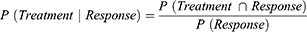Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
Cost-Effectiveness of Repository Corticotropin Injection for the Treatment of Acute Exacerbations in Multiple Sclerosis
Authors Hunter SF , Bindra J, Chopra I, Niewoehner J, Panaccio MP, Wan GJ
Received 22 July 2021
Accepted for publication 22 September 2021
Published 11 October 2021 Volume 2021:13 Pages 883—892
DOI https://doi.org/10.2147/CEOR.S330118
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Samuel F Hunter,1 Jas Bindra,2 Ishveen Chopra,3 John Niewoehner,4 Mary P Panaccio,4 George J Wan4
1Advanced Neurosciences Institute, Franklin, TN, USA; 2Falcon Research Group, North Potomac, MD, USA; 3Manticore Consultancy, Bethesda, MD, USA; 4Mallinckrodt Pharmaceuticals, Hampton, NJ, USA
Correspondence: George J Wan
Mallinckrodt Pharmaceuticals, 53 I-78 Frontage Road, Hampton, NJ, 08827, USA
Email [email protected]
Background: Relapses are common among patients with multiple sclerosis (MS) despite treatment with disease-modifying therapies. Repository corticotropin injection (RCI, Acthar® Gel), plasmapheresis (PMP), and intravenous immunoglobulin (IVIg) are alternative therapies for MS relapse. There is a dearth of economic assessments of these therapies for the acute exacerbations of MS. This study estimated the cost-effectiveness of RCI compared to PMP or IVIg.
Methods: A Markov state-transition model compared outcomes (costs, relapses, remission, and utilities) with RCI versus PMP or IVIg for the acute exacerbations in MS. The model was developed from the United States (US) payer and societal perspectives over one to three years. Patients initiated on alternative therapies were evaluated in one-day increments for the first 30 days during treatment. The model assumes the natural history of MS after treatment in the first month, adjusting for the effect of treatment. Incremental cost-effectiveness ratios (ICERs) were estimated as cost per quality-adjusted life-year (QALY) gained. The uncertainty in model parameters was evaluated in probabilistic sensitivity analyses.
Results: In the base case, RCI has an ICER of USD 42,078 per QALY compared to PMP over one year from the payer perspective and is dominant over two and three years; RCI is dominant compared to PMP from the societal perspective over all three years. Compared to IVIg, RCI is a dominant strategy from both payer and societal perspectives over all three years. Probabilistic sensitivity analysis supports the base case findings, suggesting that RCI may be cost-effective versus PMP and IVIg for acute exacerbations in MS.
Conclusion: RCI is a cost-effective alternative treatment for MS relapses compared to PMP and IVIg from the US payer and societal perspectives.
Keywords: Acthar® Gel, cost-effectiveness analysis, acute exacerbation, multiple sclerosis, quality-adjusted life-year, repository corticotropin injection
Introduction
Multiple sclerosis (MS) is a chronic autoimmune and inflammatory disease of the central nervous system. MS is characterized by episodes of reversible relapses.1 There is variability in the clinical course and severity of symptoms in MS, with the most common symptoms being bladder or bowel dysfunction, blurred vision, cognitive impairment, impaired balance, numbness and tingling, fatigue, vertigo, and weakness.2,3 It is estimated that approximately 400,000 adults in the United States (US) may have MS; however, the epidemiology of MS may be underestimated.4 Relapses are common among patients with MS despite treatment with disease-modifying therapies; with 55.7% of the patients having at least one relapse (based on annualized relapse rate).5 Studies have shown that about one-fifth of patients may experience more than two relapses per year.6 Continued relapses result in considerable functional impairment and have a negative impact on the patient’s quality of life.7
