Back to Journals » Journal of Inflammation Research » Volume 15
CREST Syndrome in Systemic Sclerosis Patients – Is Dystrophic Calcinosis a Key Element to a Positive Diagnosis?
Authors Bobeica C , Niculet E , Craescu M, Parapiru EL, Musat CL, Dinu C , Chiscop I, Nechita L, Debita M , Stefanescu V , Stefanopol IA , Nechifor A , Pelin AM , Balan G, Chirobocea S , Vasile CI , Tatu AL
Received 9 February 2022
Accepted for publication 4 May 2022
Published 9 June 2022 Volume 2022:15 Pages 3387—3394
DOI https://doi.org/10.2147/JIR.S361667
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Ning Quan
Carmen Bobeica,1,* Elena Niculet,1,2 Mihaela Craescu,1 Elena-Laura Parapiru,3,* Carmina Liana Musat,1,* Ciprian Dinu,4,* Iulia Chiscop,5,* Luiza Nechita,3,* Mihaela Debita,6,* Victorita Stefanescu,6,* Ioana Anca Stefanopol,1,7,* Alexandru Nechifor,3,* Ana Maria Pelin,8,* Gabriela Balan,3,9,10,* Silvia Chirobocea,11,* Claudiu Ionut Vasile,3,* Alin Laurentiu Tatu2,3,12
1Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 2Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID), ‘Dunărea de Jos’ University, Galați, Romania; 3Clinical Medical Department, Faculty of Medicine and Pharmacy, Dunărea de Jos University, Galați, Romania; 4Dental Department, Faculty of Medicine and Pharmacy, Dunărea de Jos University, Galați, Romania; 5Clinical Surgical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 6Medical Department, Faculty of Medicine and Pharmacy, Dunărea de Jos University, Galați, Romania; 7Department of Pediatrics, Clinical Emergency Hospital for Children “Sf. Ioan”, Galati, Romania; 8Department of Pharmaceutical Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 9Department of Gastroenterology, “Sf. Apostol Andrei” County Emergency Clinical Hospital, Galați, Romania; 10Research Center in the Field of Medical and Pharmaceutical Sciences, “Dunărea de Jos” University, Galați, Romania; 11Department of Neurology, Municipal Emergency Hospital, Moinești, Romania; 12Dermatology Department, “Sf. Cuvioasa Parascheva” Clinical Hospital of Infectious Diseases, Galați, Romania
*These authors contributed equally to this work
Correspondence: Elena Niculet; Mihaela Craescu, Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University of Galați, 35 Alexandru Ioan Cuza Street, Galați, 800008, Romania, Tel +40741398895 ; +40751869864, Email [email protected]; [email protected]
Introduction: CREST syndrome is a clinical entity associated with systemic sclerosis, which meets at least three of the five clinical features: calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia. Three of these clinical features (Raynaud’s phenomenon, sclerodactyly and esophageal dysmotility) are often present in classical subsets of SSc: limited and diffuse, and their presence in association does not define CREST syndrome. Calcinosis seems to be less common in SSc and its association with other clinical features is characteristic of CREST syndrome. Therefore, it can be appreciated that calcinosis is the key element of CREST syndrome.
Methods: This study included a number of 37 candidates with SSc, diagnosed with the help of the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2013 criteria.
Results and Discussions: These three elements (calcinosis, Raynaud’s phenomenon, esophageal dysmotility) were recorded both in the limited subset of SSc, but especially in the subset of diffuse SSc, contrary to the data in the literature.
Conclusion: We appreciate that CREST syndrome is a clinical entity that can overlap with both subsets of SSc. Given the divergent views of the authors on the classification of CREST syndrome, future studies may contribute to a reassessment of SSc classification.
