Back to Archived Journals » Comparative Effectiveness Research » Volume 7
Cost-effectiveness of sunitinib as second-line treatment for gastrointestinal stromal tumor in the People’s Republic of China
Authors Li J, Ren HY, Zhang J, Dong P, Wang Y, Stevens AL, Han Y, Huang M
Received 13 April 2016
Accepted for publication 21 July 2016
Published 23 January 2017 Volume 2017:7 Pages 1—9
DOI https://doi.org/10.2147/CER.S110509
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Francesco Chiappelli
Jian Li,1 Hong Ye Ren,2 Juanjuan Zhang,2 Peng Dong,2 Yan Wang,3 Andrea L Stevens,3 Yi Han,3 Min Huang4
1Laboratory of Carcinogenesis and Translational Research for the Ministry of National Education, Department of GI Oncology, Peking University School of Oncology, Beijing Cancer Hospital & Institute, 2Pfizer Inc., Beijing, People’s Republic of China; 3WG Consulting, New York, NY, USA; 4School of Pharmacy, Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China
Objective: To evaluate the cost-effectiveness of sunitinib as a second-line treatment in patients with advanced gastrointestinal stromal tumors that no longer respond to imatinib 400 mg/d, compared with imatinib 600 mg/d, 800 mg/d, or best supportive care (BSC) in the People’s Republic of China.
Methods: This study was conducted from the government payer’s perspective with a time horizon of 5 years. Three health states were considered: progression-free survival, disease progression survival, and death, with a cycle length of 6 weeks. Probabilities of disease progression and death were estimated based on survival functions using exponential distribution and progression survival data in the clinical trials. Drug costs were based on drug retail prices and the patient assistance program in the People’s Republic of China, and adverse event management costs were based on published data and/or expert opinion. Uncertainties for parameters in the study were addressed through one-way deterministic and probabilistic sensitivity analysis.
Results: When sunitinib was compared with imatinib 600 mg/d and BSC, the incremental cost-effectiveness ratio was RMB75,715 with RMB121,080 per quality-adjusted life-year (QALY) gained. Sunitinib demonstrated lower costs and higher QALYs than imatinib 800 mg/d. In the probabilistic sensitivity analysis, the willingness-to-pay per QALY gained was set to be three times the per capita gross domestic product of the People’s Republic of China, that is, RMB46,510 in 2014. Sunitinib was demonstrated to be cost-effective compared with imatinib 600 mg/d, imatinib 800 mg/d, and BSC, with probabilities of 82.3%, 95.6%, and 78.2%, respectively.
Limitations: Clinical data for imatinib 800 mg/d and BSC in the analysis were based upon studies in non-Chinese populations. Because of the unavailability of utility data from Chinese gastrointestinal stromal tumor patients, the analysis used the utility estimates from studies performed in other countries.
Conclusion: Sunitinib provides greater clinical benefit than high-dose imatinib or BSC as a second-line treatment. In the Chinese setting, sunitinib is estimated to be cost-effective compared with imatinib 800 mg/d, imatinib 600 mg/d, or BSC.
