Back to Journals » Hepatic Medicine: Evidence and Research » Volume 15
COVID-19-Associated Liver Injury
Authors Gildea DT , Woo SM , O'Connor CE, Rangnekar AS
Received 20 December 2022
Accepted for publication 11 February 2023
Published 21 February 2023 Volume 2023:15 Pages 1—9
DOI https://doi.org/10.2147/HMER.S384108
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Daniel T Gildea,1 Stephanie M Woo,2 Corinne E O’Connor,3 Amol S Rangnekar4
1Department of Internal Medicine, MedStar Georgetown University Hospital, Washington, DC, USA; 2Department of Gastroenterology, MedStar Georgetown University Hospital, Washington, DC, USA; 3Georgetown University School of Medicine, Washington, DC, USA; 4MedStar Georgetown Transplant Institute, MedStar Georgetown University Hospital, Washington, DC, USA
Correspondence: Daniel T Gildea, Tel +1 302-985-7777, Email [email protected]
Abstract: This review analyzes data regarding liver injury associated with COVID-19 infection. We discuss reported effects on the liver from both COVID-19 and COVID-19 treatment as well as pathophysiology, review the potential role of drug-induced liver injury as an etiology of COVID-19-associated liver injury, and touch on other reports of significant outcomes including COVID-19 cholangiopathy and autoimmune hepatitis. Finally, we review the implications of COVID-19 infection in liver transplant recipients.
Keywords: COVID-19, cholangiopathy, DILI, hepatitis, transplant, liver
Introduction
During the initial stages of the COVID-19 pandemic, an apparent connection was identified between COVID-19 infection and liver injury. This relationship is complicated, and obtaining a clearer viewpoint involves understanding the initial reports of COVID-19 associated liver injury (CALI), studying the pathophysiology of SARS-CoV-2 infection and the distribution of ACE-2 receptors (a viral target) throughout the hepatobiliary system, evaluating other factors involved in the course of COVID-19 illness, and reviewing longer-term CALI outcomes including cholangiopathy, issues surrounding liver transplantation, and autoimmune hepatitis.
COVID-19-Related Liver Injury
Early in the pandemic, reports emerged of transaminase elevations and other markers of liver injury being associated with SARS-CoV-2 infection. For instance, reviews of liver injury in the setting of COVID-19 reported CALI in anywhere between 16% and 53% of the patients1,2 noted liver injury as a prognostic indicator for severe COVID-19,3–6 and in general discussed CALI to be a potential outcome of both primary and multiple possible secondary processes.7 The work that has yet to be completed is disentangling the various proposed mechanisms of injury and understanding what pathophysiologic processes are most commonly causing liver injury – for example, differentiating ischemic hepatitis from drug-induced liver injury (DILI). Studies cite multiple possible precipitating factors of injury: systemic inflammation,8 hypoxia-reperfusion, DILI, viral-induced injury,9 high PEEP during intubation,10 sepsis, polypharmacy, exacerbation of pre-existing disease11 and shock.12
The first step is the differentiation of primary (ie, direct viral effect) from secondary causes of liver injury in infected patients. To what extent SARS-CoV-2 infection directly causes liver injury remains unclear. One single-center study, for example, noted an association between liver injury and severity of disease.13 While there is some listing of various potential causes of liver injury, the main conclusion is that COVID-19 infection in some way causes liver injury and is predictive of severity. In this study, it was found that the patients with liver injury were taking a larger amount of medications by a wide statistically significant margin.13 However, this was not an area of focus, and no Roussel Uclaf Causality Assessment Method (RUCAM) score – a scoring system which quantifies the likelihood of DILI based on factors including time of onset, known risk, other potential explanations, and more – to assess the possibility of a DILI etiology was completed. A larger meta-analysis drew a similar conclusion, noting an association of AST and ALT elevations with COVID-19 infection and the severity of disease.14 Again, DILI is briefly mentioned as one of many possible etiologies, and no RUCAM score is calculated. The study concludes with a recommendation for intensive liver monitoring in patients with severe COVID-19.14 Mishra’s 2020 study noted the association between COVID-19, liver injury, and worse outcomes, which was attributed to an inflammatory syndrome caused by COVID-19; this connection led to a recommendation of trending liver enzymes to identify patients who may need care escalation.15 Interestingly, the cohort with liver injury received statistically significantly more medications including hydroxychloroquine, tocilizumab, antibiotics, and steroids.15 Other reviews have reached similar conclusions.16,17