Corticosteroids are recommended as first-line agents for managing MS relapses.2 Patients who fail corticosteroid therapy may be susceptible to worsening disease. These patients may require hospitalization and/or rehabilitation for disease management. Further, for some patients, high-dose corticosteroids may be contraindicated. Following corticosteroid failure, these patients transition to alternative therapies to alleviate acute exacerbations. Alternative therapies to address exacerbations among patients who fail oral corticosteroids comprise repository corticotropin injection (RCI, Acthar® Gel), plasmapheresis (PMP), and intravenous immunoglobulin (IVIg).8 The American Academy of Neurology recommends PMP as a second-line treatment for steroid-resistant exacerbations in relapsing forms of MS.9,10 Although PMP is a conventional treatment approach, the potential effectiveness of plasma exchange in the treatment of MS remains unclear.11 IVIg is a pooled preparation of normal immunoglobulin G derived from the plasma of healthy donors. IVIg is occasionally used as an off-label treatment for patients non-responsive to corticosteroids.12,13 Limited clinical trial experience, small sample size, heterogeneity among study subjects, and variability in the dose provide little evidence to support the efficacy of IVIg in the treatment of relapses.14
RCI is a naturally sourced complex mixture of adrenocorticotropic hormone analogs and other pituitary peptides.15 RCI is the only non-corticosteroid therapy indicated for the treatment of acute exacerbations of MS in adults by the US Food and Drug Administration.15 The unique mechanism of action of RCI is distinct from glucocorticoids. Therefore, RCI may have a different adverse event profile than glucocorticoids, ensuing in the removal of language referring to the similarity of adverse events of RCI and glucocorticoids.15 The efficacy and safety of RCI in acute exacerbations in MS has been demonstrated in controlled clinical trials.7,16 Further, clinically meaningful improvements in MS Impact Scale 29 item version 1 (MSIS-29v1) physical subscale were observed in a prospective observational study of patients with treatment-refractory MS on RCI.17 Statistically significant improvements in clinician-rated scales (Expanded Disability Status Scale [EDSS] and Clinical Global Impression of Improvement [CGI-I]) were also observed.17 A retrospective claims analysis comparing RCI and alternative therapies supported the effectiveness of RCI in reducing relapses in MS.8,18 Further, a recent literature review corroborates the efficacy of RCI in MS.16 Initial economic analyses indicated that RCI was cost-saving compared to PMP or IVIg in resolving MS relapses from a payer perspective.19,20 Considering the societal burden of MS relapses,21 it is important to evaluate these treatments considering this perspective to optimize patient care. Further, there is a dearth of economic assessments on the cost-effectiveness of RCI, PMP, and IVIg for the acute exacerbations in MS. Cost-effectiveness analyses serve as a foundation to integrate clinical, real-world, economic, and humanistic evidence to support decision-making. This study estimated the cost-effectiveness of RCI for the treatment of acute exacerbations in MS compared to alternative therapies (PMP and IVIg) from the US payer and societal perspectives.
Methods
The analysis was conducted consistent with the International Society for Pharmacoeconomics and Outcomes Research Good Practice Guidelines for cost-effectiveness analysis22 and recommendations on cost-effectiveness in health and medicine.23
Model Overview
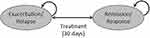
The model is a probabilistic state-transition model that evaluates the economic and quality-of-life outcomes of treating patients with MS. The model comprises of two states—relapse/exacerbation and remission/response—mirroring those in the life of a patient with MS (Figure 1). The model included only those patients with active relapses. Each health state is further based on the EDSS state (EDSS 0–8+) in one-point increments. The EDSS classification is widely used in clinical trials to define MS progression.24 Patients are allowed to move between exacerbation or response/remission state within an EDSS state, but cannot move among EDSS states. The transition of patients in the model is consistent with the treatment recommendations;25 RCI, PMP, and IVIg are used for the treatment of acute exacerbations in MS and not for slowing disease progression, measured using EDSS. Therefore, we did not consider the movement of patients across EDSS states. EDSS states were included to account for variability in clinical and pharmacoeconomic outcomes among patients with varying disease severity. The model was developed from the US payer and societal perspectives over three years in Microsoft® Excel 2019 (Redmond, Washington, US).