Keywords: systemic sclerosis, dystrophic calcinosis, CREST syndrome, telangiectasia, Raynaud’s phenomenon, sclerodactyly
Introduction
According to the criteria of the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2013, CREST syndrome is a form of systemic sclerosis (SSc) that meets at least three of the five clinical elements: calcinosis, Raynaud’s phenomenon, impaired esophageal motility, sclerodactyly and telangiectasia.1–5 Valenzuela estimates that CREST syndrome is present in more than a quarter of patients with SSc. CREST syndrome is associated with a long disease course and positive titers of anticentromere antibodies, which are known to be specific for the limited SSc subset.6–8 Meyer revealed that more than half of patients with CREST syndrome have elevated titers of anticentromere antibodies.9 Also, CREST syndrome appears to be associated with positive anti-PM/Scl-70 antibody titers.6 Although the evolution of this syndrome is often benign, it can rarely present with complications like pulmonary hypertension after a long duration of more than 10 years or gangrene, requiring finger amputation.9
In 1964, Winterbauer used the acronym CREST to describe the syndrome with the five clinical elements: dystrophic calcinosis in the subcutaneous tissue, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly and telangiectasias.6,10 CREST syndrome is also called acrosclerosis or Thibierge-Weissenbach syndrome after the French doctors Thibierge and Weissenbach who first reported the case in 1910.6,9 The etiopathogenesis of CREST syndrome is unknown, but studies have shown that a number of local factors may be involved, such as chronic local inflammation, repeated local trauma, capillary deoxygenation, bone matrix damage. CREST syndrome is associated with acroosteolysis and digital ulceration. Studies have shown that more than half of CREST syndrome cases have elevated titers of anticentromere antibodies.6 Ohira appreciates that CREST syndrome is often incomplete. Raynaud’s phenomenon, sclerodactyly and telangiectasia are the most common clinical features identified in association, while calcinosis and esophageal dysmotility were found less frequently in patients.11
Afifi et al identified the presence of coronary calcifications in a group of patients with SSc without cardiac symptoms. The coronary and extra-coronary calcium index was assessed using the multidetection computed tomography. Patients with limited SSc had higher scores than those with diffuse SSc. The study showed that patients with SSc have a cardiovascular risk induced by calcification of the coronary atheroma plaque.12 Atherosclerosis is the result of an inflammatory process mediated by monocytes and macrophages, T lymphocytes, cytokines and autoantibodies. Autoimmune diseases are caused by chronic inflammation that involves a cardiovascular risk.13
The Japanese study led by Takahashi noted that CREST syndrome may be associated with other autoimmune diseases. It records the case of a 64-year-old patient diagnosed with several immunopathies: recurrent myelitis with high titers of anti-aquaporin antibodies 4 (AQP4), primary biliary cirrhosis, Sjogren’s syndrome and CREST syndrome.14 The presence of an autoimmune disease seems to predispose to the addition of other immunopathies in the context of the disruption of the immune system as other authors have shown.15–17 Autoimmune fibrosis is a common mechanism between primitive biliary cirrhosis and SSc with high titers of anticentromere antibodies and has been reported by several authors.18,19 Fibrosis stems from an increased level of pro-inflammatory cytokines (IL6, IL1, IL17, TNFα) with pro-fibrotic potential released by inflammatory cells.11,20–22 This characteristic fibrosis is translated by skin thickness (revealed through the modified Rodnan score) and can be evaluated with the help of ultrasound elastography and high-frequency ultrasound, revealing the skin involvement in SSc patients, maybe with possible detection of calcifications at this level.23,24
Materials and Methods
We have introduced a number of 37 candidates with SSc, diagnosed with the help of the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2013 criteria.5,25 These candidates come from the south-east of Romania and were hospitalized in a university clinic in Bucharest, Romania. We obtained the agreement of the Ethics Committee number 5213 from 04.04.2019 of the Clinical Hospital “St. Maria” from Bucharest, in compliance with the Declaration of Helsinki.
