Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Cost Utility of Bronchial Thermoplasty for Severe Asthma: Implications for Future Cost-Effectiveness Analyses Based on Phenotypic Heterogeneity
Authors Keim-Malpass J , Malpass HC
Received 24 February 2022
Accepted for publication 7 June 2022
Published 17 June 2022 Volume 2022:14 Pages 427—437
DOI https://doi.org/10.2147/CEOR.S362530
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Jessica Keim-Malpass,1– 3 H Charles Malpass4
1University of Virginia School of Nursing, Charlottesville, VA, USA; 2Department of Pediatrics, University of Virginia School of Medicine, Charlottesville, VA, USA; 3University of Virginia Center for Advanced Medical Analytics, Charlottesville, VA, USA; 4Department of Pulmonary and Critical Care Medicine, University of Virginia School of Medicine, Charlottesville, VA, USA
Correspondence: Jessica Keim-Malpass, University of Virginia School of Nursing, PO Box 800782, Charlottesville, VA, 22908, USA, Email [email protected]
Background: Asthma is a disease with tremendous phenotypic heterogeneity, and the patients who are most severely impacted by the disease are high utilizers of the United States healthcare system. In the past decade, there has been many advances in asthma therapy for those with severe disease, including the use of a procedure called bronchial thermoplasty (BT) and the use of biologic therapy for certain phenotypes, but questions remain regarding the long-term durability and cost effectiveness of these therapies. The purpose of this analysis was (1) to assess the cost utility of BT relative to usual care (base case) and (2) to assess the cost utility of BT relative to usual care plus biologic therapy (omalizumab) (scenario analysis) based on updated 10-year clinical trial outcomes.
Methods: A Markov cohort model was developed and used to estimate the cost utility of BT to estimate the costs and quality-of-life impact of BT versus the comparisons over a 10-year time frame using a limited societal perspective, which included both direct health utilization costs and indirect costs associated with missed days of work, among those with severe persistent asthma.
Results: In the base case and the scenario analysis, BT was the dominant treatment strategy compared to usual care alone and usual care plus biologic therapy. The net monetary benefit for BT was $483,555.49 over a 10-year time horizon.
Conclusion: Cost-utility models are central to policy decisions dictating coverage, and can be extended to inform the patient and provider, during clinical decision-making, of the relative trade-offs of therapy, assessing long-term clinical and cost outcomes. Phenotypic classification of severe asthma is central to patient management and should also be integrated into economic analysis frameworks, particularly as new biologic agents are developed that are specific to a phenotype. Despite a larger upfront cost of BT therapy, there is a durable clinical and economic benefit over time for those with severe asthma.
Keywords: asthma, bronchial thermoplasty, cost-effectiveness analysis, economic evaluation, heterogeneity, cost-utility analysis, phenotype
Introduction
Asthma is a chronic inflammatory disease that impacts more than 34 million people in the USA, contributes to nearly 2 million unexpected visits to the emergency department (ED), and is the cause of more than 3000 deaths annually.1 Asthma is a heterogeneous disease and an estimated 5–10% of asthma patients have severe asthma.2 Asthma is characterized by the difficulty in achieving adequate symptom control even when patients are on long-term inhaled corticosteroids and long-acting beta agonists (which represents standard of care).2 Patients with severe asthma are high utilizers of healthcare services.3 This small group consumes roughly 50–60% of healthcare costs attributed to asthma care, which is estimated to be five times more than patients with mild disease.3 Beyond the direct healthcare costs attributed to medication management, outpatient and ED visits, and hospital utilization, there are also significant spillover impacts in terms of reduced health-related quality of life, missed days of work and school, limitation in activities of daily living, and impacts on other productivity costs, as well as the cumulative lifetime emotional impact of self-management for a chronic condition.4–8