ACE-2 Receptors and COVID-19 Pathophysiology
Early research into COVID-19 infection found that the angiotensin-converting enzyme 2 (ACE-2), receptor is a significant target of the virus, helping to explain its predilection for the lungs, where pneumocytes express ACE-2 receptors in significant amounts.18 Hepatocytes, on the other hand, have a much lower ACE-2 expression – about 20-fold less than pneumocytes. As such, many have concluded that liver damage caused by direct infection of hepatocytes is unlikely with SARS-CoV-2.18
Notably, though, there are ACE-2 receptors on cholangiocytes in comparable levels to that of pneumocytes,18 representing a possible avenue of viral attack (Figure 1). While there are some reports of cholestatic-type liver injury associated acutely with COVID-19,19 the significant majority of acute COVID-19-associated liver injury demonstrates hepatocellular, rather than biliary, injury.14,20 Li et al’s review echoes the notion that viral targeting of cholangiocytes may be less likely as most patients demonstrate a hepatocellular pattern of liver injury.21 Some authors, notably Boeckmans early on, have raised the question of whether other etiologies for liver injury such as DILI may have been present in some cases.22 McConnell too notes the controversial nature of the viral-action hypothesis, and posits other possibilities including platelet activation and endotheliopathy.23 In this hypothesis, it is thought that the pro-inflammatory effect of SARS-CoV-2 via both binding and internalizing ACE-2 receptors prevalent in the endothelium while also inducing IL-6, tipping the coagulation balance to favor further inflammatory cytokines and subsequent endothelial damage with resultant release of tissue factor, platelet aggregation, and fibrin generation.23
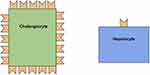 |
Figure 1 Cholangiocytes contain about 20× more ACE-2 receptors than hepatocytes. |
While the novel nature of SARS-CoV-2 has prompted rigorous evaluation of its potential effects throughout the body, a re-evaluation of the available data over these past few years reveals the use of hepatotoxic medications in a number of cases. One interesting example of a potential demarcation between what may be the less common viral-induced injury and the apparently more common drug-induced injury is shown in a report on five patients by Zampino et al.24 These patients were initially on lopinavir/ritonavir, and all but one was also receiving hydroxychloroquine.25,26 While on these treatments, four of the five patients developed significantly increased bilirubin levels.24 They were then transitioned from lopinavir/ritonavir to remdesivir with a subsequent abrupt decrease in bilirubin levels and a concomitant rise in aminotransferases.24 Most of these patients were on one or more other medications as well, including continued use of hydroxychloroquine. While no RUCAM was performed, DILI remains a possibility based on timing. The initial cholestatic insult may be reflective of either viral-induced liver injury in the setting of non-efficacious treatment or cholestatic DILI secondary to lopinavir/ritonavir.27,28
DILI and COVID-19
Interestingly, similar to cases of CALI, DILI itself has been repeatedly shown to have a predominantly hepatocellular pattern of injury.29,30 One recent review of hundreds of CALI cases suggests that DILI features should be removed as a potential confounding factor when defining the characteristics of SARS-CoV-19 infection.29 DILI may have been caused by empiric use of various antiviral medications, a common feature especially early in the pandemic when data were lacking regarding effective COVID-19 treatment.29
Chew et al analyzed 834 patients hospitalized with COVID-19, finding an incidence of significant liver injury of 12.6%.31 Both ischemia and tocilizumab administration were independent predictors of liver injury. Notably, this liver injury was not associated with risk of death. However, risk of death did have an association with ischemic, hypercoagulable, and hyperinflammatory disease states.31 As such CALI may be related to secondary insults, namely ischemia or DILI.31 A relatively early retrospective analysis by Ruan et al of 331 COVID-19 patients in the ICU found that antiviral drug use is statistically associated with liver injury, and advised caution with antiviral usage when considering both effectiveness and potential adverse effects.32 However, as is often the case particularly in the early data, no RUCAM was performed.