Keywords: cost-effectiveness analysis, gastrointestinal stromal tumor, imatinib, sunitinib, best supportive care, second-line
Corrigendum for this paper has been published
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common nonepithelial tumors of the gastrointestinal tract. They are typically defined as tumors whose behavior is driven by mutations in the Kit gene or PDGF RA gene, and may or may not stain positively for Kit.1 Traditionally, GISTs are treated by surgical resection, but recurrence and metastases are common, with prognosis influenced by mitotic rate, tumor size, and anatomical site.2 With the development of new drugs such as imatinib and sunitinib, the prognosis for patients with GISTs has improved3 significantly.4
Imatinib and sunitinib are the main drugs available on the Chinese market for the treatment of GISTs. Imatinib mesylate, a selective inhibitor of the kinase activities of KIT and platelet-derived growth factor receptor (PDGFR), has been demonstrated as an effective treatment for advanced GIST. However, some patients exhibit primary resistance to imatinib, and many patients develop acquired resistance during the course of treatment.5
In the past few years, sunitinib has been found to be effective in treating patients with GISTs who have failed treatment by imatinib.6 Sunitinib is a multitargeted receptor tyrosine kinase inhibitor that has antiangiogenic and antitumor effects. It works by inhibiting cellular signaling of multiple targets, including the PDGFRs, the vascular endothelial growth factor receptors (VEGFRs), stem cell growth receptor (c-Kit), FMS-like tyrosine kinase 3 (FLTs), and neurotropic factor receptor.7 Sunitinib belongs to a different chemical class than that of imatinib and has different binding characteristics and affinities. Sunitinib is more potent than imatinib in controlling tumor-related angiogenesis because it inhibits the VEGFR kinases, while imatinib does not.6
Sunitinib was first introduced as second-line treatment for GIST patients who were intolerant to, or progressed after, imatinib.6 The efficacy and acceptable safety of sunitinib as a second-line treatment for GISTs have been documented by previous studies.7,8 The cost-effectiveness of sunitinib is now the critical issue for defining clinical treatment guidelines and reimbursement policy and the one that most concerns doctors and the authorities.9 Sunitinib has been demonstrated as cost-effective in a number of different settings; according to a review by Blanke and Muse,9 sunitinib was cost-effective in treating patients with advanced GISTs who were intolerant/resistant to imatinib in countries such as Spain and Mexico. In a Spanish setting, the incremental cost-effectiveness ratio (ICER) of sunitinib compared to best supportive care (BSC) was €49,090/quality-adjusted life-year (QALY) gained.10 In a study in Mexico, sunitinib was cost-saving, with longer progression-free survival (PFS) time compared to high-dose imatinib as a second-line treatment.11
Currently, there are no published economic studies on treatments for GISTs among Chinese patients. Given the different socioeconomic conditions across countries, economic findings from other countries may not be applicable to the Chinese setting. This study aimed to evaluate the cost-effectiveness of sunitinib as a second-line treatment in patients with advanced GISTs that no longer responded to imatinib 400 mg/d treatment by considering clinical and economic factors specific to the People’s Republic of China.
Methods
Three health states were considered for patients who were assumed to take imatinib or sunitinib: PFS, disease progression survival (DPS), and death (Figure 1). Patients were assumed to stay on second-line treatment until tumor progression was confirmed by the physician. After confirmation of progression, patients would be switched to third-line treatment or BSC. In this model, patients with disease progression will be treated with BSC. Two health states were considered for patients who were assumed not to receive imatinib or sunitinib, that is, receive BSC as the second-line treatment: PFS and death (Figure 1). Patients were assumed to move to the death state if their condition progressed under BSC treatment. The probabilities of disease progression and death changes at different times were estimated based on survival functions with exponential distribution and median PFS and median overall survival (OS) data reported in clinical trial studies.
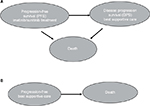  | Figure 1 Disease states for different treatment options. Notes: (A) Disease states for imatinib or sunitinib treatment. (B) Disease states for BSC treatment. Abbreviation: BSC, best supportive care. |
The analysis was conducted from the government payer’s perspective. The time horizon of the analysis was the advanced GIST patient’s lifetime with a maximum of 5 years, and the cycle length equals to 6 weeks. Advanced GIST patients who no longer responded to imatinib 400 mg/d treatment enter into the starting health state of the Markov model. Costs and outcomes were estimated by modeling the transition of patients between health states over time. The different second-line treatment options compared in the analysis were sunitinib 50 mg/d (4 weeks on and 2 weeks off), imatinib 600 mg/d, imatinib 800 mg/d, and BSC in the People’s Republic of China. In the People’s Republic of China, for the BSC practices, it is normally recommended to continue to use previous effective drugs as the supportive care because the main targeted therapies (ie, imatinib and sunitinib) all have patient assistant programs. In addition to the target therapies, the supportive care also includes symptomatic treatment, for example, pain management, nutrition therapy, pleural fluid drainage, and ascites drainage.