Initially, all patients start in the exacerbation state across nine EDSS states. A natural history matrix is applied to the probability of treatment success with RCI, PMP, or IVIg to derive a treatment-specific transition from exacerbation to remission in the first 30 days (on-treatment). The 30-day criterion was selected based on a published claims-based methodology.26 This methodology comprises an inpatient or outpatient claim with a diagnosis of MS followed by receipt of a relapse treatment or procedure (RCI, PMP, or IVIg).27 Patients accrue in the exacerbation or response state for 30 days in daily increments. Following the on-treatment period, the natural history of relapsing-remitting MS is used to estimate the number of patients in each health state over three years with a cycle length of one month. Treatments are administered consistent with the current recommendations and clinical practices.15,27
Model Inputs
Clinical Probabilities
The EDSS provides a method to quantify MS severity by monitoring the level of disability in patients over time. Higher EDSS score indicates an increasing level of disease severity and worsening health outcomes.28,29 To simplify the assessment of outcomes and mitigate uncertainty in the model, the model categorized disease severity based on EDSS states; patients with EDSS ≤4.0 were considered having a mild/moderate disease, while those with EDSS >4.0 were considered having severe disease consistent with current scaling.30 Baseline patient distribution by EDSS state and relapse severity were derived from the MS Relapse Registry, titled “Observational Registry of Acthar Gel for Participants with Multiple Sclerosis Relapse,” funded by Mallinckrodt Pharmaceuticals (N = 125; Table 1).17,31 The study was conducted to monitor the course of MS and to document relapses and the recovery process, in a group of MS patients treated with RCI.
 |
Table 1 Baseline Patient Distributions by EDSS State and Relapse Severity |
During the on-treatment period, the treatment-related probability of relapse resolution in the first 30 days was used. The probabilities were sourced from a retrospective study utilizing administrative claims data between January 01, 2008 and June 30, 2015. The monthly probabilities were converted to daily probabilities as appropriate. The study evaluated the treatment effectiveness of RCI, PMP, and IVIg in patients 18–89 years of age who experienced an MS relapse. The proportion of patients achieving relapse resolution with RCI, PMP, and IVIg was 96.9%, 50.7%, and 43.9%, respectively.8 The response state was considered the absorbing state during the on-treatment period; it was assumed that patients achieving a response remain in that state for the duration of the on-treatment period.
During the off-treatment period, the probability of relapse was estimated as a function of baseline treatment and EDSS state (Table 2). The probability of response was derived from a retrospective study using the mean number of relapse episodes in the 12-month follow-up period after administration of RCI or PMP/IVIg.8
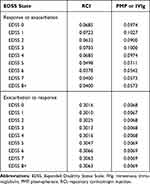 |
Table 2 Adjusted Monthly Transition Probabilities During the Off-Treatment Period |
The transition probability of exacerbation to response/remission state during the off-treatment period was similarly based on baseline treatment (Table 2) and EDSS state and was derived as follows:
Adverse Events
For each treatment, only serious adverse events (SAEs) were applied in the model since SAEs account for the greatest economic impact on the healthcare system and patients. The probabilities of SAEs for RCI were sourced from the MS Relapse Registry and the published literature for PMP and IVIg. The SAE rates are presented in Table 3.
 |
Table 3 SAEs for Treatments |
Costs
The model included treatment costs (drug and administration costs), cost of adverse events, direct medical, direct non-medical, and indirect cost of relapse, and the base cost of relapse by EDSS score (direct and indirect costs). The model assumed that all patients incur the cost of conventional therapy after the first cycle (off-treatment period).
Wholesale acquisition costs were used for the cost of drug acquisition. The administration, dose strength, and dosing were obtained from the real-world use and prescribing information.27,32 Administration costs were applied to PMP and IVIg but not RCI since RCI is a self-administered treatment. Treatment with PMP assumed an average dose of 40 mL/kg (patient weight of 79.1 kg)33 and six sessions;27 treatment with IVIg assumed an average dose of 400 mg/kg and five sessions followed by maintenance therapy every four weeks.27,34 Drug acquisition and administration costs are presented in Table 4. Direct and indirect costs of exacerbation/relapse and remission/response were categorized by disease severity (mild/moderate and severe MS). Productivity loss and absenteeism costs were estimated using the human capital approach.35 Finally, the cost of relapse of the baseline MS by EDSS state was added to the overall costs.36 All costs were discounted at 3.0% annually and reported in 2021 United States Dollars (USD).
 |
Table 4 Economic Input Parameters |
Health Utilities
The health utility of MS during exacerbation/relapse and remission/response by EDSS state was sourced from the published literature (Table 5).28 The utility decrement due to relapse was based on the disease severity—0.091 and 0.302 for mild/moderate and severe MS relapse, respectively.21 All health utilities were discounted at 3.0% annually.
