Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Cost-effectiveness of vedolizumab compared with conventional therapy for ulcerative colitis patients in the UK
Authors Wilson MR, Azzabi Zouraq I, Chevrou-Severac H, Selby R, Kerrigan MC
Received 25 February 2017
Accepted for publication 16 July 2017
Published 16 October 2017 Volume 2017:9 Pages 641—652
DOI https://doi.org/10.2147/CEOR.S135609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Michele R Wilson,1 Ismail Azzabi Zouraq,2 Helene Chevrou-Severac,2 Ross Selby,3 Matthew C Kerrigan4
1RTI Health Solutions, Research Triangle Park, NC, USA; 2Takeda Pharmaceuticals International AG, Zurich, Switzerland; 3Takeda UK Ltd., Bucks, UK; 4PHMR Limited, London, UK
Objective: To examine the clinical and economic impact of vedolizumab compared with conventional therapy in the treatment of moderately-to-severely active ulcerative colitis (UC) in the UK based on results of the GEMINI I trial.
Methods: A decision-analytic model in Microsoft Excel was used to compare vedolizumab with conventional therapy (aminosalicylates, corticosteroids, immunomodulators) for the treatment of patients with UC in the UK. We considered the following three populations: the overall intent-to-treat population from the GEMINI I trial, patients naïve to anti-TNF therapy, and those who had failed anti-TNF-therapy. Population characteristics and efficacy data were obtained from the GEMINI I trial. Other inputs (eg, unit costs, probability of surgery, mortality) were obtained from published literature. Time horizon was a lifetime horizon, with costs and outcomes discounted by 3.5% per year. One-way and probabilistic sensitivity analyses were conducted to measure the impact of parameter uncertainty.
Results: Vedolizumab had incremental cost-effectiveness ratios of £4,095/quality-adjusted life-year (QALY), £4,423/QALY, and £5,972/QALY compared with conventional therapy in the intent-to-treat, anti-TNF-naïve, and anti-TNF-failure populations, respectively. Patients on vedolizumab accrued more QALYs while incurring more costs than patients on conventional therapy. The sensitivity analyses showed that the results were most sensitive to induction response and transition probabilities for each treatment.
Conclusion: The results suggest that vedolizumab results in more QALYs and may be a cost-effective treatment option compared with conventional therapy for both anti-TNF-naïve and anti-TNF-failure patients with moderately-to-severely active UC.
Keywords: ulcerative colitis, cost-effectiveness, vedolizumab, inflammatory bowel disease
Introduction
Ulcerative colitis (UC) is an idiopathic inflammatory bowel disorder characterized as a chronic condition in which the colon mucosa becomes inflamed and ulcerated.1,2 UC affects about 0.24% of the UK population per a 2011 report by the National Institute for Health and Care Excellence.3 The symptoms of UC can lead to a substantial negative impact on patient quality of life, even when compared with other chronic conditions, such as rheumatoid arthritis.4 Patients with UC also incur a significant economic burden, including direct medical costs and indirect costs associated with absenteeism and productivity loss.5
Current pharmacologic treatments for UC are not curative. Current treatments manage acute disease symptoms and prevent relapses.6 UC is first managed by conventional therapies, such as aminosalicylates, steroids, and immunosuppressants.2 In patients for whom conventional therapy fails (either due to lack of efficacy or intolerability), another mix of conventional therapies or biologic treatments may be used to manage the disease. All current biologic therapies work by inhibiting TNF-alpha (ie, anti-TNF therapies). For patients for whom pharmacotherapy is ineffective, surgery may be an alternative.
Vedolizumab is a novel biologic treatment with a novel mechanism of action: unlike systemic anti-TNF therapies, vedolizumab is gut-specific. In the Phase III GEMINI I trial, vedolizumab patients had higher response and remission compared with those on conventional therapy.7,8 The results of the GEMINI I trial demonstrated that vedolizumab may provide a greater health benefit than conventional therapy.
