Back to Journals » Hepatic Medicine: Evidence and Research » Volume 15
COVID-Associated Cast-Forming Cholangiopathy: A Commentary on Disease Mechanism, Treatment, and Prognosis
Authors Sarkis Y, Saleem N, Vuppalanchi R, Gromski M
Received 30 October 2022
Accepted for publication 23 March 2023
Published 28 March 2023 Volume 2023:15 Pages 27—32
DOI https://doi.org/10.2147/HMER.S384176
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Yara Sarkis,1 Nasir Saleem,2 Raj Vuppalanchi,2 Mark Gromski2
1Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA; 2Division of Gastroenterology and Hepatology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA
Correspondence: Mark Gromski, Gastroenterology and Hepatology Department, Indiana University Hospital, 550 N. University Blvd, Suite 1634, Indianapolis, IN, 46202, USA, Tel +317-944-0925, Fax +317-968-1265, Email [email protected]
Abstract: The complete impact of COVID-19 infection continues to develop since the onset of the COVID-19 pandemic. COVID-19 cholangiopathy has been recently described in a subset of patients who recovered from severe COVID-19 infection. The most common phenotype of patients suffering from COVID-19 cholangiopathy had severe infection requiring a stay in the intensive care unit, mechanical ventilation and vasopressor medications. Patients with COVID-cholangiopathy present with severe and prolonged cholestatic liver injury. In cases where biliary cast formation is identified, we defined the entity as “COVID-19 cast-forming cholangiopathy”. This subset of COVID-19 cholangiopathy is not well understood and there are no standardized diagnosis or management to this date. The reported clinical outcomes are variable, from resolution of symptoms and liver test abnormalities to liver transplant and death. In this commentary, we discuss the proposed pathophysiology, diagnosis, management, and prognosis of this disease.
Keywords: COVID-19 infection, cast-forming cholangiopathy, COVID-19 cholangiopathy, long COVID syndrome
Introduction
During the last few years, the world and the medical field have struggled to combat the COVID-19 pandemic. The Sars-CoV-2 virus affects multiple organs, including the liver.1 Hepatocellular injuries complicating COVID-19 infection are the most frequent liver abnormalities (14–58% reported rates in literature)1 and correlates with disease severity.2 With decreasing mortality rate and longer follow up after recovery, “long COVID syndrome” has been described and COVID-19 cholangiopathy has been reported as a prolonged cholestatic profile after recovery from severe COVID-19 infection.3 In a subset of patients with severe COVID-19 cholangiopathy, endoscopic retrograde cholangiopancreatography (ERCP) is needed for management and identifies cast-formation in the biliary tree.1,2,4 We defined this subset of COVID-19 cholangiopathy as “COVID-19 cast-forming cholangiopathy”.
Cholangiopathies and biliary cast formation are known complications of critical illness and have been previously described in the literature after severe illness (burn, trauma, respiratory distress) or liver transplant (2.1% to 3.6% of cases).1,5,6 They are the result of biliary duct ischemia and prolonged hypotension, with secondary biliary infection in those patients.7 The presence of COVID-19 cholangiopathy in patients recovering from COVID-19 is now well recognized and it appears to have negative consequences on their health and might be associated with poor quality of life and poor outcomes such as liver transplant and death.8 Due to its rarity and novelty (first case series reported in May 20213), evidence, guidelines, and standardized evaluation and management of this entity are lacking. In this review, we discuss the disease mechanism, treatment, and prognosis of COVID-19 cholangiopathy to better understand and recognize this disease phenotype.