The aim of this study was to analyze the presence of the clinical features characteristic of CREST syndrome in the investigated SSc group of patients (both subtypes, limited and diffuse); more specifically it was to highlight the presence of those three main elements (Raynaud’s phenomenon, esophageal dysmotility and sclerodactyly) in both of the disease subtypes, and search whether the other two elements (telangiectasia and calcinosis), which are rarer, could dictate whether the SSc patients also suffer from CREST syndrome. The group of patients included mostly women (30 women and 7 men), with an 81.1% ratio, and 18.9% men. Almost a third of patients (13) were from the rural area, while 24 were from the urban one. The patients’ age varied between 28 and 76 years.
In order to establish a group of patients to be studied, the following inclusion and exclusion criteria were used: a positive diagnosis of SSc according to the 2013 revised ACR/EULAR criteria which have a higher sensitivity (Table 1), cases of scleroderma “sine scleroderma” were taken also into consideration (but there were none), dermal sclerosis according to the LeRoy criteria (which established the patients’ disease subtype – limited or diffuse), establishment of the duration between the first sign of disease and the dermal sclerosis development, and, furthermore the positive SSc diagnosis, comorbidities, toxic exposure and stressful events were also analyzed.
 |
Table 1 The New ACR/EULAR Classification Criteria from 2013 for SSc Diagnosis |
We introduced the results of this study in an Excel file; statistical analysis processing followed and used Microsoft Excel, SPSS version 24.0 (IBM Corp.), generating results presented in the form of a table. Quantitative data were outlined with the help of descriptive statistics, while qualitative data were outlined with the help of frequency distributions and contingency tables. The sample in-between comparisons were made by making use of the Chi-squared test. All of the P-values were two-tailed, and a P-value equal to 0.05 was considered to be significant.
Results and Discussions
Among the clinical features of CREST syndrome, Raynaud’s phenomenon was the most common (Figure 1). It was present in all patients with SSc (diagnosed with the help of ACR/EULAR criteria, as revealed by Table 2) except for one case of diffuse SSc.
 |
Table 2 Frequency Distribution by SSc ACR Criteria, in Both Subgroups and Total Group of Patients |
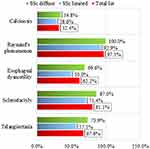 |
Figure 1 Clinical elements of CREST syndrome: Frequency distributions for the total group and by subsets of SSc. |
In order of frequency (Table 3), the next clinical element present in the analyzed group was sclerodactyly registered in most patients (81.1%). Analyzing the subset distribution frequency of sclerodactyly, its predominant presence in the diffuse SSc subset is noted (87.0%). Relatively similar percentages were recorded for telangiectasia and esophageal dysmotility (Figure 1). In the whole group, these clinical elements were present in approximately 2/3 of the patients. Equally, this time the diffuse subset of SSc records higher percentages of patients with telangiectasia and esophageal dysmotility (73.9% and 69.9%). Of the five clinical features of CREST syndrome, calcinosis was the least reported, identified in only 1/3 of patients in the entire group (32.4%). The frequency distribution of subsets of calcinosis was similar to that of sclerodactyly. Thus, calcinosis was mainly present in the subset of patients with diffuse SSc (34.8%), in the subset of limited SSc the share being slightly lower (in 28.6% of patients with limited SSc).