The study objective was to estimate the cost-effectiveness of vedolizumab compared with conventional therapy in patients with moderately-to-severely active UC in the UK, as seen in the GEMINI I trial. The abstract of this paper was presented at the 23rd United European Gastroenterology Week Conference in 2015 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in UEG Journal.9
Methods
Model structure
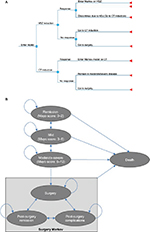
To examine the costs and outcomes associated with vedolizumab and conventional therapy in a moderately-to-severely active UC population, we developed a decision-analytic model based on a model developed by Tsai et al.10,11 Specifically, we created a model including a decision-tree and long-term Markov framework (Figure 1). The model considers three on-treatment health states based on Mayo scores: remission (Mayo <3); mild UC (Mayo 3–5), and moderate-severe UC (Mayo ≥6). In addition, we included three health states related to surgery: surgery; post-surgery remission; and post-surgery complications. The basic model structure is consistent with an indirect comparison of vedolizumab with other biologics.12
The induction phase of the model represents the GEMINI I trial’s 6-week induction period. During this phase, patients with moderate-severe disease initiate treatment with either vedolizumab or conventional therapy and are monitored for response at the end of 6 weeks, as seen in the GEMINI I trial. Patients responding to vedolizumab in induction and who do not experience discontinuation resulting from adverse event intolerability then enter a long-term Markov model for maintenance therapy in one of the three Mayo-score health states (Figure 1B). Patients who respond to treatment may remain on therapy moving through these health states. Patients who fail to respond in induction, who subsequently lose response, or who experience intolerability to adverse events are assumed to discontinue vedolizumab and switch to conventional therapy.
Patients in the conventional therapy arm (whether at the onset of the model or after switching from vedolizumab) face a similar decision-tree for induction as vedolizumab patients. However, those who fail to respond to conventional therapy are assumed to remain in the moderate-severe health state until they require surgery.
Patients in either arm of the model in moderate-severe disease incur a risk of surgery. Those who require surgery move to the surgery health state and are assumed to discontinue pharmacotherapy (Figure 1B). Following surgery, these patients may transition among the surgery-related health states in each subsequent cycle: post-surgery remission (free of complications); post-surgery complications (experiencing complications); or surgery (requiring another surgery).
All patients incur a risk of death in any cycle in the model, regardless of their current health state or pharmacotherapy.
The model was populated with data from the GEMINI I trial and the published literature, and estimated costs and outcomes from the National Institutes of Health and Personal Social Services perspective over a patient’s lifetime. Costs and outcomes were presented in 2013/2014 British pounds, and we assumed an annual discount rate of 3.5% for both as specified by the National Institute for Health and Care Excellence.3
Patient population
The patients in this analysis were from the GEMINI I trial. Specifically, we included patients with moderately-to-severely active UC (ie, Mayo score ≥6) “who have had an inadequate response with, lost response to, or are intolerant to either a conventional therapy or an anti-TNF”.7 Patients in the trial were a mix of anti-TNF-naïve and anti-TNF-failure, with 51.8% of patients being anti-TNF-naïve. The modeled population averaged 40.25 years of age and 73.43 kg in weight, with 58% being male as seen in the GEMINI I trial.7
We examined costs and outcomes in the following three populations:
- mixed population: patients in this population represent the entire GEMINI I trial population (includes both anti-TNF-naïve and anti-TNF-failure patients, representing the intent-to-treat [ITT] population of the GEMINI I trial);
- anti-TNF-naïve subgroup: patients who have never received a biologic treatment;
- anti-TNF-failure subgroup: patients who have previously failed an anti-TNF treatment.
Treatments
Treatment with vedolizumab (300 mg) at baseline, week 2, week 6, and every 8 weeks thereafter in combination with conventional therapy (a combination of treatments such as azathioprine, 6-mercaptoprurine, methotrexate, 5-aminosalicylate, sulfasalazine, oral mesalamine, prednisolone or budesonide, and antibiotics) was compared with conventional therapy alone. Though patients receiving vedolizumab could also receive conventional therapy, we assumed vedolizumab patients took lower doses of conventional therapy than those taking only conventional therapy. Details on the proportion of patients receiving each treatment comprising conventional therapy can be seen in the Supplementary materials.
Patients responding to vedolizumab were then treated for 1 year, as in the GEMINI I trial. However, we do not have long-term efficacy data for vedolizumab. As such, there is great uncertainty regarding treatment efficacy over a patient’s lifetime. Due to the lack of data beyond 1 year, for the base case we assumed that after 1 year any patients still on vedolizumab would then switch to conventional therapy alone, and incur the costs and transition probabilities associated with conventional therapy.