Clinical inputs
Clinical data were obtained from four studies (Table 1). Li et al12 reported the efficacy of oral imatinib 600 mg/d as second-line treatment among Chinese GIST patients. Data on the efficacy of imatinib 800 mg/d among Chinese patients were not available, and a study conducted in North American patients by Blanke et al9 was used as the alternative source. Data on the efficacy of sunitinib were obtained from the Phase IV trial conducted by Pfizer Inc. in the People’s Republic of China.13 In the study, patients were given oral sunitinib 50 mg/d for 4 weeks followed by 2 weeks off-treatment in 6-week cycles. Data on the efficacy of BSC as a second-line treatment were based on a Phase III trial by Demetric et al,7 and the efficacy of BSC is assumed to be equal to the efficacy of placebo reported in this study.
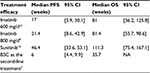  | Table 1 Treatment efficacy Abbreviations: PFS, progression-free survival; OS, overall survival; BSC, best supportive care; NA, not available; CI, confidence interval. |
The model included the following severe adverse events (AEs) (ie, with grade ≥3) related to treatment: anemia (and other hematological serious AEs) (with incidence rates of 3.8% for imatinib 600 mg/d, 14.0% for imatinib 800 mg/d, 18.7% for sunitinib and 3.0% for BSC as the second-line treatment), hypertension (with incidence rate of 3.4% for sunitinib), diarrhea, vomiting, nausea (with incidence rates of 1.9% for imatinib 600 mg/d, 16.0% for imatinib 800 mg/d, 1.7% for sunitinib and 2.0% for BSC as the second-line treatment), rash (with incidence rate of 3.8% for imatinib 600 mg/d), mucosal inflammation (with incidence rate of 1.7% for sunitinib), fatigue (with incidence rates of 1.9% for imatinib 600 mg/d, 5.1% for sunitinib and 2.0% for BSC as the second-line treatment), and hand-foot syndrome (with incidence rate of 10.2% for sunitinib). These AEs have been most commonly reported in the clinical studies.7,9,10,12 The incidence rate of AE per cycle is assumed to be constant within the overall study period.
Cost and utility
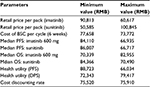
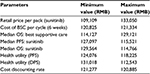
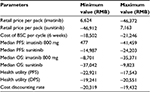
Costs were calculated for each cycle in the model for imatinib, sunitinib, and BSC, respectively. Costs were expressed in 2015 Chinese RMB. Retail prices of imatinib and sunitinib were RMB25,500 per pack/12,000 mg for imatinib and RMB13,100 per pack/350 mg for sunitinib. The actual drug cost for the course of treatment was estimated based on patient assistance programs in the People’s Republic of China for sunitinib and imatinib. For imatinib, each year, patients will pay out-of-pocket for months 1–3 and receive the drug for free for months 4–12,14 and for sunitinib, patients pay out-of-pocket for the first eight packs (ie, 350 mg/pack), and if the drug proves to be effective, patients will receive the remaining drug for free until the end of their treatment course.15 In the analysis, it is assumed that all patients will participate in the Patient Assistance Program programs, and the government payer will cover out-of-pocket drug costs and treatment-related AE costs for the patients.
For the state of PFS under imatinib or sunitinib treatment, costs of drugs and AE treatment were included. The treatment cost per incidence for hand–foot syndrome was based on expert opinion and estimation, including the cost of outpatient visits and medication; the treatment cost per incidence of hypertension was also based on expert opinion with an estimation of three hospital days; and the treatment cost per incidence of fatigue was the cost of one outpatient visit based on expert opinion. Other AE treatment costs were estimated based on the data reported in the cost-effectiveness study for lung cancer treatment in the People’s Republic of China.16 These costs are listed in Table 2. The cost of BSC in the People’s Republic of China mainly depends on patients’ willingness to get treatment, and there is no estimation available in the literature review. For the two health states under BSC (ie, disease progressed after treated with imatinib or sunitinib or BSC as the second-line treatment), costs of BSC may include acute care, long-term care, hospice stay, medical staff visits, laboratory tests, and radiological tests and were estimated as one average cost based on the palliative care cost reported in the study.10 All costs are adjusted to price level January 2015 RMB.