As recently as two decades ago, there were very few options for patients with severe asthma exacerbations beyond systemic corticosteroids, which were associated with significant treatment toxicity and morbidity.7,9 Within the past 10 years, there have been substantial efforts made to understand the phenotypic heterogeneity of the disease, particularly among those patients with severe asthma and who are refractory to front-line therapies.7,10 As more focus has been placed on defining phenotypic biomarkers, there has also been a rapid influx of targeted biologic therapies approved for severe asthma, including monoclonal antibodies that target immunoglobulin E (IgE) (omalizumab), interleukin-5 (IL-5) (mepolizumab/reslizumab), the receptor IL-5Ra (benralizumab), and anti-IL-4Ra (dupilumab).10–12 Omalizumab, the first biologic treatment approved for asthma, has been available the longest and has demonstrated efficacy, but not all patients are responsive to treatment and patients may have to change biologic therapy if they are refractory to the initial treatment regimen.11 There is also a high economic cost associated with lifetime continuous use of biologic therapy.7,9
Bronchial thermoplasty (BT) is an intervention delivered through a bronchoscope that targets the airway smooth muscle. During BT, radiofrequency ablation is sequentially applied to the airways via a small catheter.13 The therapeutic intent of BT is to target hypertrophic airways by decreasing airflow obstruction, leading to long-lasting atrophy of the airway smooth muscle layer while simultaneously not damaging the mucosal layer.14,15 Typically, BT requires an outpatient procedure with three separate bronchoscopic procedure visits.16 Two large randomized controlled trials have demonstrated the safety and efficacy of BT compared to standard treatment (inhaled corticosteroid and long-acting beta agonist) for moderate to severe asthma, and have published long-term follow-up data.1,16–18 A Cochrane Review evaluating BT for those with moderate to severe asthma concluded that the intervention provided a modest clinical benefit for those at risk of asthma exacerbation but did not significantly impact patient-reported asthma control scores or health-related quality of life between exacerbations.19 Unlike the persistent and lifelong costs associated with biologic therapy, BT is associated with a high up-front cost and no costs associated with the therapy after the patient recovers from treatment.20 Further, while more data are needed, it is thought by some that those who undergo BT can successfully be weaned off other asthma medications over time.18
Head-to-head comparisons of BT and biologics have not yet been made through randomized controlled trials, and there are limited data comparing the effectiveness using observational or real-world data.21 The initial randomized controlled trials demonstrating the safety and efficacy of BT (the RISA and AIR2 trials) have published 10-year follow-up data,1,18 but long-term utilization data beyond the 10-year mark are lacking. Further, the RISA17 and AIR216 trials included patients with baseline forced expiratory volume in 1 second (FEV1) greater than 50–60% of predicted, and there have been recent efforts to study the safety and efficacy of severely obstructed asthma patients with FEV1 less than 50% of predicted.15 In an observational cohort study, Langton et al15 found that severely obstructed asthmatics who underwent BT had a similar safety and efficacy profile, and lower medication use over time, compared to less obstructed patients. It is unclear whether those who initiate a biologic therapy and then undergo BT could eventually be taken off their biologic therapy over time. In addition, the average age of the those enrolled in AIR2 was 41 years old, and although we know that asthma exacerbations and unplanned hospitalizations can impact days off from work and indirect costs of treatment, we know very little about the long-term impacts beyond the clinical trial period.16
Questions remain about the cost effectiveness of BT, given the phenotypic heterogeneity of the disease, durability of treatment effect over time, direct and indirect costs of the therapy, and the various biologics that are only targeted towards specific phenotypes.22 Previous research has investigated the cost effectiveness of BT in moderate to severe asthma, but very few studies included severe asthma with a biologic as a comparator.1,20,23–25 When moderate asthma has been included in the model, the incremental cost-effectiveness ratio (ICER) exceeded a $50,000 willingness-to-pay (WTP) threshold.13,20,23,24 When the comparator was the early biologic therapy omalizumab, BT was cost effective relative to biologic therapy using a WTP of $100,000/quality-adjusted life-year (QALY).20 In addition, previous cost-effectiveness analyses have failed to include indirect costs associated with lost work days. The purpose of this analysis was to extend the previous work by (1) assessing the cost utility of BT relative to usual care and (2) assessing the cost utility of BT relative to usual care plus biologic therapy (omalizumab), while also accounting for a limited societal perspective (costs associated with missed days of work).