Inputs
Treatment efficacy
We used treatment response (defined as a decrease in Mayo score ≥3) and remission (Mayo ≤2) at 6 weeks (end of induction) and at the 52 weeks (maintenance), as seen in the GEMINI I trial (Final Clinical Study Report C13006, unpublished data, 2012).7 The estimated response and remission probabilities for each of the three subpopulations are presented in Table 1.
  | Table 1 Probability of response and remission for each treatment Notes: Data from Feagan et al.7 a Probability of response and remission is among those who responded at 6 weeks. Abbreviation: ITT, intent-to-treat. |
For responding patients, the health state that a patient transitions to is based on response or remission and what his or her Mayo score was prior to response or remission. We obtained the percentage of responders remaining in moderate-severe disease state from the trial data (13.2%, 10.1%, and 20.9% of responders for the mixed [ITT] population, anti-TNF-naïve population, and anti-TNF-failure population, respectively).
To estimate disease progression in the maintenance phase and beyond, we optimized transition probabilities such that the modeled proportion of patients in remission and mild UC at the end of the maintenance phase most closely approximated what we would expect given the GEMINI I trial data. Due to lack of long-term (>1 year) trial data at the time of model development, we assumed the derived transition probabilities would be similar over time. The probabilities of each health state transition for each treatment can be seen in the Supplementary materials.
Patients requiring surgery were assumed to permanently discontinue pharmacotherapy. These patients then transitioned among the three surgery and post-surgery health states. We estimated the health state transition probabilities for the surgery and post-surgery health states from previously published studies. These probabilities can be seen in the Supplementary materials.
Clinical safety and discontinuation
Patients on vedolizumab may discontinue due to lack of efficacy or due to adverse events. We assumed all patients who did not respond in induction discontinued after the induction phase. The data for discontinuations in the maintenance phase for patients on vedolizumab were obtained from the clinical trial data (36.89%, 26.39%, and 48.84% for the mixed, naïve, and failure populations, respectively). After 1 year, any patient who lost response was assumed to discontinue. We did not assume any adverse event-related discontinuation after 1 year. Patients remained on conventional therapy for their remaining lifetime unless they required surgery.
Mortality
Because UC has not been shown to increase the risk of mortality, patients in the analysis are assumed to have mortality similar to the general population. Age- and sex-specific all-cause mortality data were obtained from the Office for National Statistics.13 Mortality risk was assumed to increase as patients aged over time in the model.
Utility
Quality-adjusted life-years (QALYs) were estimated by applying utility weights (ranging from 0 for death to 1 for perfect health) to each modeled health state. Health state utilities for remission, mild disease, and moderate-to-severe disease were obtained from a pair of studies by Tsai et al and Punekar and Hawkins,10,11 which presented utility weights based on EuroQol five dimensions data from a UK population.
Treatment-specific adverse event rates, along with utility decrements for selected events (eg, serious infection, tuberculosis, lymphoma, hypersensitivity reactions, and skin reactions), were obtained from the published literature. Unfortunately the previous economic models in UC did not include adverse events, so utility data were obtained where available from the published literature (Table 2).
  | Table 2 Model inputs Notes: aData from Tsai et al.10 bUnit costs for all resource use other than for surgery were obtained from National Health Service (NHS) reference costs.16 Cost of surgery was obtained from Buchanan et al17 and inflated to 2013/2014 using pay and price index.19 cProbabilities of adverse events were obtained from Feagan et al.7 dAdverse event costs are based on NHS reference costs for each condition.16 eData sources for adverse event disutilities are as follows: serious infection,20 tuberculosis,21 lymphoma,22 acute hypersensitivity reactions,23 and skin site reactions.24 All were adjusted by a mean age-related utility adjustment factor of 0.91. |
Costs
We considered only direct medical costs in this study. These included drug acquisition and administration costs, medical costs specific to each health state, and adverse event-related medical costs (Table 2). Vedolizumab’s cost was assumed to be £1,500 per 300 mg vial and £328 per intravenous administration. Conventional therapy was assumed to have an average cost of £102 per day based on expert clinical opinion.14 Dosing and unit costs were obtained from the British National Formulary.15 We assumed that the conventional therapy costs for patients taking vedolizumab were half that of the cost incurred by patients taking only conventional therapy.