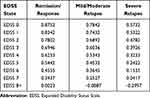 |
Table 5 Health Utilities of Remission/Response and Exacerbation/Relapse for Baseline MS |
Sensitivity Analyses
Probabilistic sensitivity analyses (PSA) were performed to account for the uncertainty around model parameters and to test the robustness of the model using appropriate distributions.
Results
Base Case Analysis
Considering the payer perspective over one year, RCI results in an incremental quality-adjusted life-years (QALY) gain of 0.115 at an additional average cost of $4839, which translates to an incremental cost-effectiveness ratio (ICER) of $42,078 per QALY compared to PMP (Table 6). However, RCI is a dominant treatment strategy from the payer perspective over two and three years with an average cost savings of $6827 to $18,551 and an average QALY gain of 0.241 to 0.368 compared to PMP. RCI is a dominant treatment strategy compared to IVIg from the payer perspective with an average cost savings of $79,867, $177,939, and $275,954 over year 1, year 2, and year 3, respectively. Considering the societal perspective, RCI is a dominant treatment strategy over all three years compared to both PMP and IVIg. A detailed breakdown of costs is provided in Table 7.
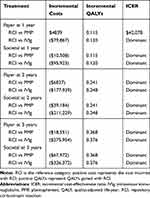 |
Table 6 Cost-Effectiveness of RCI Compared to PMP or IVIg |
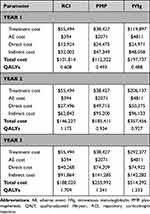 |
Table 7 Breakdown of Costs |
Sensitivity Analyses
The PSA randomly sampled the selected distributions during each of the 1,000 iterations (Figure 2). RCI was cost-effective compared to PMP or IVIg, consistent with the base case findings. RCI was cost-effective at a willingness-to-pay (WTP) threshold of $100,000. Rebates and drug price discounts may further enhance the cost-effectiveness of RCI.
Discussion
Relapses are common among patients with MS, which may result in a high economic burden on the patients and adversely impact the health-related quality of life and functional ability of the patients.37 Corticosteroids are recommended as the first-line agents for managing MS relapses.2 Patients who fail corticosteroid therapy may be at a high risk of worsening disease, requiring hospitalization and/or rehabilitation for disease management. These patients may be eligible to receive alternative therapy; however, there is no definitive guidance in the US for the use of these therapies. RCI is the only non-corticosteroid therapy indicated for the treatment of acute exacerbations of MS in adults approved by the US Food and Drug Administration.15 PMP is recommended by the American Academy of Neurology,9,10 whereas IVIg is an off-label treatment for MS relapses, with limited supporting evidence.12,13 There is a lack of data regarding the costs and benefits of RCI in clinical practice in the US. This is the first study to evaluate the cost-effectiveness of RCI versus PMP and IVIg. Cost-effectiveness analyses are important tools to compare different healthcare interventions and aid in decision-making.
Our analyses suggest that RCI is a cost-effective treatment strategy compared to PMP and IVIg. Considering the payer perspective over one year, the use of RCI is cost-effective (ICER: $42,078 per QALY) compared to PMP at the commonly used WTP threshold of $100,000 to $150,000 per QALY. RCI is dominant compared to PMP over two and three years. The findings suggest that the initial cost of RCI is offset by long-term cost savings and gain in QALYs. RCI is a dominant treatment strategy over IVIg due to the high cost of IVIg treatment and limited effectiveness in treating MS relapses. These results are consistent with previous economic evaluations where RCI was cost-saving compared to PMP or IVIg.19,20 However, these prior economic evaluations only focused on the direct cost of treatment and did not consider the clinical characteristics of patients with MS relapse.