Methods
Overview
Two Markov cohort models were developed to estimate the cost utility of (1) a base case of BT plus usual care versus usual care alone and (2) a scenario analysis of BT plus usual care versus usual care plus biologic therapy (omalizumab). The modeling approach was used to account for state transitions associated with severe asthma exacerbation and subsequent healthcare utilization. Usual care is defined as the use of inhaled corticosteroids and long-acting beta agonists, with the use of additional controller medications as needed (for example, leukotriene modifiers). Each of the cycle lengths was 1 year and the overall length of the analysis was for a period of 10 years. Model inputs were based predominantly on published clinical trial findings and supplemented with additional data from the literature and publicly accessible websites. A limited societal perspective was utilized because the model accounts for costs associated with days missed from work along with costs associated with treatment itself and asthma exacerbations. We utilized and adhered to the Consolidated Health Evaluation Reporting Standards (CHEERS) for economic evaluations related to health interventions.26 No institutional board review approval was needed because model parameters were based on aggregate publicly available data. Analytic decisions were maintained in an audit trail to increase rigor and reproducibility in the modeling process.
Model Structure and Overall Analysis Schema
Figure 1 represents the health states associated with the Markov cohort model. Combined consensus guidelines from the International Society of Pharmacoeconomics and Outcomes Research and the Society for Medical Decision Making on state-transition modeling were consulted for guidance in best practice recommendations.27 The same health states were used for both the base case and the scenario analysis. The cycle length was 1 year, so annual estimates of exacerbation and utilization were used to construct this model.28 For every time epoch, patients could be considered healthy, experiencing a severe exacerbation requiring a physician visit (assuming additional controller medications required), experiencing a severe exacerbation requiring an emergency department (ED) visit (assuming discharged home from ED), or experiencing a severe exacerbation requiring hospitalization. The exacerbation requiring hospitalization could result in a well state/discharge home, or death due to exacerbation. Underlying population-level risk of death was incorporated into the analysis and the model assumes that in order to die from asthma, a patient must be admitted to the hospital first. Every other health state assumes that the patient either remains well or successfully recovers from their asthma exacerbation.
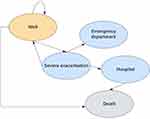 |
Figure 1 Markov state diagram. |
The model structure (Figure 2) and analysis was completed using TreeAge Pro Health (Williamston, MA). Models were built for both the base case and scenario analysis, where the comparator for usual care included biologic therapy. The cost-utility analysis was conducted using a Markov model that evaluated a hypothetical cohort of severe asthma patients for a period of 10 years. The characteristics of the base case of the cohort were developed based on the characteristics of patients enrolled in the AIR2 trial (NCT01350414), where the safety and efficacy of BT plus usual care was evaluated versus a sham bronchoscopy procedure plus usual care, and there are associated 10-year follow-up outcomes.1,16 The AIR2 trial was a randomized controlled trial where all subjects had to: (1) have severe persistent asthma; (2) have a baseline Asthma Quality of Life Questionnaire score of 6.25 or lower; (3) have an FEV1 greater than 60% of predicted; (4) have at least 2 days of asthma symptoms during a 4-week baseline period despite the use of asthma controller medications; and (5) be a non-smoker for at least a year, with less than a 10-year pack-year smoking history, to enroll.16 The RISA trial also evaluated the safety and efficacy of BT plus usual care versus usual care in a similar population with FEV1 greater than 50% of predicted and eligible patients currently taking up to 30 mg of corticosteroids with associated long-term outcomes. The RISA trial did not evaluate utilization secondary to asthma exacerbation in a way that characterizes ED use or hospitalization.17,18 Despite this, the RISA trial characteristics and outcomes were ascertained to confirm the modeling strategy and inputs with very similar patient characteristics and impact on exacerbations. For the scenario analysis, omalizumab was chosen as the biologic therapy comparator, in part because it has been used in the severe asthma population for the longest amount of time. There are no direct comparisons of BT versus biologics in the clinical trial setting, so inputs of omalizumab versus standard therapy were informed by the literature.4,20,29 Therapy constituting usual care was defined by Steps 3–4 of the Global Initiative for Asthma (GINA) treatment strategy.6,30 Both the base case and the scenario analysis assume that patients in the BT arm remain on all usual-care medications for their underlying severe asthma, which is a conservative approach given that the AIR2 trial demonstrated a reduction in underlying usual-care medication over time.1,16