To estimate the per-cycle cost for each health state, health state-specific resource use was obtained from Tsai et al,10 who reported annual resource use for each health state as estimated by a panel of UK gastroenterologists. Unit costs from the National Institutes of Health Reference Cost database16 and Buchanan et al17 were multiplied by per-cycle resource use and updated to 2013/14 British pounds (Table 2). Adverse event costs were assumed to be the weighted average costs for codes from the National Health Service Reference Cost schedule16 (Table 2).
Calculations
The model estimated costs (drug, other medical, and total) as well as life-years and QALYs. In addition, the incremental cost-effectiveness ratios (ICERs) were calculated as the difference in costs of care divided by the difference in QALYs between the two strategies. We generated these results for each of the three patient populations.
Parameter uncertainty was examined through one-way and multivariate probabilistic sensitivity. Uncertainty around model parameter estimates was based on the 95% CI using calculated or reported patient counts, standard errors, or ranges, where available. When such data were not available, we assumed the 95% CI to be ±20% of the base-case estimate. Costs and risk multipliers were varied ±20% assuming a gamma distribution; utility weights, adverse event risks, and discontinuations were varied assuming a 95% CI with a beta distribution; transition probabilities followed a Dirichlet distribution. For the probabilistic sensitivity analysis, we performed a second-order Monte-Carlo simulation with 3,000 simulations.
Results
Base-case results
In the base-case analysis, treatment with vedolizumab resulted in more QALYs than conventional therapy regardless of patient population (Table 3). Overall costs were higher for patients on vedolizumab than patients on conventional therapy (Table 3). Vedolizumab was cost-effective (ICER <£30,000/QALY gained) in all patient populations, with ICER values of £4,095/QALY and £4,423/QALY, and £5,972/QALY, in the mixed, anti-TNF-naïve, and anti-TNF-failure populations, respectively.
  | Table 3 Deterministic results Abbreviations: ICER, incremental cost-effectiveness ratio (incremental costs/incremental QALYs); ITT, intent-to-treat; QALY, quality-adjusted life-year. |
One-way sensitivity analysis
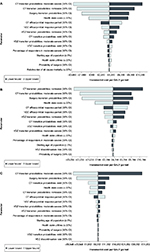
Figures 2A–C illustrate one-way sensitivity analyses comparing vedolizumab with conventional therapy in each subpopulation. Results were most sensitive to induction response during induction and the health state transition probabilities from remission and from surgery.
Multivariate probabilistic sensitivity analysis
In the probabilistic sensitivity analysis, vedolizumab was cost-effective compared with conventional therapy in over 99% of cases in both the mixed population and anti-TNF-naïve populations, as shown in the cost-effectiveness acceptability curve (Figure 3). Vedolizumab was cost-effective in 89.9% of simulations in the anti-TNF-failure subgroup. In all simulations, vedolizumab was more effective than conventional therapy. Vedolizumab was also cost-saving in approximately 19%–20% of simulations.
  | Figure 3 Probabilistic sensitivity analysis results: vedolizumab versus conventional therapy. |
Discussion
We developed a model to compare vedolizumab plus conventional therapy with conventional therapy in patients with moderately-to-severely active UC in a UK population. We considered the full GEMINI ITT population (which includes anti-TNF-naïve and anti-TNF-failure patients) and the anti-TNF-naïve and anti-TNF-failure populations individually. In all analyses, vedolizumab was the most cost-effective treatment.
Our model results are aligned with the results of previous UC models.10,18 In the anti-TNF-naïve population, Tsai et al10 estimated 3.838 QALYs over 10 years for patients on conventional therapy. Similarly, the National Institute for Health and Care Excellence health technology assessment submission for infliximab estimated 3.828 QALYs for patients treated with conventional therapy.18 When using utility weights from Tsai et al,10 assuming no health state effect on mortality, and using a 10-year time horizon, our model estimated 3.830 QALYs for patients on conventional therapy. No previous models considered a mixed population or an anti-TNF-failure population, so a comparison could not be drawn for these analyses.
A recently-presented meta-analysis of real-world cohort studies of vedolizumab in UC found that 43% of patients achieved response by week 6 and 24% achieved remission.25 Our model assumed 47% response and 17% remission at 6 weeks for vedolizumab for the mixed population. Similarly, at 1 year, the meta-analysis found that 64% of vedolizumab patients achieved response at 12 months with 51% in remission, whereas our study assumed 56% response at 12 months and 42% remission. As such, our model assumptions and results align quite well with the real-world evidence for vedolizumab in UC.