 |
Table 3 CREST Syndrome Elements – Patient Group and Disease Subgroups Frequency Distribution |
In particular, in our study we recorded the presence of calcifications more frequently in patients with diffuse SSc, calcinosis not being a feature of this subset.26 Jacobsen noted that forms of SSc with high titers of anticentromere antibodies associate the presence of calcinosis with digital ulceration and telangiectasia.26 Anticentromere antibodies are known to have limited SSc specificity and have a better survival rate than diffuse forms.7,27
Based on the statements of some researchers who classified CREST syndrome as equivalent or identical to the limited subset of SSc or as a particular form of limited SSc, we appreciate that our study does not support this assumption. We identified calcinosis in both subsets of SSc, but especially in the subset of diffuse SSc. If we consider the opinions of other authors who classify CREST syndrome as a distinct form of SSc, different from the limited and diffuse SSc subsets,10 our study does not support this assumption either, as elements of CREST syndrome were present in both subsets of SSc. Our study proves that CREST syndrome is a clinical entity that can overlap with both subsets of SSc. Somewhat similar, Johnson noted that CREST syndrome may be present in most cases of limited and diffuse ScS,28 while Steen reported in 2008 that CREST syndrome might be a milder form, with improved survival.29
Four of the five clinical features of CREST syndrome (sclerodactyly, esophageal dysmotility, telangiectasia and especially Raynaud’s phenomenon) were recorded in high percentages in patients with diffuse and limited SSc. Only calcinosis was recorded in a lower percentage and this creates the difference. Because Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia are part of the clinical picture of diffuse and limited SSc and are often associated with each other, regardless of the disease subset.4,5 We appreciate that CREST syndrome is the association of at least three of the five clinical elements in which the presence of calcinosis is essential and mandatory. We can conclude that calcinosis is the obligatory clinical element of CREST syndrome.
Calcinosis in CREST syndrome is identified in the form of subcutaneous calcifications and is formed against the background of local ischemia and dystrophy in SSc. Dystrophic calcinosis is a type of calcification characteristic of connective tissue diseases. Devitalized tissues of SSc are prone to calcium deposition under conditions of normal plasma calcium and phosphate levels. SSc vasculopathy causes local tissue hypoxia followed by damage to collagen and elastin fibers. Local inflammation promotes calcification of devitalized tissues. In other words, ectopic mineralization occurs with localization in the peri-articular subcutaneous tissue. Insoluble calcium deposits consist of amorphous calcium phosphate and hydroxyapatite crystals and have the appearance of agglomerations of inorganic crystalline materials with localized or extended disposition. At the periphery of calcium deposits there is a granulomatous inflammatory reaction followed by the formation of foreign body granuloma and fibrosis1 These deposits formed on the background of local inflammation maintain, in turn, recurrent inflammation. Therefore, a vicious circle is created. Moreover, dystrophic calcinosis favors the appearance of skin ulcers that can become infected, and in the long run produces joint contracture and muscle atrophy.6 At the same time, local ischemia reduces the layer of subcutaneous fat.1
Calcinosis is a source of pain and disability. It can be clinically or radiologically evidenced and drug treatment is often ineffective.6 Colchicine and calcium channel blockers are the first-line treatment for subcutaneous calcification in SSc, but have not been shown to be effective.30 Similarly, bisphosphonates, thalidomide, and sodium thiosulfate did not have encouraging results, and rituximab treatment led to divergent opinions.31 In 2019, Gordon conducted a study in which children with Hutchinson-Gilford progeria were enrolled. They showed calcifications consisting of hydroxyapatite crystals with extraskeletal disposition. Zoledronic acid, pravastatin and lonafarnib were ineffective.32 Therefore, the main treatment remains the surgical excision of calcium deposits.6 However, some studies have shown that statins could be used due to their anticalcification and antiinflammatory, antithrombotic and antioxidant effect.33,34 A study that used mice as experimental models of pseudoxanthoma elasticum (PXE), a genetic disease in which ectopic mineralization occurs in the arterial walls, obtained favorable results after administration of atorvastatin. Therefore, statins prevent abnormal mineralization, but do not cause their resolubilization.35 Also, persistent skin ulcers by the area of calcinosis or in the acral regions require treatment to avoid infection, being difficult to manage and sometimes it can be useful to combine topical medication.36 Of particular interest is the finding highlighted by Draganescu and collaborators regarding the risk of HIV patients to develop skin lesions. The inflammatory and immune status of this disease, even in the setting of antiviral treatment, might be a preset to subcutaneous calcification development.37
Winterbauer has classified CREST syndrome since 1964 as a distinct form of SSc with a better prognosis.10,28 In 2001, the classification was changed and CREST syndrome was seen as a particular form of limited SSc. Johnson finds Le Roy’s classification in the two SSc subsets, limited and diffuse, convenient and predictable. Johnson also notes that most cases of limited and diffuse SSc have clinical items characteristic of CREST syndrome.28,38 Recently, Adiguin appreciated that CREST syndrome is identical to the limited subset of SSc.39 Based on the divergent opinions regarding the classification of CREST syndrome, several authors consider that a new classification of SSc is necessary.6,28,39
We appreciate that the microcalcifications (small calcium deposits) in the SSc are much more frequent than we can objective clinically, ultrasound or radiologically because there is a subclinical stage in which they cannot be identified by clinical examination. Nishikawa et al identified the presence of calcifications at the titanium piece-bone mass limit using laser scanning confocal microscopy.40 Given this observation, We anticipate that calcium micro-deposits in the devitalized subcutaneous tissue of SSc could be identified at an early stage by this more reliable method long before deposits are large enough to be clinically identified. The same early detection will have to be studied in the future and with the help of coherent optical tomography.