 |
Figure 2 Model structure for the bronchial thermoplasty arm (the usual-care arm has the same Markov structure). |
Model Inputs
Model inputs for the BT plus usual care arm and the usual-care arm were based on rates of exacerbations as published by the AIR2 trial, and can be found in Table 1.1,16 Rates of exacerbation were modeled as constant for the usual-care patients. Rates for patients in the BT arms included a higher rate of hospitalization/complications directly following the procedure, followed by a constant rate of exacerbation for the remainder of the 10 years. Rates of exacerbation were converted into transition probabilities using the equation 1−e−rt. QALYs and disutility adjustments were assessed using utility weights ranging from 0 to 1, and were abstracted from the extant literature. Even though the interventions are aimed at reducing severe asthma exacerbation, none of them is expected to be curative, and thus the highest utility weight/QALY a patient will experience in the well state is 0.77. Disutilities were associated with events (exacerbation, emergency department use, hospitalization) and were subtracted from the baseline well state (0.77) during the cycle in which the event occurred. All annual utility weights and disutility adjustments were derived from the literature and are presented in Table 1.
 |
Table 1 Model Parameters |
Cost inputs were chosen in accordance with the limited societal perspective to include direct costs associated with intervention, usual-care medication, and exacerbations requiring a physician visit, ED visit, or hospitalization; and indirect costs associated with days missed from work. There is a dramatic difference in costs associated with a hospitalization where the patient survives and is discharged home versus a patient who dies in the hospital, which mirrors other end-of-life utilization studies.31–33 Cost data utilized the perspective of those with private insurance, given that the age of the hypothetical base-case patient was 41 years, and therefore not yet eligible for Medicare benefits.25 BT was entered as a one-time upfront cost within the Markov model, and annual medication costs were used. The cost estimates used were based on 2013 US dollars, so they were converted to 2020 US dollars by multiplying by the rate of inflation using the medical services component of the consumer price index. Days missed from work were also determined from the AIR2 trial and this figure was multiplied by the average daily US salary in 2020 US dollars. There has been no substantial decrease in intervention or medication costs during the time period from 2013 to 2020. Costs and utilities were discounted at 3%, in accordance with best practice recommendations.34
Sensitivity Analyses
One-way and two-way sensitivity analyses were conducted to estimate the parameter uncertainty on the model base case and the scenario analysis. In the one-way sensitivity analysis, variables were varied either on known ranges of outcomes or as greater/less than 10% of the model parameter when a range was not known (Table 1).
Results
Baseline Results
Overall cost-utility model results for the base case and the scenario analysis are shown in Table 2. BT was considered the dominant strategy in both the base case and scenario analysis. For the base case over the 10-year model period, BT increased QALYs by 0.53 over time. Costs in the usual-care arm were lower and totaled $43,643 compared to $54,113 in the BT arm over the 10-year period. Ten-year costs for the biologic arm were estimated at $300,483 and costs for BT at $54,113. The cumulative percentage of each cohort who experienced the health states at 10 years was similar, which likely drives the relatively similar effectiveness/QALY rankings seen between strategies. The net monetary benefit for BT was $483,555 (Figure 3).
 |
Table 2 Cost-Utility Rankings for Each Comparison and Strategy |
 |
Figure 3 Net monetary benefit (NMB) vs willingness to pay. |
Sensitivity Analyses
As seen in the one-way sensitivity analysis and tornado diagram (shown for the scenario analysis related to biologic therapy in Figure 4), parameters related to the transition probabilities contributed the most to model uncertainty.