This analysis has several limitations. First, we used response and remission data from the GEMINI I trial, which was a clinical trial conducted in multiple countries. It is important to note that clinical trial efficacy may be higher than what we would expect in a real-world setting where treatment compliance may be lower. However, it is unclear whether this would bias the results in any particular direction. Additionally, because the trial is a multinational trial, there may be differences in efficacy among patients in the UK as compared with other countries. The impact of this limitation is uncertain.
An additional clinical data limitation is the lack of long-term efficacy data. This data limitation makes it challenging to project costs and outcomes beyond 1 year. We took the approach of setting the maximum duration of therapy to 1 year in estimating our results. To test this assumption, we allowed responders to continue vedolizumab indefinitely assuming efficacy consistent with the maintenance phase. In this scenario analysis, we found that the ICER did not exceed £20,000/QALY in any population. However, one would expect that patients who have responded to vedolizumab for 1 year would exhibit better continued response than those over the course of the maintenance phase following induction (which included some induction responders who ultimately lost response). As such, by assuming similar efficacy to that observed in the maintenance phase, we may actually be underestimating the longer-term efficacy of vedolizumab for those who achieved and maintained response for 1 year. But even in this conservative assumption, vedolizumab remained cost-effective.
Limited data are available regarding parameter estimates for the post-surgical health states and adverse events. Transition probabilities for the post-surgical health states were estimated based on available data, however the transition among these health states is unclear. The results were not sensitive to these transition probabilities: eliminating the probability of surgery from the model (and thus eliminating entry to the post-surgical health states) resulted in an ICER of below £10,000/QALY for vedolizumab in all populations. Additionally, previous economic analyses of UC did not include adverse events, which made obtaining data for the costs and disutilities difficult. Furthermore, some of the adverse events were extremely rare, making it difficult to accurately estimate their probability based on short-term trials with relatively small patient counts. However, assuming an extreme scenario of 5% annual probability of lymphoma and tuberculosis for vedolizumab did not result in an ICER above £20,000/QALY. Conversely, zeroing out the risk of adverse events had minimal impact on the results. As such, the concerns regarding these data limitations are minimal.
Finally, we assumed patients are 100% adherent to their treatment. However, in the real world, patients on vedolizumab may skip a scheduled infusion (ie, drug holiday). The assumption of 100% treatment compliance may overstate the benefits of the more effective treatment. However, the assumption of full compliance may also be conservative with respect to vedolizumab, as we may be overestimating the costs of drug acquisition/administration.
Conclusion
Vedolizumab has shown better clinical response than conventional therapy in UC, as seen in the GEMINI I trial. The results of this analysis echo these results from an economic perspective: treatment with vedolizumab improves clinical outcomes (ie, greater QALYs, more time spent in remission and response, fewer surgeries) compared with conventional therapy for patients with moderately-to-severely active UC. When considering both costs and efficacy over a patient’s lifetime, vedolizumab was found to be a cost-effective treatment.
The results of this study also illustrate a potential benefit of vedolizumab as another alternative to surgery. Previously, failure of biologic therapy would leave patients without alternatives other than surgery for treatment of their disease. Surgery can be quite costly and result in substantial reduction in quality of life.5 As such, the introduction of a novel, effective biologic treatment with an alternative mechanism of action than anti-TNF treatments provides patients with UC with another alternative to delay or avoid unwanted surgery.
Acknowledgment
The model and manuscript development were funded by Takeda.
Disclosure
Mr Wilson is employed by RTI Health Solutions, which received funding from Takeda to conduct this research. Mr Azzabi Zouraq and Dr Chevrou-Severac were employees of Takeda Pharmaceuticals International AG at the time of the model and manuscript development. Mr Selby is employed by Takeda UK. Dr Kerrigan is an employee of PHMR Associates, which received funding from Takeda for this research. The authors report no other conflicts of interest in this work.