Definition and Clinical Features
Several case reports and case series have been recently published describing new-onset cholestatic liver injury in patients with a presumably normal liver at baseline, who have a history of severe COVID-19 infection typically requiring ICU stay with pressor support (Table 1). The abnormal liver profile is recognized a mean of 3 months after COVID-19 recovery.2 Patients present to medical care with symptoms of nausea, vomiting, pruritus, or jaundice. In some cases, patients present with gallstone pancreatitis or choledocholithiasis.9 Liver tests typically show a cholestatic pattern with elevations in alkaline phosphatase (including up to 10 times higher than the upper limit of normal), gamma-glutamyl transferase and at times bilirubin.8 The typical phenotype is a patient that has recovered from acute severe COVID-19 infection, requiring prolonged intensive care unit (ICU) stay, multiple pressors and immunomodulator drugs, invasive ventilation, and antibiotics. High-resolution cross-sectional imaging with magnetic resonance cholangiopancreatography (MRCP) may show bile duct abnormalities with beaded appearance (dilation and stricture), similar to sclerosing cholangitis. When performed, endoscopic retrograde cholangiopancreatography (ERCP) often identifies pliable intraluminal biliary casts, stones, and sludge (Figures 1–3). In some patients, biliary casts were large and unique, reaching 7 cm in length and with an appearance mirroring the branching of the biliary system. In others, they were small but multiple, and required several ERCPs with balloon extraction for complete clearance.10 Prior MRCP does not always identify filling defects within the extrahepatic bile duct, despite the presence of biliary cast in that location.
 |
Table 1 Major Case Reports and Case Series Describing Covid-19 Cholangiopathy |
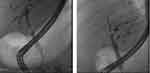 |
Figure 1 Segmental biliary stricture on ERCP – fluoroscopic view. |
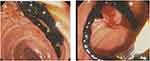 |
Figure 2 Biliary cast seen on ERCP – endoscopic view. |
 |
Figure 3 Biliary cast, stent placement on ERCP – endoscopic view. |
We termed this subset of COVID-19 cholangiopathy with biliary cast formation as “COVID-19 cast-forming cholangiopathy”. Its clinical features and imaging characteristics are comparable to those described in critical illness-associated secondary sclerosing cholangitis (SSC)6,7 and its prognosis, although difficult to define clearly given the paucity of availability of data, is similarly reserved. Patients with COVID-19 cast-forming cholangiopathy have been reported to have varying degrees of bile duct injury. Some have disease limited to the intrahepatic ducts, while others have irregularity, dilation and/or stenosis in the main bile duct.1,2 Bile duct casts have been detected in most patients undergoing intervention with ERCP.1,8,10 A subset of patients described have had a rapidly progressive course of biliary damage, as evidenced by worsening biliary structural appearance on subsequent MRCP or ERCP procedures.
Liver biopsy findings have been diverse, but showed features common to primary and secondary sclerosing cholangitis: large duct obstruction, fibrosis, inflammatory infiltrate, degenerative cholangiocyte injury, and prominent keratin 7 staining of hepatocytes (typical of chronic cholestatic liver disease).2,4,6 Furthermore, in some patients who were transplanted, explant findings demonstrated cholestasis and findings of early cirrhosis with nodule formation.11 Liver biopsy likely does play a prominent role in the diagnosis of this condition and could help understand its pathophysiology.
COVID-19 cast forming cholangiopathy appears to be a new subset of cholestatic liver injury with cast formation and nonspecific MRCP, ERCP and biopsy findings that is diagnosed in patients who recovered from a severe COVID-19 infection requiring ICU stay. There are no definitive diagnostic criteria, and the constellation of signs and symptoms in the appropriate clinical environment allows for this diagnosis.