Discussion
This study compared the cost utility of (1) BT versus usual care and (2) BT versus biologic therapy (omalizumab)/usual care over a 10-year time horizon among patients with severe asthma. Among those with severe asthma requiring biologic therapy (omalizumab) and among those in the base case/standard of care asthma therapies, BT was the dominant and favored strategy for treatment.
There have been several cost-effectiveness studies related to the use of BT in severe asthma, with varied results. For example, Cangelosi et al25 found BT to be cost effective compared to usual care, but their model assumed that someone in the BT arm would return to a better than severe asthma utility state following treatment. Our study assumed a more conservative modeling approach, given that the utilities were based on exacerbation and not underlying improvement in health-related quality of life associated with improved symptomatology (owing to limitations in the comparison of health-related quality of life data in the AIR2 and RISA clinical trials). When modeled from a Singapore perspective, Nguyen et al24 did not find BT to be either cost effective or the favored strategy compared to usual care. Nguyen et al did account for both a health system and a societal perspective, and included costs associated with days missed from work, but did not account for the updated long-term outcomes. Zein et al23 modeled the cost effectiveness of BT versus usual care from both a 5- and 10-year time horizon and found BT to be cost effective, but did not include the biologic therapy comparison. Zafari et al20 published the only previous cost-effectiveness analysis that included a comparator of biologic therapy in the usual-care arm (specifically omalizumab). Similar to our analysis, they found that when the comparator included biologic therapy, BT became the dominant and favored strategy. Our analysis further extends the work by Zafari et al by including days missed from work and a first step of a societal perspective in the model, and extends the model horizon, given the long-term clinical outcomes published by Chaudhuri et al in 2021.35
There are several limitations of our model design owing to scant inclusion of the limited societal perspective, and uncertainty associated with parameter inputs due to the gaps in available evidence, that must be addressed. First, long-term exacerbation and event rates were collected only in the BT arm and not in the usual-care arm at the 5- and 10-year assessment following the AIR2 trial, and could not be included in meaningful way because of the lack of a usual-care comparator. Therefore, it is very likely that the model inputs underestimate the relative rate of exacerbations experienced in the usual-care arm, with a chance to further favor BT over time.1 Further, the costs associated with removing asthma medication in the BT arm were not included owing to a lack of a comparator assessment. Importantly, a recent 10-year outcome update assessed the durability of response among participants in both the AIR2 and RISA clinical trials (n=136) compared to 56 participants who were in the sham/control arms of the trials, on an intention-to-treat basis (n=18 or 32% of the sham/control arm patients received BT after the previous clinical trial concluded).35 This follow-up analysis demonstrated that in the year prior to the 10-year assessment, there was a higher rate of severe exacerbation in the BT arms than during the 5-year assessment, but similar in rate to the 1-year post-BT assessment, indicating a potential small loss of durability of effectiveness of the intervention over time, but an overall stable trajectory.35 Also, the 1-year follow-up assessments are unclear about the proportion of those who received BT and sham/control patients who ultimately required biologic therapy, so it is very difficult to extrapolate these results.35 In addition, there were no head-to-head clinical trials assessing BT versus biologic therapy. As such, there is uncertainty in interpreting our cost-utility analysis results as definitive, given the potential baseline clinical characteristics and differences in populations who require biologic therapy, along with the use of purely observational data for the parameter estimates.