Another more recent study by Pazgan-Simon et al analyzed 450 patients (88 of which had liver injury) who were hospitalized with COVID-19. The majority of those with liver injury had a mixed-type picture, with only a small portion (7%) having a cholestatic injury pattern. The authors note that, as with many other reports, this is inconsistent with the known ACE-2 distribution predominantly on cholangiocytes.33 All 16 patients who died from the disease in this study did not achieve normalization of aminotransferases or ALP/GGT, which was attributed to multiple organ failure perhaps exacerbated by DILI or hypoxia.33 Additionally, liver injury may not correlate with a higher risk of COVID-19 mortality.33,34
Naseralallah et al also delved into the uncertainty surrounding elevated liver enzymes in the setting of COVID-19, noting that many different combinations of medications were used to treat COVID-19 infection, especially early in the pandemic.35 This recent study assessed the effectiveness of the updated RUCAM analysis in determining DILI in cases of CALI. In a cohort of 72 patients, they found that RUCAM-based assessment had high inter-rater agreement and reliability, concluding that RUCAM was successful and useful in determining possible or probable cases of DILI in patients with COVID-19.35 They also noted that 91.6% of these DILI cases were due to antimicrobial medications.35 This risk of iatrogenic liver injury serves as a reminder of the risk which must be fully understood, and balanced against potential benefit, when it comes to selecting and utilizing medications in the setting of SARS-CoV-19 infection (Figure 2).
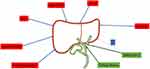 |
Figure 2 Suspected etiologies of hepatobiliary injury. |
The American Association for the Study of Liver Diseases (AASLD) in October 2022 updated a consensus statement regarding COVID-19 and liver injury. It discusses the common association of elevated aminotransferases in the setting of COVID-19 infection, noting that the majority of cases are only mildly abnormal and cholestatic in nature.36 The statement also comments on the diagnostic difficulty inherent in these cases, specifically whether the abnormal findings are due to viral infection itself, complications, or DILI. In light of this, the authors recommend investigating other, non-COVID-19-related causes of elevated liver biochemistries, including other types of viral hepatitis, pancreaticobiliary disease, DILI, myositis, cardiac injury, ischemia, cytokine release syndrome, and cholangiopathy related to critical illness. Regular monitoring of liver values is important in COVID-19 patients, particularly in those receiving potentially hepatotoxic therapy.36
COVID-19 Cholangiopathy
Given the predominance of ACE-2 receptors in the biliary system as compared to the liver (Figure 3), it is not surprising that some patients develop significant biliary injury or cholangiopathy in the setting of severe infection (Table 1). Additionally, ischemic injury due to respiratory failure or hypotension may be a significant contributing etiology. While this finding has been less common than hepatocellular injury in the setting of COVID-19, it is associated with significant morbidity and mortality. COVID-19-associated cholangiopathy appears to be a late manifestation of severe COVID-19 infection, often appearing well after recovery from other manifestations and in some cases over 100 days after initial infection.37 Affected patients present with laboratory evidence of cholestatic liver injury and jaundice. Imaging can reveal dilation and irregularity of the intrahepatic bile ducts consistent with secondary sclerosing cholangitis.37 Patients are also at risk of developing ascending cholangitis.