Pathophysiology and Disease Mechanism
Several theories for the mechanism of injury in COVID-19 associated cast forming cholangiopathy have been offered in the literature and point to a potential “multiple-hit” mechanism.12
The SARS-CoV-2 virus enters the cells through the angiotensin-converting enzyme 2 (ACE2) receptor. This receptor is abundant in endothelial cells, which helps to explain the COVID-19 associated hypercoagulable state.2 COVID-19 microangiopathy and hypercoagulable state have been associated with hepatic artery swelling, portal vein phlebitis and sinusoidal obstruction syndrome. Moreover, patients with severe COVID-19 infection requiring ICU stay have prolonged systemic and hepato-splanchnic hypotension due to the use of vasopressors and proning maneuvers, as well as high positive expiratory pressure for management of acute respiratory distress syndrome (ARDS). Microangiopathies and hypotension seen with severe COVID-19 likely lead to direct ischemic injury to biliary tree and resultant cellular death. These features are likely contributing pathophysiologic factors in the biliary damage seen in COVID-19 associated cholangiopathy. Additionally, COVID-19 is associated with a hyperactivation of the immune system and subsequent cytokine release syndrome with high levels of circulating interleukin 6 (IL-6) that may also contribute to inflammation in the biliary tree.2,8 IL-6 plays an important role in the inflammatory cascade of COVID-19 and contributes to the endothelial injury in this disease.13 Some studies have shown better outcomes in vaccinated patients which confirms the role of immune system activation and inflammatory response in this disease pathogenesis.11
The above mechanisms justify the similarities between COVID-19 associated cast-forming cholangiopathy and the cholestatic syndrome in critically ill patients.1,2 When comparing a population of patients with SSC from COVID-19 to ICU-associated SSC, Hunyadi et al showed comparable clinical parameters between the two groups and a similar transplant-free survival.14 However, despite common features, SSC encountered in critically ill individuals is likely different than the described COVID-19 associated cast-forming cholangiopathy. For instance, critical illness SSC has been known to preserve the distal common bile duct unlike other diffuse cholangiopathies including those associated to the COVID infection.6
Other mechanisms have been hypothesized to contribute to the pathogenesis of COVID-19 cast-forming cholangiopathy, notably direct cytopathic effect of the SARS-CoV-2 virus and drug-induced liver injury (DILI).2,8 COVID-19 virus disrupts the gut mucosa by binding to ACE2 receptors and penetrates the blood and the bile through the portal system.15 Despite being rare in hepatocytes, ACE2 receptors are highly expressed in cholangiocytes, which could explain directly mediated damage to the biliary epithelium and the cytopathic effect of the virus on the biliary cells.3 In critically ill patients with COVID-19 infection, many drugs previously associated with DILI have been used for direct antiviral treatment, blood pressure support and sedation. Ketamine infusion, particularly, has been associated with the development of cholestatic liver injury in patients with COVID in a single center study in Zurich.16 In fact, there was a positive correlation between the infusion duration-effect and the dose-effect of ketamine infusion and rising bilirubin level.
Diabetic patients seem to be more at risk of developing COVID-19 cholangiopathy as evidenced by a recent study by Wendel-Garcia et al.16 Metabolic syndrome and particularly type 2 diabetes mellitus were found to be risk factors for COVID-19 associated cast-forming cholangiopathy.13 It is unclear how insulin resistance and high blood sugar contributes to the destruction of the bile duct, but diabetic microangiopathy with ensuing capillary remodeling and occlusion might be the culprit and could potentially explain this correlation.
Treatment and Prognosis
Gastroenterologists and advanced endoscopists are often consulted to consider whether an ERCP is indicated in the setting of profound cholestasis and bile duct irregularity on cross-sectional imaging. In the COVID-related cases reported in the literature, ERCP was performed almost universally. Cast removal, bile duct dilation, stent placement, sphincterotomy and repeat ERCP were performed when needed. Despite successful interventions, liver function tests remain elevated in several cases with continued progression of the disease, as evidenced by repeat MRCP or ERCP.2 Occasionally, ERCP treatment resulted in stabilization of the disease and no further complications.10
In some centers, non-invasive medical treatment with ursodeoxycholic acid and cholestyramine was used for symptomatic management. These medications had mild benefit on pruritus and did not appear to prevent disease progression.8,17 In some instances, ursodeoxycholic acid did not show any benefit9 and a combination with obeticholic acid has been attempted.12 Plasma exchange has been attempted in some institutions due to its prior role in cholestatic liver disease. It had minimal reported benefits and patients who did not improve were directed to liver transplant evaluation.11
Like other types of sclerosing cholangiopathy, biliary infections are common and constitute an added obstacle in the recovery of those patients.14 Physicians should have a low threshold to treat and broaden antibiotics to avoid worsening bile duct destruction and increased mortality risk. Peri-procedural antibiotics at the time of ERCP are essential.