The QALY/utility estimates for this analysis were based on exacerbations severe enough to seek care through an outpatient visit, ED visit, or hospitalization for severe asthma. Intrusive asthma symptoms have a marked impact on health-related quality of life, and impact productivity and the ability to work as well as leisure activities.6,8 While our analysis did attempt to include a societal perspective with the indirect costs associated with missed working days, there is a major gap in the lack of inclusion of utilities associated with variations in health-related quality of life and symptoms that are intrusive to activities of daily living. Other societal perspective inputs should include caregiving burden, impacts on recreational activity, and impacts on child outcomes, given that most of these patients are young adults (and have young children) at the time of initial treatment. Future work should also include longer time horizons and estimates of life-years gained, particularly given the age at diagnosis and severity onset.36
The AIR2 and RISA trials included patients with a baseline FEV1 greater than 60% and 50% of predicted, respectively, and there is emerging evidence to suggest that BT can be safely offered in patients with FEV1 less than 50% of predicted.15 When these severely obstructed patients were assessed in a cohort study, it was found that following BT they had significant improvements in health-related quality of life, reduction in exacerbation frequency, decreased use of medication, and decreased requirement for oral steroids.15 Patients who are more severely obstructed at baseline are likely the patients with the highest utilization and those who are more likely to be on biologic therapy. Further, patients with more severe asthma may be more likely to be refractory to biologic therapy; in other words, to fail to achieve symptom control. Our model did not include estimates of those who would not achieve any symptom control through the biologic comparator within this present analysis. For these reasons, it is imperative that future cost-effectiveness analyses are able to take heterogeneous phenotypes, symptomatology, and disease severity into account when conducting analyses. Because clinical trials cannot possibly compare all combinations of interventions or follow-up on patients beyond the clinical trial period, it is important that we use real-world data to discern head-to-head comparisons and long-term follow-up data when the relevant clinical trials do not exist.37,38
Beyond relevance to policy, coverage, and reimbursement, cost-utility models offer relevance to the patient and provider during clinical decision-making to initiate conversations relative to the trade-offs of therapy and long-term clinical and cost outcomes.39 These models can also be used to educate patients about the importance of adherence to asthma medication, given that model outcomes are often based on clinical trial data and that higher levels of adherence are noted in trials than in the general population.40 Further, while cost-utility or cost-effectiveness models are often conducted through the lens of the payor or healthcare system, more work can be done to build them from the perspective of the patient, which is currently difficult owing to the lack of transparency regarding private insurance costs in the US payer context. These models can offer a visual approach to direct cost impacts from the perspective of the patient. If a patient and family knew what their direct costs were estimated to be prior to clinical decision-making, these models could help inform their shared decisions. Here, we chose to conduct two separate analyses (usual care only and usual care plus biologic) as proof of concept that in the future these models can be tailored to an individual’s phenotype (and associated biologic therapy, if applicable) to help influence patient-centered decisions in the setting of phenotypic heterogeneity. Given the vast phenotypic heterogeneity in moderate-to-severe asthma and the tremendous implications for shared clinical decision-making in the realm of BT and biologic therapy, economic models will continue to be central in this clinical area, particularly as novel therapies continue to enter the market.
Funding
JKM is supported as a Betty Irene Moore Nurse Fellow funded by the Gordon and Betty Moore Foundation (GBMF9048).
Disclosure
The authors report that are no financial or other conflicts of interest to disclose.