 |
Table 1 Summarized Data on Cholangiopathy in COVID-19 Patients |
 |
Figure 3 Scant distribution of ACE-2 receptors in the liver. |
Patients with underlying chronic liver disease (CLD) may be at higher risk for COVID-19 associated liver injury. Up to 20% of the patients with CLD may develop progressive cholestasis. Among patients with cholestatic liver failure marked by higher bilirubin levels, up to nearly two-thirds may subsequently evolve into secondary sclerosing cholangitis. Secondary sclerosing cholangitis occurs more commonly in patients with NAFLD and metabolic risk factors.38–40
As with other manifestations of CALI, the etiology of the cholangiopathy is not fully understood. The ACE-2 receptor distribution may play a role, but other causes including host inflammatory response, thrombosis,41 bile duct ischemia, and DILI have also been postulated.46 Interestingly, treatment with ketamine has also been suggested as a risk factor,42 which is not necessarily uncommon given the need for sedation with intubation with severe COVID-19 disease.
Medical treatments that have been attempted so far include ursodeoxycholic acid and obeticholic acid with variable results.40 Vaccination, as with other COVID-19 complications, has been shown to improve outcomes.43 ERCP has been employed as well, especially in severe cases. Some have recommended that ERCP be considered as part of standard management in COVID-19 cholangiopathy cases specifically to relieve obstruction by removing bile casts.41,44 COVID-19-associated cholestasis may resolve over time. However, liver transplantation has been required for severe cases of COVID-19 cholangiopathy occurring many months after initial illness.40,45 Although many patients with COVID-19 cholangiopathy may not be suitable candidates for transplantation due to comorbidities or degree of overall illness, cases of successful liver transplant for this indication have been reported.42,45
Liver Transplantation and COVID-19
The SARS-CoV-19 pandemic has led to the cancellation or delay of countless surgeries since it began in earnest in March 2020. Considerations of safety and risk have been at the forefront of discussion and are especially important in the setting of the required immunosuppression following liver transplant. One recent review studied liver transplant outcomes in patients with high MELD or fulminant hepatitis diagnosed with COVID-19 just prior to transplant (median 19 days before initiation), and found no increase in mortality compared to normal transplant patients.47 Routine screening for COVID-19 is recommended in both donors and recipients. Although transplant is contraindicated in the setting of active infection, patients can be considered for liver transplantation 14–21 days after the initial infection, particularly if repeat testing is negative.
In a similar vein, some controversy still exists regarding post-transplant immunosuppression in the setting of COVID-19 infection. Some data suggest that patients infected soon after transplant have no increased mortality risk, and in general immunosuppressed patients may not have higher mortality from COVID-19.48 Tacrolimus has been associated with better survival, perhaps due to T-cell inhibition or direct antiviral effect.48 Mycophenolate mofetil, on the other hand, is an independent predictor of severe COVID-19 in liver transplant recipients.47 As such, optimal management regarding immunosuppression in the setting of COVID-19 is unclear. Reducing the overall level of immunosuppression is reasonable in post-transplant recipients with COVID-19 infection. While antimetabolites such as mycophenolate may be dose reduced or held, calcineurin inhibitors should be continued. Antiviral therapy may be used for treatment, although caution should be used with nirmatrelvir/ritonavir given interactions with calcineurin inhibitors.
Autoimmune Hepatitis and COVID-19
Environmental triggers have been associated with multiple autoimmune conditions. Several viruses have been implicated in the development of autoimmune hepatitis (AIH). Whether the SARS-CoV-19 virus correlates with AIH may not be evident for some time as there is often a long latency period to development of AIH after viral infection. Currently, there are some reports of AIH that appear to be have been triggered by SARS-CoV-19 infection itself.49–51
There are some additional reports of AIH that may be triggered or exacerbated by COVID-19 vaccines.52–54 In the majority of cases, vaccine-associated AIH resolved with steroid treatment.55 Given the overall paucity of evidence, it remains unclear whether these cases are due to an inflammatory response from infection or vaccination versus other associated factors such as direct infection itself or idiosyncratic injury from vaccination.