Progression of COVID-19-associated cast-forming cholangiopathy is unpredictable. Prognosis varies from mild improvement post-ERCP to complete liver failure and death. In instances where patients continue to worsen despite ERCP and/or noninvasive treatment, a liver transplant evaluation is warranted.18 There are different types of liver transplant pursued and reported in the literature for this condition. Aside from standard deceased orthotopic transplant,19–23 living donor orthotopic transplant was done in two cases as reported by Kulkarni et al.11 Those patients were followed for 5 and 8 months and were placed on triple immunosuppression. Their post-operative course has been unremarkable. Moreover, one patient underwent right lobe auxiliary partial orthotopic liver transplant (APOLT) and had good outcome at 6 months follow up with signs of native liver regeneration.19 In fact, based on prior evidence, APOLT is considered an alternative to orthotopic liver transplant in acute liver failure to allow for native liver regeneration when possible. Irrespective of the type of liver transplant, the reported cases of transplant for COVID-19 cholangiopathy have shown success with the caveat that follow-up has been <1 year.11,19–22
Conclusion
COVID-19-associated cast-forming cholangiopathy is a newly described syndrome in a subset of survivors of severe COVID-19 requiring ICU stay. The etiology of this disease is likely multifactorial, and the precise pathogenesis is not well understood at this time. There is a spectrum of severity of disease, from abnormalities of alkaline phosphatase alone to liver failure and death. ERCP may provide benefit for those with recurrent cholangitis or jaundice. The precise treatment algorithm for this disease state is still a work in progress, and further study of the entity is warranted.
Disclosure
Yara Sarkis and Nasir Saleem are co-primary authors. Dr Mark Gromski is a consultant for Boston Scientific, received research support from Olympus and Cook Medical, outside the submitted work; None of the authors disclose a conflict of interest relative to this work.
References
1. Saleem N, Li BH, Vuppalanchi R, Gawrieh S, Gromski MA. Critical illness cholangiopathy in COVID-19 long-haulers. Tech Innov Gastrointest Endosc. 2022;24:351–353. doi:10.1016/j.tige.2022.05.006
2. Faruqui S, Okoli FC, Olsen SK, et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am J Gastroenterol. 2021;116(7):1414–1425. doi:10.14309/ajg.0000000000001264
3. Roth NC, Kim A, Vitkovski T, et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116(5):1077–1082. doi:10.14309/ajg.0000000000001154
4. Morley JE. Editorial: COVID-19 - the long road to recovery. J Nutr Health Aging. 2020;24(9):917–919. doi:10.1007/s12603-020-1473-6
5. Lemmers A, Pezzullo M, Hadefi A, et al. Biliary cast syndrome after liver transplantation: a cholangiographic evolution study. J Gastroenterol Hepatol. 2021;36(5):1366–1377. doi:10.1111/jgh.15318
6. Laurent L, Lemaitre C, Minello A, et al. Cholangiopathy in critically ill patients surviving beyond the intensive care period: a multicentre survey in liver units. Aliment Pharmacol Ther. 2017;46(11–12):1070–1076. doi:10.1111/apt.14367
7. Gelbmann CM, Rümmele P, Wimmer M, et al. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102(6):1221–1229. doi:10.1111/j.1572-0241.2007.01118.x