  | Table 2 Treatment cost for severe AEs Note: Data from Wu et al.16 Abbreviations: AE, adverse event; SAE, serious adverse event. |
The utility of each health state measures the quality of life corresponding to that state on a scale of 0–1, where 0 indicates death and 1 indicates perfect health. To model the change in the quality of life over the course of treatment, two utility values were used: the utility associated with the state of PFS and the utility associated with the state of disease progression. The utility values used in the base-case analysis were retrieved from the study by Paz-Ares et al17 (Table 3).
  | Table 3 Utility estimates of the health states Abbreviations: PFS, progression-free survival; DPS, disease progression survival. |
A deterministic model was run with the base-case input values. A 3.5% annual discount rate after the first year was applied to both costs and outcomes (ie, progression life-year [LY], LY, and QALY). The ICERs between treatments with sunitinib versus other treatment options were calculated.
Sensitivity analysis
One-way sensitivity analysis and probabilistic sensitivity analysis (PSA) were performed. The one-way sensitivity analysis was conducted with ±10% modifications of the base-case values for variables in the model. Variables in the one-way sensitivity analysis uded treatment costs (imatinib, sunitinib, and BSC), median PFS/OS of all treatment options, health utility of PFS/DPS, and the cost discounting rate. The PSA was performed on the basis of 1,000 simulations, sampling values from predefined distributions for parameters with reported standard errors in the analysis. Gamma distribution was used for median PFS/OS of the three treatment options (ie, imatinib 600 mg/d, imatinib 800 mg/d, and sunitinib). Other variables with uncertainties that can impact the analysis results were excluded in the PSA as standard errors of these variables were not reported in the corresponding studies. Results of PSA were presented as ICER planes. Microsoft Office Excel 2014 was used for modeling and analysis.
Results
Base-case results
The results from the base-case analyses were presented in Table 3. Treatment with sunitinib versus imatinib 600 mg/d resulted in 0.744 progression-free LY gained, 0.422 LY gained, and 0.398 QALYs gained at an incremental cost of RMB30,165. The ICER was RMB75,715 per QALY gained. Treatment with sunitinib was dominant compared with imatinib 800 mg, with lower costs and higher QALYs. Treatment with sunitinib versus BSC resulted in patient benefits of 0.257 progression-free LY gained, 1.357 LY gained, and 0.836 QALYs gained at an incremental cost of RMB101,268. The ICER was RMB121,080 per QALY gained (Tables 4 and 5).
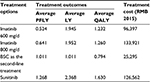  | Table 4 Treatment outcomes and costs of all treatment options Abbreviations: PFLY, progression-free life-year; LY, life-year; QALY, quality-adjusted life-year; BSC, best supportive care. |
One-way sensitivity analysis
Input values for one-way sensitivity analysis are presented in Table 6. The results of the one-way sensitivity analyses of ICER per QALY gained comparing sunitinib versus imatinib 600 mg/d, imatinib 800 mg/d, and BSC are presented in Tables 7–9 with the tornado diagrams in Figures 2–4, respectively.
The change of ICER per QALY gained comparing sunitinib versus imatinib 600 mg from the base-case ICER level is also shown in Figure 2.
The change of ICER per QALY gained comparing sunitinib versus BSC is shown in Figure 3.
The change of ICER per QALY gained comparing sunitinib versus imatinib 800 mg/d is shown in Figure 4.