References
Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–1657. | ||
National Institutes of Health [homepage on the Internet]. Ulcerative colitis. NIH; 2010 [updated May 24, 2017]. Available from: https://medlineplus.gov/ulcerativecolitis.html. Accessed July 20, 2017. | ||
National Institute for Health and Care Excellence [homepage on the Internet]. Clinical Guidelines CG166: Ulcerative Colitis. NICE; 2010. Available from: https://www.nice.org.uk/guidance/cg166/chapter/introduction. Accessed July 20, 2017. | ||
Rubin DT, Dubinsky MC, Panaccione R, Siegel CA, Binion DG, Kane SV, Hopper J. The impact of ulcerative colitis on patients’ lives compared to other chronic diseases: a patient survey. Dig Dis Sci. 2010;55(4):1044–1052. | ||
Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31(7):693–707. | ||
Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters. Am J Gastroenterol. 2010;105(3):501–523. | ||
Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. | ||
Sandborn W, Sands B, Rutgeerts P, et al. Sustained therapeutic benefit of vedolizumab throughout 1 year in ulcerative colitis in GEMINI I, a randomized, placebo-controlled, double-blind, multicenter trial. J Crohns Colitis. 2013;7:S138–S139. | ||
Wilson M, Kerrigan M, Smyth M, Chevrou-Severac H, Bergman A, Selby R. Cost-effectiveness of vedolizumab compared with conventional therapy for the treatment of moderately-to-severely active ulcerative colitis in the United Kingdom. UEG Journal. 2015;3 Suppl 1:A608. | ||
Tsai HH, Punekar YS, Morris J, Fortun P. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2008;28(10):1230–1239. | ||
Punekar YS, Hawkins N. Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis. Eur J Health Econ. 2010;11(1):67–76. | ||
Wilson MR, Bergman A, Chevrou-Severac H, Selby R, Smyth M, Kerrigan MC. Cost-effectiveness of vedolizumab compared with infliximab, adalimumab, and golimumab in patients with ulcerative colitis in the United Kingdom. Eur J Health Econ. Epub 2017 Mar 8. | ||
Office for National Statistics [homepage on the Internet]. Death registrations summary tables, England and Wales 2010. ONS; 2011. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathregistrationssummarytablesenglandandwalesreferencetables. Accessed August 07, 2017. | ||
Royal College of Physicians. National clinical audit of biological therapies: UK inflammatory bowel disease (IBD) audit. Available from: https://www.rcplondon.ac.uk/projects/outputs/national-clinical-audit-report-biological-therapies-adult-report-2013. Accessed August 07, 2017. | ||
British National Formulary. No. 66. 2014 [cited 2014 Dec]. Available from: http://www.bnf.org/bnf/go?bnf/current/. | ||
Department of Health. NHS reference costs 2013 to 2014. NHS; 2013 [updated March 9, 2015]. Available from: https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014. Accessed July 20, 2017. | ||
Buchanan J, Wordsworth S, Ahmad T, et al. Managing the long term care of inflammatory bowel disease patients: the cost to European health care providers. J Crohns Colitis. 2011;5(4):301–316. | ||
Bryan S, Andronis L, Hyde C, Connock M, Fry-Smith A, Wang D. Infliximab for the treatment of acute exacerbations of ulcerative colitis. Health Technol Assess. 2010;14 Suppl 1:9–15. | ||
Personal Social Services Research Unit. Curtis L. Unit costs of health and social care 2014. PSSRU; 2014. Available from: http://www.pssru.ac.uk/archive/pdf/uc/uc2014/full-with-covers.pdf. Accessed July 20, 2017. | ||
Brown RE, Hutton J, Burrell A. Cost effectiveness of treatment options in advanced breast cancer in the UK. Pharmacoeconomics. 2001;19(11):1091–1102. | ||
Porco TC, Lewis B, Marseille E, Grinsdale J, Flood JM, Royce SE. Cost-effectiveness of tuberculosis evaluation and treatment of newly-arrived immigrants. BMC Public Health. 2006;6:157. | ||
Hornberger J, Reyes C, Lubeck D, Valente N. Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma. Leuk Lymphoma. 2008;49(2):227–236. | ||
Beusterien KM, Davies J, Leach M, Meiklejohn D, Grinspan JL, O’Toole A, Bramham-Jones S. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50. | ||
Beusterien KM, Szabo SM, Kotapati S, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer. 2009;101(3):387–389. | ||
European Crohn’s and Colitis Organisation [homepage on the Internet]. Schreiber S, Dignass A, Peyrin-Biroulet L, Hather G, Demuth D, Khalid JM, Loftus EV. Real world effectiveness of vedolizumab over one year in inflammatory bowel disease: a meta-analysis. Digestive Disease Week (DDW) 2017. Poster 1700. Available from: https://www.ecco-ibd.eu/publications/congress-abstract-s/abstracts-2017/item/p466-real-world-effectiveness-of-vedolizumab-over-one-year-in-inflammatory-bowel-disease-a-meta-analysis-2.html. Accessed August 07, 2017. |
Supplementary materials
Conventional therapy in the model includes a combination of aminosalicylates, corticosteroids, and immunomodulators. The proportion of treatments defining conventional therapy was based on expert clinical opinion.1 The estimated treatment cost of conventional therapy is based on the doses and unit costs reported in the British National Formulary.2
The treatment options, dosing assumptions, and estimated treatment mix for patients receiving the conventional therapy strategy are summarized in Table S1. The percentages sum to greater than 100% because patients may be on multiple therapies. We assume that the resource-use cost of conventional therapy for patients taking biologics is half that of the costs of the conventional therapy strategy alone. We tested this assumption in a scenario analysis in which we assumed conventional therapy costs for the biologic regimens are equivalent to those for the conventional therapy regimen.