COVID-19 Vaccination and Liver Injury
COVID vaccination programs began in earnest in late 2020. In general, vaccination is considered to be safe and effective.56 However, there have been reports of liver injury due to mRNA vaccines.57 Cases have been reported at a median of 15 days after both the first and second doses with a hepatocellular pattern of injury.58,59 Nearly two-thirds of patients had prior comorbidities and a similar number were taking regular medications.60 Almost 80% of these patients presented with jaundice, the most common finding, and almost 90% received steroids. The majority had complete recovery, with death in 4.3% of the patients.60
A considerable portion of patients with vaccine-induced liver injury have demonstrated features of immune-mediated hepatitis. These COVID-19 vaccines may induce autoimmunity by triggering the interferon pathway. Autoantibodies, often ANA, were present in 95% of the patients. Corticosteroids were given more often to patients with severe liver injury and evidence of immune-mediated hepatitis. Liver injury resolved in the majority of cases (regardless of corticosteroid use) within 6 months.59
Given the low incidence of liver injury due to available vaccines, the AASLD COVID-19 guidance statement continues to recommend vaccination for patients with chronic liver disease. This recommendation extends to patients who have undergone liver transplant as well. Liver transplant recipients with elevated liver enzymes following vaccination should be evaluated for acute cellular rejection as well as viral-mediated injury.36 This reinforces the fact that vaccination against COVID is critically important, as the myriad short- and long-term risks of infection, discussed in part throughout this paper, certainly outweigh the rare vaccination risk.
Conclusions
Over the past 3 years, our understanding of the SARS-CoV-19 virus has significantly improved. This knowledge has led to the development of effective vaccines and treatment options as well as reduced mortality. However, with time, it has become apparent that liver injury including cholangiopathy may occur in the setting of severe COVID-19 infection. Further investigation is needed to elucidate whether liver injury is due to direct viral effects or other etiologies. As more time progresses, additional complications of COVID-19 infection such as autoimmune hepatitis may become evident. Liver transplantation is an option for selected patients with severe COVID-19 associated cholangiopathy. Liver transplant recipients with COVID-19 infection may benefit from reduction in immunosuppression and antiviral therapy. Patients who recover from COVID-19 infection can safely proceed to liver transplantation after recovery. Vaccination is considered safe and effective, although rare instances of liver injury may occur.
Funding
No financial support was provided for this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Idalsoaga F, Ayares G, Arab JP, Díaz LA. COVID-19 and indirect liver injury: a narrative synthesis of the evidence. J Clin Transl Hepatol. 2021;9(5):760–768. doi:10.14218/JCTH.2020.00140
2. Labenz C, Toenges G, Wörns MA, Sprinzl MF, Galle PR, Schattenberg JM. Liver injury in patients with severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33(9):1194–1200. doi:10.1097/MEG.0000000000001827
3. Dawood DRM, Salum GM, El-Meguid MA. The Impact of COVID-19 on Liver Injury. Am J Med Sci. 2022;363(2):94–103. doi:10.1016/j.amjms.2021.11.001
4. Wong YJ, Tan M, Zheng Q, et al. A systematic review and meta-analysis of the COVID- 19 associated liver injury. Ann Hepatol. 2020;19(6):627–634. doi:10.1016/j.aohep.2020.08.064
5. Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25(1):54. doi:10.1186/s40001-020-00454-x
6. Li G, Yang Y, Gao D, Xu Y, Gu J, Liu P. Is liver involvement overestimated in COVID-19 patients? A meta-analysis. Int J Med Sci. 2021;18(5):1285–1296. doi:10.7150/ijms.51174