Probabilistic sensitivity analysis
The ICER planes (ie, incremental cost vs incremental QALY) based on 1,000 simulations are shown in Figure 5 for 1) sunitinib versus imatinib 600 mg/, 2) sunitinib versus imatinib 800 mg/d, and 3) sunitinib versus BSC. In each ICER plane, x-axis is the incremental QALY gained using sunitinib compared with the comparator treatment and the y-axis is the incremental cost using sunitinib compared with the comparator treatment. The willingness-to-pay (WTP) value per QALY gained is set to be three times the gross domestic product per capita, with the per capita gross domestic product of the People’s Republic of China, based on recommendation by the Commission on Macroeconomics and Health of the World Health Organization.18 The per capita gross domestic product of the People’s Republic of China was approximately RMB46,510 in 2014,19 and based on the WTP, sunitinib is cost-effective versus imatinib 600 mg with the probability of 82.3%, versus imatinib with the probability of 95.6%, and versus BSC with the probability of 78.2%.
Discussion
Traditionally, patients rely on BSC as second-line treatment for advanced GISTs. Technology development has made new therapeutics available.18 GISTs are expensive to treat, and the unit cost of high-dose imatinib or sunitinib tends to be high.11
This study compared the costs and effectiveness of sunitinib, high-dose imatinib, and BSC.
Among patients with advanced GISTs who failed imatinib 400 mg/d as the first-line treatment, we estimated the ICER of sunitinib treatment to be RMB75,715/QALY gained and RMB121,080/QALY gained compared with imatinib 600 mg and BSC, respectively. Sunitinib was dominant compared with imatinib 800 mg. While these estimates can be affected by variations in input values, particularly the median PFS of sunitinib, the range of ICER comparing sunitinib with imatinib 600 mg/d estimated from the one-way sensitivity analysis did not exceed the societal WTP threshold recommended by the Commission on Macroeconomics and Health of the World Health Organization.19 The Commission recommended the use of three times gross domestic product per capita as the WTP threshold, and the per capita gross domestic product of the People’s Republic of China was approximately RMB46,510 in 2014.20
The ICER of sunitinib compared to BSC calculated in this study is lower than the €49,090/QALY gained found in a study by Paz-Ares et al.10 Paz-Ares et al also found the efficacy and the unit cost of sunitinib to be the most influential variables for the results, which is consistent with this study (Figure 2). Contreras-Hernández et al11 studied the cost-effectiveness of sunitinib compared with imatinib 800 mg/d and BSC in Mexico. The ICER of sunitinib versus BSC was $46,108.89 per LY gained, and the overall direction is consistent with findings in this study. Contreras-Hernández et al11 also found that sunitinib was cost-saving over high-dose imatinib as second-line treatment and delivered greater survival benefits to patients, which is consistent with this study. It should be noted that their study included the cost of BSC in the drug therapy arms, while this study did not.
This study is subject to several limitations. First, there are limitations of the data and assumptions used to estimate the range for the variables used in the modeling. The clinical data for imatinib 800 mg/d and BSC are based on studies in non-Chinese populations. The cost data for some AEs are largely based on expert opinion given the lack of published data. Second, because of the lack of genotype prevalence data in the People’s Republic of China, this study did not assess the impact of response rate for patient cohorts with different genotypes. Another study limitation was the unavailability of utility data from Chinese GIST patients and hence the use of utility estimates from studies in other countries. The sensitivity analyses found that uncertainty in clinical data (particularly median PFS of sunitinib treatment) had a greater influence on the results than did the uncertainty in utility values. In this study, we showed that sunitinib was cost-effective compared with other treatment options based on the criterion recommended by the Commission on Macroeconomics and Health of the World Health Organization. The actual WTP threshold adopted by a specific decision maker based on local socioeconomic conditions may determine the best treatment option for particular patients.
Third-party payers in different countries have chosen to pay for some new oncology drugs even though the ICERs are higher than their established efficiency threshold.10 Findings from this study support the consideration of sunitinib as cost-effective or cost-saving with greater survival benefits alternative for the second-line treatment of GISTs in Chinese patients.
Conclusion
Among patients with advanced GISTs who have failed imatinib 400 mg/d as the first-line treatment, sunitinib provides greater clinical benefit than high-dose imatinib or BSC. In the Chinese setting, sunitinib is estimated to be cost-effective compared with imatinib 800 mg/d, imatinib 600 mg/d, or BSC.