References
1. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295–1302. doi:10.1016/j.jaci.2013.08.009
2. Hekking -P-PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042
3. Hanratty CE, Matthews JG, Arron JR, et al. A randomised pragmatic trial of corticosteroid optimization in severe asthma using a composite biomarker algorithm to adjust corticosteroid dose versus standard care: study protocol for a randomised trial. Trials. 2018;19(1):5. doi:10.1186/s13063-017-2384-7
4. Mansur AH, Srivastava S, Mitchell V, Sullivan J, Kasujee I. Long term clinical outcomes of omalizumab therapy in severe allergic asthma: study of efficacy and safety. Respir Med. 2017;124:36–43. doi:10.1016/j.rmed.2017.01.008
5. Stróżek J, Samoliński BK, Kłak A, et al. The indirect costs of allergic diseases. Int J Occup Med Environ Health. 2019;32(3):281–290. doi:10.13075/ijomeh.1896.01275
6. Settipane RA, Kreindler JL, Chung Y, Tkacz J. Evaluating direct costs and productivity losses of patients with asthma receiving GINA 4/5 therapy in the United States. Ann Allergy Asthma Immunol. 2019;123(6):564–572.e3. doi:10.1016/j.anai.2019.08.462
7. Denlinger LC, Heymann P, Lutter R, Gern JE. Exacerbation-prone asthma. J Allergy Clin Immunol Pract. 2020;8(2):474–482. doi:10.1016/j.jaip.2019.11.009
8. Lucas C, Aly S, Touboul C, Sellami R, Guillaume X, Garcia G. Patient-reported outcome in two chronic diseases: a comparison of quality of life and response profiles in severe migraine and severe asthma. Patient Relat Outcome Meas. 2020;11:27–37. doi:10.2147/PROM.S222597
9. Wang AL, Tantisira KG. Personalized management of asthma exacerbations: lessons from genetic studies. Expert Rev Precis Med Drug Dev. 2016;1(6):487–495. doi:10.1080/23808993.2016.1269600
10. Delgado J, Dávila IJ, Domínguez-Ortega J; Group of Severe Asthma (SEAIC). Clinical recommendations for the management of biological treatments in severe asthma patients: a consensus statement. J Investig Allergol Clin Immunol. 2020. doi:10.18176/jiaci.0638
11. Domingo C, Pomares X, Morón A, Sogo A. Dual monoclonal antibody therapy for a severe asthma patient. Front Pharmacol. 2020;11:587621. doi:10.3389/fphar.2020.587621
12. Bermejo I, Stevenson M, Cooper K, et al. Mepolizumab for treating severe eosinophilic asthma: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(2):131–144. doi:10.1007/s40273-017-0571-8
13. Canadian Agency for Drugs and Technology in Health. Bronchial Thermoplasty for Severe Asthma: A Review of the Clinical and Cost-Effectiveness, and Guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015.
14. Langton D, Lee P. Bronchial thermoplasty: redefining its role. Respirology. 2020;25(9):981–986. doi:10.1111/resp.13887
15. Langton D, Ing A, Fielding D, et al. Safety and effectiveness of bronchial thermoplasty when FEV1 is less than 50. Chest. 2020;157(3):509–515. doi:10.1016/j.chest.2019.08.2193
16. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116–124. doi:10.1164/rccm.200903-0354OC
17. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185–1191. doi:10.1164/rccm.200704-571OC
18. Pavord ID, Thomson NC, Niven RM, et al. Safety of bronchial thermoplasty in patients with severe refractory asthma. Ann Allergy Asthma Immunol. 2013;111(5):402–407. doi:10.1016/j.anai.2013.05.002
19. Torrego A, Solà I, Munoz AM, et al. Bronchial thermoplasty for moderate or severe persistent asthma in adults. Cochrane Database Syst Rev. 2014;(3):CD009910. doi:10.1002/14651858.CD009910.pub2
20. Zafari Z, Sadatsafavi M, Marra CA, Chen W, FitzGerald JM. Cost-effectiveness of bronchial thermoplasty, omalizumab, and standard therapy for moderate-to-severe allergic asthma. PLoS One. 2016;11(1):e0146003. doi:10.1371/journal.pone.0146003
21. Langton D, Sha J, Guo S, et al. Bronchial thermoplasty versus mepolizumab: comparison of outcomes in a severe asthma clinic. Respirology. 2020;25(12):1243–1249. doi:10.1111/resp.13830
22. O’Reilly A, Lane S. What is the role of bronchial thermoplasty in the management of severe asthma? Ther Adv Respir Dis. 2018;12:1753466618792410. doi:10.1177/1753466618792410
23. Zein JG, Menegay MC, Singer ME, et al. Cost effectiveness of bronchial thermoplasty in patients with severe uncontrolled asthma. J Asthma. 2016;53(2):194–200. doi:10.3109/02770903.2015.1072552
24. Nguyen HV, Bose S, Mital S, et al. Is bronchial thermoplasty cost-effective as treatment for problematic asthma patients? Singapore’s perspective on a global model. Respirology. 2017;22(6):1102–1109. doi:10.1111/resp.13027
25. Cangelosi MJ, Ortendahl JD, Meckley LM, et al. Cost-effectiveness of bronchial thermoplasty in commercially-insured patients with poorly controlled, severe, persistent asthma. Expert Rev Pharmacoecon Outcomes Res. 2015;15(2):357–364. doi:10.1586/14737167.2015.978292
