhibitor, and oral hormonal contraceptives levonorgestrel/ethinyl estradiol in healthy volunteers. Clin Pharmacol Drug Dev. 2021;10(4):376–383. doi:10.1002/cpdd.870
25. Feagan BG, Danese S, Loftus EV, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397(10292):2372–2384. doi:10.1016/S0140-6736(21)00666-8
26. Feagan B, Matsuoka K, Rogler G, et al. P491 - Efficacy and safety outcomes of long-term treatment with filgotinib 200 mg among patients with Ulcerative Colitis: an interim analysis of SELECTIONLTE. J Crohn’s Colitis. 2022;16(Supplement_1):i456. ECCO Congress 2022.
27. Vermeire S, Feagan B, Peyrin-Biroulet L, et al. P517 - Re-treatment with filgotinib in patients withUlcerative Colitis following treatment interruption: analysis of the SELECTIONand SELECTIONLTE studies. J Crohn’s Colitis. 2022;16(Supplement_1):i473. ECCO Congress 2022.
28. Schreiber S, Feagan B, Peyrin-Biroulet L, et al. OP07 - Exploring disease control by combining clinical, biological, and health-related quality of life remission with endoscopic improvements among Ulcerative Colitis patients treated with filgotinib: a post-hoc analysis from the SELECTION trial. J Crohn’s Colitis. 2022;16(Supplement_1):i007. ECCO Congress 2022.
29. Danese S, Feagan B, Schreiber S, et al. POSA350 effect of filgotinib on EQ-5D-5L and work productivity and activity impairment among patients with ulcerative colitis: results from the phase 2B/3 selection trial. Value Health. 2022;25(1):S217–8. doi:10.1016/j.jval.2021.11.1061
30. Schreiber S, Luftus EV, Maaser C, et al. Efficacy and safety of filgotinib in patients with ulcerative colitis stratified by age: post hoc analysis of the phase 2b/3 SELECTION and SELECIONLTE studies - DDW ePoster - Su1565. Gastroenterol Supplement. 2022;162(7):
31. Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1554–1573.e12. doi:10.1053/j.gastro.2020.01.001
32. FDA adds black box warning to JAK inhibitors. cites heart-related issues, cancer, death [Internet]. Available from: https://www.healio.com/news/rheumatology/20210901/fda-adds-black-box-warning-to-jak-inhibitors-cites-heartrelated-issues-cancer-death.
33. Solitano V, Fiorino G, D’Amico F, Peyrin-Biroulet L, Danese S. Thrombosis in IBD in the Era of JAK Inhibition. Curr Drug Targets. 2021;22(1):126–136. doi:10.2174/1389450121666200902164240
34. European Medicines Agency.EMA Jyseleca Public Assessment Report. Available from: https://www.ema.europa.eu/en/documents/assessment-report/jyseleca-epar-public-assessment-report_en.pdf. 2020.
35. Hellstrom WJG, Dolhain RJEM, Ritter TE, et al. MANTA and MANTA-RAy: rationale and Design of Trials Evaluating Effects of Filgotinib on Semen Parameters in Patients with Inflammatory Diseases. Adv Ther. 2022;39(7):3403–3422. doi:10.1007/s12325-022-02168-4
36. Berinstein JA, Sheehan JL, Dias M, et al. Tofacitinib for biologic-experienced hospitalized patients with acute severe ulcerative colitis: a retrospective case-control study. Clin Gastroenterol Hepatol. 2021;19(10):2112–2120.e1. doi:10.1016/j.cgh.2021.05.038
37. Panaccione R, Collins EB, Melmed GY, et al. Efficacy and safety of advanced induction and maintenance therapies in patients with moderately to severely active ulcerative colitis: an indirect treatment comparison using Bayesian Network meta-analysis - DDW ePoster - Tu1449. Gastroenterol Supplement. 2022;162(7):S–965. doi:10.1016/S0016-5085(22)62287-X
38. Filgotinib for treating moderate to severe rheumatoid arthritis | guidance | NICE [Internet]. Available from: https://www.nice.org.uk/guidance/ta676/chapter/2-Information-about-filgotinib.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.