Health state transitions
We derived the health state transition probabilities for the Mayo-score-based health states using response and remission data from the induction and maintenance phases of the GEMINI I trial. The transition probabilities were calibrated to align as closely as possible to the modeled proportion of patients in remission and mild disease at 54 weeks, with the expected proportion of patients in these states given the GEMINI I trial data. Table S2 presents the Mayo-score health state transition probabilities for each treatment and for each health state.
We estimated the health state transition probabilities for the three surgery-related health states using published data (Table S5).
  | Table S1 Doses and unit costs of conventional therapy Notes: Data from British National Formulary2 for unit costs; UK IBD Audit Steering Group1 for percentage use. |
  | Table S2 Probabilities of transition among health states: mixed population Notes: Calibrated to clinical data from Feagan et al.3 Risk of surgery from the moderate-severe health state estimated from Frolkis et al.4 Abbreviation: UC, ulcerative colitis. |
  | Table S3 Probabilities of transition among health states: naïve population Notes: Calibrated to clinical data from Feagan et al.3 Risk of surgery from the moderate-severe health state estimated from Frolkis et al.4 Abbreviation: UC, ulcerative colitis. |
  | Table S4 Probabilities of transition among health states: failure population Abbreviation: UC, ulcerative colitis. Notes: Calibrated to clinical data from the Feagan et al.3 Risk of surgery from the moderate-severe health state estimated from Frolkis et al.4 |
  | Table S5 Surgery and post-surgery health state transition probabilities Notes: aThe probability of mortality is not included in the above transition probabilities. Mortality-adjusted probabilities are derived by the formula (1 – p(morths,y)), where p(morths,y) is the mortality risk for health state (hs) in time period (y). bConverted from a 6-month risk of 0.153 from Loftus et al.5 cAssumed to be the remainder of 100% minus all other possible transitions. dEstimated based on an annual probability of 0.84 from Xie et al.6 eConverted from a monthly risk of 0.31 from Loftus et al.5 f Converted from the proportions of patients with late complications (0.457) from Loftus et al.5 |
References
Royal College of Physicians. National clinical audit of biological therapies: UK inflammatory bowel disease (IBD) audit. Available from: http://www.rcplondon.ac.uk/sites/default/files/national_clinical_audit_report_of_ biological_therapies_-_adult_report._29_august_2013.pdf.August 2013. | ||
British National Formulary. No. 66. 2014 [cited 2014 Dec]. Available from: http://www.bnf.org/bnf/go?bnf/current/. | ||
Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. | ||
Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterol. 2013;145(5):996–1006. | ||
Loftus EV Jr, Delgado DJ, Friedman HS, Sandborn WJ. Colectomy and the incidence of postsurgical complications among ulcerative colitis patients with private health insurance in the United States. Am J Gastroenterol. 2008;103(7):1737–1745. | ||
Xie F, Blackhouse G, Assasi N, Gaebel K, Robertson D, Goeree R. Costutility analysis of infliximab and adalimumab for refractory ulcerative colitis. Cost Eff Resour Alloc. 2009;7:20. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.