Conclusion
Calcinosis was found in both diffuse and limited SSc. Therefore, calcinosis does not appear to be a particular form of limited SSc and no equivalent form of SSc, but rather a clinical entity superimposed over both subsets of SSc.
Since Raynaud’s phenomenon, sclerodactyly and esophageal dysmotility are often present in SSc, these three sufficient clinical elements in the five cannot be classified as CREST syndrome, we appreciate that the key element of the syndrome is dystrophic calcinosis. We emphasize that we cannot talk about CREST syndrome in the absence of calcinosis, contrary to the existing data in the literature. In conclusion, the CREST syndrome without calcinosis raises and proposes the need to reformulate the terminology.
We estimate that dystrophic calcinosis is much more common in SSc than the statistics report, given that the early stages of calcium accumulation can only be evident when the deposit reaches dimensions that allow clinical objectification.
Abbreviations
SSc, systemic sclerosis; CO, carbon monoxide, NO2, nitrogen dioxide, SO2, sulfur dioxide; ESR, erythrocyte sedimentation rate; CRP, C reactive protein; ANA, antinuclear antibodies; ACA, anticentromere antibodies; ACR, American College of Rheumatology; EULAR, European League Against Rheumatism.
Data Sharing Statement
The information will be granted access to under reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Clinical Hospital “St. Maria” from Bucharest, with the number 5213 from 04.04.2019 and is in compliance with the Declaration of Helsinki.
Consent for Publication
Each patient signed an informed consent for the publication of data and/or photographs, and it represents part of the medical chart.
Acknowledgments
The authors wish to acknowledge that the present study was academically supported by the ‘Dunarea de Jos’ University of Galati, Romania, through the research center – Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID).
Author Contributions
CB, EN, MC and ALT were involved in the conception of the study and had major contribution in the writing and revising of the manuscript. CB, ELP, CLM, CD, IC, LN, MD, VS, IAS, AN, AMP, GB, SC and CIV contributed in the acquisition, analysis and interpretation of the data. All authors have substantially revised and critically reviewed this article. All authors have agreed on the journal to which the article will be submitted and agree to take responsibility and be accountable for the contents of the article. All authors reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
Funding
The article publishing charge was paid by the “Dunarea de Jos” University of Galati, Romania.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Boulman N, Slobodin G, Rozenbaum M, Rosner I. Calcinosis in rheumatic diseases. Semin Arthritis Rheum. 2005;34(6):805–812. doi:10.1016/j.semarthrit.2005.01.016
2. Morgan ND, Shah AA, Mayes MD, et al. Clinical and serological features of systemic sclerosis in a multicenter African American cohort: analysis of the genome research in African American scleroderma patients clinical database. Medicine. 2017;96(51):e8980. doi:10.1097/MD.0000000000008980
3. Arana-Guajardo A, Villarreal-Alarcón M. CREST syndrome: clinical expression of the disease. J Clin Rheumatol. 2017;23(5):285. doi:10.1097/RHU.0000000000000491
4. Masi AT. Preliminary criteria for the classifications of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association diagnostic and therapeutic criteria committee. Arthritis Rheum. 1980;23(5):581–590. doi:10.1002/art.1780230510
5. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–1755. doi:10.1136/annrheumdis-2013-204424
6. Valenzuela A, Song P, Chung L. Calcinosis in scleroderma. Curr Opin Rheumatol. 2018;30(6):554–561. doi:10.1097/BOR.0000000000000539
7. Haustein UF. Systemic sclerosis-scleroderma. Dermatol Online J. 2002;8(1):3. doi:10.5070/D30VD8P0XW
8. Bobeica C, Niculet E, Halip AI, et al. Predictive value of immunological markers in systemic sclerosis. Exp Ther Med. 2021;22(3):1–5. doi:10.3892/etm.2021.10426