7. Łykowska-Szuber L, Wołodźko K, Rychter AM, Szymczak-Tomczak A, Krela-Kaźmierczak I, Dobrowolska A. Liver injury in patients with coronavirus disease 2019 (COVID-19)-A narrative review. J Clin Med. 2021;10(21):5048. doi:10.3390/jcm10215048
8. Effenberger M, Grander C, Grabherr F, et al. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis. 2021;53(2):158–165. doi:10.1016/j.dld.2020.08.004
9. Yahia AIO. Liver Injury and Dysfunction Associated with COVID-19: a review article. Clin Lab. 2022;68(1). doi:10.7754/Clin.Lab.2021.210535
10. Gabrielli M, Franza L, Esperide A, et al. Liver injury in patients hospitalized for COVID-19: possible role of therapy. Vaccines. 2022;10(2):192. doi:10.3390/vaccines10020192
11. Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int off J Int Assoc Study Liver. 2021;41(1):20–32. doi:10.1111/liv.14730
12. Singh A, Premkumar M, Singh V. Liver injury in COVID-19 – the culprit may not be COVID-19! J Hepatol. 2021;75(3):739–740. doi:10.1016/j.jhep.2021.03.010
13. Elemam NM, Hannawi H, Naeem KB, Hannawi S. A single centered study reveals association between liver injury and COVID-19 infection. Saudi J Biol Sci. 2021;28(10):6017–6022. doi:10.1016/j.sjbs.2021.06.064
14. Wijarnpreecha K, Ungprasert P, Panjawatanan P, et al. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33(7):990–995. doi:10.1097/MEG.0000000000001817
15. Mishra K, Naffouj S, Gorgis S, et al. Liver injury as a surrogate for inflammation and predictor of outcomes in COVID-19. Hepatol Commun. 2021;5(1):24–32. doi:10.1002/hep4.1586
16. Harapan H, Fajar JK, Supriono S, et al. The prevalence, predictors and outcomes of acute liver injury among patients with COVID-19: a systematic review and meta-analysis. Rev Med Virol. 2022;32(3):e2304. doi:10.1002/rmv.2304
17. Mohammed SA, Eid KM, Anyiam FE, et al. Liver injury with COVID-19: laboratory and histopathological outcome-systematic review and meta-analysis. Egypt Liver J. 2022;12(1):9. doi:10.1186/s43066-022-00171-6
18. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. biorxiv. 2020. doi:10.1101/2020.02.03.931766
19. Voiosu A, Roman A, Pop R, et al. Characteristics and outcomes of patients with COVID-19 and liver injury: a retrospective analysis and a multicenter experience. Romanian J Intern Med Rev Roum Med Interne. 2022;60(1):49–55. doi:10.2478/rjim-2021-0027
20. Schattenberg JM, Labenz C, Wörns MA, et al. Patterns of liver injury in COVID-19 - a German case series. United Eur Gastroenterol J. 2020;8(7):814–819. doi:10.1177/2050640620931657
21. Li P, Liu Y, Cheng Z, Yu X, Li Y. COVID-19-associated liver injury: clinical characteristics, pathophysiological mechanisms and treatment management. Biomed Pharmacother Biomedecine Pharmacother. 2022;154:113568. doi:10.1016/j.biopha.2022.113568
22. Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94(4):1367–1369. doi:10.1007/s00204-020-02734-1
23. McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Covid-19 and liver injury: role of inflammatory endotheliopathy, platelet dysfunction, and thrombosis. Hepatol Commun. 2022;6(2):255–269. doi:10.1002/hep4.1843
24. Zampino R, Mele F, Florio LL, et al. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14(5):881–883. doi:10.1007/s12072-020-10077-3
25. Kashour Z, Riaz M, Garbati MA, et al. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2021;76(1):30–42. doi:10.1093/jac/dkaa403
26. Patel TK, Patel PB, Barvaliya M, Saurabh MK, Bhalla HL, Khosla PP. Efficacy and safety of lopinavir-ritonavir in COVID-19: a systematic review of randomized controlled trials. J Infect Public Health. 2021;14(6):740–748. doi:10.1016/j.jiph.2021.03.015
27. Tang H, Zhou L, Li X, et al. Drug-induced liver injury associated with lopinavir-ritonavir in patients with COVID-19: a disproportionality analysis of U.S. food and drug administration adverse event reporting system (FAERS) data. Int J Clin Pharm. 2021;43(4):1116–1122. doi:10.1007/s11096-021-01311-5
28. Lopinavir. In: liverTox: clinical and research information on drug-induced liver injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Available from: http://www.ncbi.nlm.nih.gov/books/NBK547961/.