8. Bartoli A, Cursaro C, Andreone P. Severe acute respiratory syndrome coronavirus-2-associated cholangiopathies. Curr Opin Gastroenterol. 2022;38(2):89–97. doi:10.1097/MOG.0000000000000808
9. Soldera J, Balbinot RA, Balbinot SS. Billiary casts in post-COVID-19 cholangiopathy. Gastroenterol Hepatol. 2022. doi:10.1016/j.gastrohep.2022.08.008
10. Dhaliwal A, Dhindsa BS, Esquivel RG. COVID bile duct: biliary cast syndrome as a complication of SARS-CoV-2 infection. J Gastrointest Surg off J Soc Surg Aliment Tract. 2022;26(8):1806–1807. doi:10.1007/s11605-022-05297-x
11. Kulkarni AV, Khlegi A, Sekaran A, et al. Post COVID-19 cholestasis: a case series and review of literature. J Clin Exp Hepatol. 2022;12:1580–1590. doi:10.1016/j.jceh.2022.06.004
12. Caballero-Alvarado J, Zavaleta Corvera C, Merino Bacilio B, Ruiz Caballero C, Lozano-Peralta K. Post-COVID cholangiopathy: a narrative review. Gastroenterol Hepatol. 2022. doi:10.1016/j.gastrohep.2022.09.004
13. Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. doi:10.1002/rmv.2141
14. Hunyady P, Streller L, Rüther DF, et al. Secondary sclerosing cholangitis following COVID-19 disease: a multicenter retrospective study. Clin Infect Dis off Publ Infect Dis Soc Am. 2022. doi:10.1093/cid/ciac565
15. Bethineedi LD, Suvvari TK. Post COVID-19 cholangiopathy - A deep dive. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2021;53(10):1235–1236. doi:10.1016/j.dld.2021.08.001
16. Wendel-Garcia PD, Erlebach R, Hofmaenner DA, et al. Long-term ketamine infusion-induced cholestatic liver injury in COVID-19-associated acute respiratory distress syndrome. Crit Care Lond Engl. 2022;26(1):148. doi:10.1186/s13054-022-04019-8
17. Linneweber L, Mann AB, Denk G, Kraft E, Weber S. Cholangiopathy in early rehabilitation after intensive care treatment of patients with COVID-19. Am J Gastroenterol. 2022;117(1):197–198. doi:10.14309/ajg.0000000000001511
18. Tafreshi S, Whiteside I, Levine I, D’Agostino C. A case of secondary sclerosing cholangitis due to COVID-19. Clin Imaging. 2021;80:239–242. doi:10.1016/j.clinimag.2021.07.017
19. Rela M, Rajakannu M, Veerankutty FH, Vij M, Rammohan A. First report of auxiliary liver transplantation for severe cholangiopathy after SARS-CoV-2 respiratory infection. Am J Transplant off J Am Soc Transplant Am Soc Transpl Surg. 2022;22(12):3143–3145. doi:10.1111/ajt.17165
20. Lee A, Wein AN, Doyle MBM, Chapman WC. Liver transplantation for post-COVID-19 sclerosing cholangitis. BMJ Case Rep. 2021;14:8. doi:10.1136/bcr-2021-244168
21. Klindt C, Jensen BE, Brandenburger T, et al. Secondary sclerosing cholangitis as a complication of severe COVID-19: a case report and review of the literature. Clin Case Rep. 2021;9(5):e04068. doi:10.1002/ccr3.4068
22. Durazo FA, Nicholas AA, Mahaffey JJ, et al. Post-covid-19 cholangiopathy-A new indication for liver transplantation: a case report. Transplant Proc. 2021;53(4):1132–1137. doi:10.1016/j.transproceed.2021.03.007
23. Meersseman P, Blondeel J, De Vlieger G, van der Merwe S, Monbaliu D; Collaborators Leuven Liver Transplant program. Secondary sclerosing cholangitis: an emerging complication in critically ill COVID-19 patients. Intensive Care Med. 2021;47(9):1037–1040. doi:10.1007/s00134-021-06445-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