Acknowledgments
The abstract of this study was presented at the ISPOR 18th Annual European Congress as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Value In Health 2015 November issue.21
Disclosure
Yan Wang, Andrea L Stevens and Yi Han are employees of WG Consulting, which received funding for this study from Pfizer; Hong Ye Ren and Peng Dong are employees of Pfizer China, which funded this study. The authors have no other conflict of interest.
References
Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466–1478. | ||
Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33(5):478–483. | ||
Conlon KC, Casper ES, Brennan MF. Primary gastrointestinal sarcomas: analysis of prognostic variables. Ann Surg Oncol. 1995;2(1):26–31. | ||
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–58. | ||
Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480. | ||
Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann Oncol. 2007;18(suppl 10):x3–x10. | ||
Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–1338. | ||
George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45(11):1959–1968. | ||
Blanke CD, Huse DM. Cost effectiveness of tyrosine kinase inhibitor therapy in metastatic gastrointestinal stromal tumors. J Med Econ. 2010;13(4):681–690. | ||
Paz-Ares L, García del Muro X, Grande E, González P, Brosa M, Díaz S. Cost-effectiveness analysis of sunitinib in patients with metastatic and/or unresectable gastrointestinal stroma tumours (GIST) after progression or intolerance with imatinib. Clin Transl Oncol. 2008;10(12):831–839. | ||
Contreras-Hernández I, Mould-Quevedo JF, Silva A, et al. A pharmaco-economic analysis of second-line treatment with imatinib or sunitinib in patients with advanced gastrointestinal stromal tumours. Br J Cancer. 2008;98(11):1762–1768. | ||
Li J, Gong JF, Li J, Gao J, Sun NP, Shen L. Efficacy of imatinib dose escalation in Chinese gastrointestinal stromal tumor patients. World J Gastroenterol. 2012;18(7):698–703. | ||
Shen L, Qin SK, Sun Y, et al. Sunitinib in Chinese patients with advanced gastrointestinal stromal tumor (GIST). J Clin Oncol. 2015;33(suppl):abstre15208. | ||
Glivec International Patient Assistance Program [webpage on the Internet]. China Charity Federation Glivec Patient Assistance Programs Introduced. Available from: https://www.gipap.org.cn/Tipap-Gipap_Mgmts/system/gfiledownload.jsp. Accessed April 15, 2015. | ||
Cancer Foundation of China. Introduction to the Patients Assistant Program for Sutent. Available from: http://www.cfchina.org.cn/show.php?contentid=74. Accessed April 15, 2015. | ||
Wu B, Chen H, Shen J, Ye M. Cost-effectiveness of adding rh-endostatin to first-line chemotherapy in patients with advanced non-small-cell lung cancer in China. Clin Ther. 2011;33(10):1446–1455. | ||
Paz-Ares L, del Muro JG, Grande E, Díaz S. A cost-effectiveness analysis of sunitinib in patients with metastatic renal cell carcinoma intolerant to or experiencing disease progression on immunotherapy: perspective of the Spanish National Health System. J Clin Pharm Ther. 2010;35(4):429–438. | ||
Tourneau CL, Raymond E, Faivre S. Sunitinib: a novel tyrosine kinase inhibitor. A brief review of its therapeutic potential in the treatment of renal carcinoma and gastrointestinal stromal tumors (GIST). Ther Clin Risk Manag. 2007;3(2):341–348. | ||
WHO. Commission on Macroeconomics and Health. Macroeconomics and Health: Investing in Health for Economic Development. Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001. | ||
Li Q [webpage on the Internet]. National Bureau of Statistics Released Information on the Gross Domestic Production of China. Available from: http://politics.people.com.cn/n/2015/0226/c70731-26599998.html. Accessed April 15, 2015. | ||
Ren H, Zhang J, Dong P. Cost-effectiveness of sunitinib as second-line treatment for gastrointestinal stromal tumor (Gist) In China. Value Health. 2015;(7):A455. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.