29. Teschke R, Méndez-Sánchez N, Eickhoff A. Liver injury in COVID-19 patients with drugs as causatives: a systematic review of 996 dili cases published 2020/2021 based on RUCAM as causality assessment method. Int J Mol Sci. 2022;23(9):4828. doi:10.3390/ijms23094828
30. Woo SM, Alhaqqan DM, Gildea DT, Patel PA, Cundra LB, Lewis JH. Highlights of the drug-induced liver injury literature for 2021. Expert Rev Gastroenterol Hepatol. 2022;16(8):767–785. doi:10.1080/17474124.2022.2101996
31. Chew M, Tang Z, Radcliffe C, et al. Significant liver injury during hospitalization for COVID-19 is not associated with liver insufficiency or death. Clin Gastroenterol Hepatol. 2021;19(10):2182–2191.e7. doi:10.1016/j.cgh.2021.05.022
32. Ruan X, Lu X, Wang K, et al. Liver injury after antiviral treatment of critically ill patients with COVID-19: a single-centered retrospective cohort study. Ann Palliat Med. 2021;10(3):2429–2438. doi:10.21037/apm-20-1581
33. Pazgan-Simon M, Serafińska S, Kukla M, et al. Liver injury in patients with COVID-19 without underlying liver disease. J Clin Med. 2022;11(2):308. doi:10.3390/jcm11020308
34. Sikkema BJB, Sint Nicolaas JJ, van Wijngaarden PP. No association between COVID-19 related liver injury and the course of disease: a retrospective study. Scand J Gastroenterol. 2021;56(1):68–71. doi:10.1080/00365521.2020.1842489
35. Naseralallah LM, Aboujabal BA, Geryo NM, et al. The determination of causality of drug induced liver injury in patients with COVID-19 clinical syndrome. PLoS One. 2022;17(9):e0268705. doi:10.1371/journal.pone.0268705
36. Fix OK, Fontana RJ, Chu J, et al. AASLD expert panel consensus statement: COVID-19 clinical best practice advice for hepatology and liver transplant providers. AASLD. 2022. Available from: https://www.aasld.org/sites/default/files/2022-10/AASLD%20COVID-19%20Guidance%20Document%2010.06.2022F.pdf.
37. Caballero-Alvarado J, Corvera CZ, Bacilio BM, Caballero CR, Lozano-Peralta K. Post COVID cholangiopathy: a narrative review. Gastroenterol Hepatol. 2022. doi:10.1016/j.gastrohep.2022.09.004
38. Hartl L, Haslinger K, Angerer M, et al. Progressive cholestasis and associated sclerosing cholangitis are frequent complications of COVID-19 in patients with chronic liver disease. Hepatology. 2022;76(6):1563–1575. doi:10.1002/HEP.32582
39. Bütikofer S, Lenggenhager D, Wendel Garcia PD, et al. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41(10):2404–2417. doi:10.1111/LIV.14971
40. Meersseman P, Blondeel J, de Vlieger G, et al. Secondary sclerosing cholangitis: an emerging complication in critically ill COVID-19 patients. Intensive Care Med. 2021;47(9):1037–1040. doi:10.1007/S00134-021-06445-8/FIGURES/1
41. Saleem N, Li BH, Vuppalanchi R, Gawrieh S, Gromski MA. Critical Illness Cholangiopathy in COVID-19 Long-haulers. Tech Innov Gastrointest Endosc. 2022;24(4):351–353. doi:10.1016/j.tige.2022.05.006
42. Bauer U, Pavlova D, Abbassi R, et al. Secondary sclerosing cholangitis after COVID-19 pneumonia: a report of two cases and review of the literature. Clin J Gastroenterol. 2022;15(6):1124–1129. doi:10.1007/s12328-022-01687-5
43. Kulkarni AV, Khlegi A, Sekaran A, et al. Post COVID-19 cholestasis: a case series and review of literature. J Clin Exp Hepatol. 2022;12(6):1580–1590. doi:10.1016/j.jceh.2022.06.004