9. Meyer O. [CREST syndrome]. Ann Med Intern. 2002;153(3):183–188. French.
10. Winterbauer RH. Multiple telangiectasia, Raynaud’s phenomenon, sclerodactyly, and subcutaneous calcinosis: a syndrome mimicking hereditary hemorrhagic telangiectasia. Bull Johns Hopkins Hosp. 1964;114:361–383.
11. Ohira H, Watanabe H. Pathophysiology and recent findings of primary biliary cirrhosis complicated by systemic sclerosis. Hepatol Res. 2014;44(4):377–383. doi:10.1111/hepr.12285
12. Afifi N, Khalifa MM, Al Anany AA, Ali hassan HG. Cardiac calcium score in systemic sclerosis. Clin Rheumatol. 2022;41(1):105–114. doi:10.1007/s10067-021-05887-1
13. Magda S, Mincu R, Mihai C, Cinteza M, Vinereanu D. Atherosclerosis in systemic sclerosis: a modern controversy. Maedica. 2015;10(3):248–256.
14. Takahashi M, Nagata R, Ozaki A, Kaneko S, Saiki H, Matsumoto S. A case of anti-AQP4 antibody-positive recurrent myelitis overlapped with autoimmune disorders including incomplete CREST syndrome revealed multiple discontinuous cord lesions. Rinsho Shinkeigaku. 2009;49(2–3):115–118. doi:10.5692/clinicalneurol.49.115
15. Tatu AL, Ionescu MA. Multiple autoimmune syndrome type 3-thyroiditis, vitiligo and alopecia areata. Acta Endocrinol. 2017;13(1):124–125. doi:10.4183/aeb.2017.124
16. Brănișteanu DE, Pintilie A, Dimitriu A, et al. Clinical, laboratory and therapeutic profile of lichen planus. Rev Med Chir Soc Med Nat Iasi. 2017;121:25–32.
17. Tatu AL, Nwabudike LC The treatment options of male genital lichen sclerosus et atrophicus. Short Title for a Running Head: treatments of genital lichen sclerosus.
18. Imura-Kumada S, Hasegawa M, Matsushita T, et al. High prevalence of primary biliary cirrhosis and disease-associated autoantibodies in Japanese patients with systemic sclerosis. Mod Rheumatol. 2012;22(6):892–898. doi:10.3109/s10165-012-0607-z
19. Horino T, Ichii O. Distal phalanx osteolysis and subcutaneous calcinosis in CREST syndrome. J Clin Rheumatol. 2021;27(1):e22. doi:10.1097/RHU.0000000000001210
20. Barsotti S, Orlandi M, Codullo V, et al. One year in review 2019: systemic sclerosis. Clin Exp Rheumatol. 2019;37Suppl 119(4):3–14.