26. Husereau D, Drummond M, Petrou S. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29:117–122.
27. Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force–3. Value Health. 2012;15(6):812–820. doi:10.1016/j.jval.2012.06.014
28. Olariu E, Cadwell KK, Hancock E, Trueman D, Chevrou-Severac H. Current recommendations on the estimation of transition probabilities in Markov cohort models for use in health care decision-making: a targeted literature review. Clinicoecon Outcomes Res. 2017;9:537–546. doi:10.2147/CEOR.S135445
29. Bousquet J, Cabrera P, Berkman N, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60(3):302–308. doi:10.1111/j.1398-9995.2004.00770.x
30. Global Initiative for Asthma - GINA. Available from: https://ginasthma.org/.
31. Reich O, Signorell A, Busato A. Place of death and health care utilization for people in the last 6 months of life in Switzerland: a retrospective analysis using administrative data. BMC Health Serv Res. 2013;13:116. doi:10.1186/1472-6963-13-116
32. Feudtner C, DiGiuseppe DL, Neff JM. Hospital care for children and young adults in the last year of life: a population-based study. BMC Med. 2003;1:3. doi:10.1186/1741-7015-1-3
33. Keim-Malpass J, Erickson JM, Malpass HC. End-of-life care characteristics for young adults with cancer who die in the hospital. J Palliat Med. 2014;17(12):1359–1364. doi:10.1089/jpm.2013.0661
34. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi:10.1001/jama.2016.12195
35. Chaudhuri R, Rubin A, Sumino K, et al. Safety and effectiveness of bronchial thermoplasty after 10 years in patients with persistent asthma (BT10+): a follow-up of three randomised controlled trials. Lancet Respir Med. 2021;9(5):457–466. doi:10.1016/S2213-2600(20)30408-2
36. Jakubiak-Lasocka J, Jakubczyk M. Cost-effectiveness versus cost-utility analyses: what are the motives behind using each and how do their results differ?-A polish example. Value in Health Regional Issues. 2014;4:66–74. doi:10.1016/j.vhri.2014.06.008
37. Lavelle TA, Kent DM, Lundquist CM, et al. Patient variability seldom assessed in cost-effectiveness studies. Med Decis Making. 2018;38(4):487–494. doi:10.1177/0272989X17746989
38. Arora P, Boyne D, Slater JJ, Gupta A, Brenner DR, Druzdzel MJ. Bayesian networks for risk prediction using real-world data: a tool for precision medicine. Value Health. 2019;22(4):439–445. doi:10.1016/j.jval.2019.01.006
39. Salvi E, Parimbelli E, Quaglini S, Sacchi L. Eliciting and exploiting utility coefficients in an integrated environment for shared decision-making. Methods Inf Med. 2019;58(1):24–30. doi:10.1055/s-0039-1692416
40. Chongmelaxme B, Chaiyakunapruk N, Dilokthornsakul P. Incorporating adherence in cost-effectiveness analyses of asthma: a systematic review. J Med Econ. 2019;22(6):554–566. doi:10.1080/13696998.2019.1572014
41. Krishnan V, Diette GB, Rand CS, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174(6):633–638. doi:10.1164/rccm.200601-007OC
42. CDC WONDER. Available from: https://wonder.cdc.gov/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