44. Soldera J, Balbinot RA, Balbinot SS. Biliary casts in post-COVID-19 cholangiopathy. Gastroenterol Hepatol. 2022. doi:10.1016/j.gastrohep.2022.08.008
45. Rela M, Rajakannu M, Veerankutty FH, Vij M, Rammohan A. First report of auxiliary liver transplantation for severe cholangiopathy after SARS-CoV-2 respiratory infection. Am J Transplant. 2022;22(12):3143–3145. doi:10.1111/ajt.17165
46. Heucke N, Keitel V. COVID-19-associated cholangiopathy: what is left after the virus has gone? Hepatol Baltim Md. 2022;76(6):1560–1562. doi:10.1002/hep.32668
47. Nacif LS, Fernandes MR, Waisberg DR, et al. Liver transplant after SARS-CoV-2 infection: a systematic review. Clin Sao Paulo Braz. 2022;77:100042. doi:10.1016/j.clinsp.2022.100042
48. Punga D, Isac S, Paraipan C, et al. Impact of COVID-19 infection on liver transplant recipients: does it make any difference? Cureus. 2022;14(2):e22687. doi:10.7759/cureus.22687
49. Montón Rodríguez C, Navarro Cortés P, Lluch García P, Mínguez Pérez M. Autoimmune hepatitis triggered by COVID-19. Rev Espanola Enfermedades Dig Organo Of Soc Espanola Patol Dig. 2022;114(1):64–65. doi:10.17235/reed.2021.8045/2021
50. Volchkova EA, Legkova KS, Topchy TB. Коронавирусная инфекция в роли триггера аутоиммунного гепатита. Клиническое наблюдение [COVID-19 as a trigger of autoimmune hepatitis. Case report]. Ter Arkh. 2022;94(2):259–264. Russian. doi:10.26442/00403660.2022.02.201374
51. Drăgănescu AC, Săndulescu O, Bilașco A, et al. Transient immune hepatitis as post-coronavirus disease complication: a case report. World J Clin Cases. 2021;9(16):4032–4039. doi:10.12998/wjcc.v9.i16.4032
52. Zin Tun GS, Gleeson D, Al-Joudeh A, Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2022;76(3):747–749. doi:10.1016/j.jhep.2021.09.031
53. Torrente S, Castiella A, Garmendia M, Zapata E. Probable autoimmune hepatitis reactivated after COVID-19 vaccination. Gastroenterol Hepatol. 2022;45(Suppl 1):115–116. doi:10.1016/j.gastrohep.2021.10.002
54. Cao Z, Gui H, Sheng Z, Xin H, Xie Q. Letter to the editor: exacerbation of autoimmune hepatitis after COVID-19 vaccination. Hepatol Baltim Md. 2022;75(3):757–759. doi:10.1002/hep.32269
55. Floreani A, De Martin S. COVID-19 and autoimmune liver diseases. J Clin Med. 2022;11(10):2681. doi:10.3390/jcm11102681
56. Wong CKH, Mak LY, Au ICH, et al. Risk of acute liver injury following the mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines. J Hepatol. 2022;77(5):1339–1348. doi:10.1016/J.JHEP.2022.06.032
57. Covid-19 vaccines. LiverTox: clinical and research information on drug-induced liver injury; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK570468/.
58. Shroff H, Satapathy SK, Crawford JM, Todd NJ, VanWagner LB. Liver injury following SARS-CoV-2 vaccination: a multicenter case series. J Hepatol. 2022;76(1):211–214. doi:10.1016/J.JHEP.2021.07.024
59. Efe C, Kulkarni AV, Terziroli Beretta-Piccoli B, et al. Liver injury after SARS-CoV −2 vaccination: features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022;76(6):1576–1586. doi:10.1002/HEP.32572
60. Roy A, Verma N, Singh S, Pradhan P, Taneja S, Singh M. Immune-mediated liver injury following COVID-19 vaccination: a systematic review. Hepatol Commun. 2022;6(9):2513–2522. doi:10.1002/HEP4.1979
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