21. Allanore Y. [Pathophysiology of systemic sclerosis]. Med Sci. 2016;32(2):183–191. French. doi:10.1051/medsci/20163202012
22. Niculet E, Chioncel V, Elisei AM, et al. Multifactorial expression of IL-6 with update on COVID-19 and the therapeutic strategies of its blockade (Review). Exp Ther Med. 2021;21(3):263. doi:10.3892/etm.2021.9693
23. Santiago T, Santiago M, Ruaro B, et al. Ultrasonography for the assessment of skin in systemic sclerosis: a systematic review. Arthritis Care Res. 2019;71(4):563–574. doi:10.1002/acr.23597
24. Ruaro B, Soldano S, Smith V, et al. Correlation between circulating fibrocytes and dermal thickness in limited cutaneous systemic sclerosis patients: a pilot study. Rheumatol. 2019;39(8):1369–1376.
25. Masi AT, Medsger TA
26. Jacobsen S, Halberg P, Ullman S, et al. Clinical features and antinuclear serum in 230 Danish patients with systemic sclerosis. Br J Rheumatol. 1998;37(1):39–45. doi:10.1093/rheumatology/37.1.39
27. Asano Y. Systemic Sclerosis. J Dermatol. 2018;45(2):128–138. doi:10.1111/1346-8138.14153
28. Johnson SR, van den Hoogen F, Devakandan K, Matucci-Cerinic M, Pope JE. Systemic sclerosis: to subset or not to subset, that is the question. Eur J Rheumatol. 2020;7(Suppl 3):S222–S227. doi:10.5152/eurjrheum.2020.19116
29. Steen VD. The many faces of scleroderma. Rheum Dis Clin North Am. 2008;34(1):1–15. doi:10.1016/j.rdc.2007.12.001
30. Bienvenu B. Treatment of subcutaneous calcinosis in systemic disorders. Rev Med Intern. 2014;35(7):444–452. doi:10.1016/j.revmed.2014.04.018
31. Dubos M, Ly K, Martel C, Fauchais AL. Is rituximab an effective treatment of refractory calcinosis? BMJ Case Rep. 2016;bcr2015213179. doi:10.1136/bcr-2015-213179
32. Gordon CM, Cleveland RH, Baltrusaitis K, et al. Extraskeletal calcifications in Hutchinson-Gilford Progeria syndrome. Bone. 2019;125:103–111. doi:10.1016/j.bone.2019.05.008
33. Nigwekar S, Bhan I, Turchin A, et al. Statin use and calcific uremic arteriolopathy: a matched case-control study. Am J Nephrol. 2013;37(4):325–332. doi:10.1159/000348806
34. Nwabudike LC, Elisei AM, Buzia OD, Miulescu M, Tatu AL. Statins. A review on structural perspectives, adverse reactions and relations with non-melanoma skin cancer. Rev Chim. 2018;69(9):2557–2562. doi:10.37358/RC.18.9.6575
35. Guo H, Li Q, Chou DW, Uitto J. Atorvastatin counteracts aberrant soft tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6⁻/⁻). J Mol Med (Berl). 2013;91(10):1177–1184. doi:10.1007/s00109-013-1066-5
36. Nwabudike LC, Tatu AL. Magistral prescription with silver nitrate and Peru balsam in difficult-to-heal diabetic foot ulcers. Am J Ther. 2018;25(6):e679–e680. (). doi:10.1097/MJT.0000000000000622
37. Draganescu M, Baroiu L, Iancu A, et al. Perspectives on skin disorder diagnosis among people living with HIV in southeastern Romania. Exp Ther Med. 2021;21(1):97. doi:10.3892/etm.2020.9529
38. LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202–205.
39. Adigun R, Goyal A, Bansal P, Hariz A. Systemic Sclerosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; January, 2022 [Updated July 3, 2021]. Available fromhttps://www.ncbi.nlm.nih.gov/books/NBK430875/.
40. Nishikawa T, Masuno K, Mori M, Tajime Y, Kakudo K, Tanaka A. Calcification at the interface between titanium implants and bone: observation with confocal laser scanning microscopy. J Oral Implantol. 2006;32(5):211–217. doi:10.1563/799.1
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