RCI is an effective and well-tolerated treatment that expedites the resolution of acute exacerbations in MS.7,8,16,18 Further, RCI use is convenient for patients as it can be self-administered.15 In contrast, both PMP and IVIg are administered in the hospital setting for acutely limited patients and are more intensive treatments. Collectively, this clinical and economic evidence demonstrates the health economic value of RCI over PMP and IVIg. Treatment costs are central to concerns of access and affordability; however, it is important to consider the clinical, economic, and humanistic value of treatments. Value is defined as the potential benefits of interventions for their costs38 and is measured using cost-effectiveness metrics. Interventions that offer substantial benefits are recognized as those with high “Care Value” (ICER ranging from $100,000 to $150,000 per QALY).39,40 This ICER threshold of 3 times nation’s per capita income is based on the recommendations of the World Health Organization’s Choosing Interventions that are Cost Effective (WHO-CHOICE).41
Despite recent advances in treatment, patients with MS experience functional disability, which affects their daily activities and work productivity.21,42 These patients may require ongoing care and support that adds to the overall economic and patient burden.21,43 In a comprehensive cost-effectiveness analysis, costs should include healthcare costs downstream of the intervention and indirect costs due to productivity loss and caregiver burden, in addition to the direct costs of the intervention.44 The lost productivity is notable for patients with MS, who have to often leave the workforce as a result of their disability.45 The current analysis includes costs accrued due to increased disability, caregiver costs, and cost due to lost productivity of patients in addition to medical and pharmacy costs. The findings indicate that RCI is a dominant treatment strategy compared to both PMP and IVIg over all three years from the societal perspective.
Besides the model assumptions, this analysis has some limitations. First, the cost of additional treatment for patients who experience relapse was not included, which may enhance the cost-effectiveness of RCI. Second, the transition of patients between EDSS states was not considered since the treatments are prescribed for acute exacerbations in MS and due to the short duration of treatment. Finally, while wholesale acquisition cost is appropriate for health economic models, price negotiations and other factors (eg, discounts, rebates, and value-based arrangements) may alter the actual cost to the US payers. Some limitations are inherent to the model. The cost and utility estimates were obtained from various literature sources, which may add uncertainty and heterogeneity to the model. Preferably, information from large, high-quality, head-to-head trials should be used to inform comparative effectiveness assessments. The PSA was conducted to account for uncertainty in the estimates and the findings were consistent with the base case analyses.
Conclusions
RCI is a cost-effective alternative treatment for MS relapses compared to PMP and IVIg from the US payer and societal perspectives. Further clinical and economic analyses should assess the long-term clinical- and cost-effectiveness of RCI, PMP, and IVIg for acute exacerbations in MS.
Data Sharing Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Compliance with Ethics Guidelines
This study does not involve any human participants, human data, and/or human material. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgments
This study has not been presented or submitted, in part or full, to any other journal or conference.
Funding
This study was sponsored by Mallinckrodt Pharmaceuticals. The article processing charges and open access fee for this publication were funded by Mallinckrodt Pharmaceuticals.
Disclosure
GJW and JN are employees at Mallinckrodt Pharmaceuticals. JB and MP were paid consultants at Mallinckrodt Pharmaceuticals for the duration of the project. IC was a research collaborator for the duration of the study. SFH is a research collaborator and has received speaker honoraria, consulting agreements, and grant/research financial support from AbbVie, Acorda, Actelion, Adamas, Alkermes, Avanir, Bayer, Biogen Idec, Bristol Meyers Squibb, EMD Serono, Janssen-Actelion, Novartis, Osmotica, Mallinckrodt, Roche, Sanofi, Synthon, and Teva. All authors declare that they have no other conflicts of interest.
References
1. Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5–7, 2010. Int J MS Care. 2012;14(3):105–114. doi:10.7224/1537-2073-14.3.105
2. National Multiple Sclerosis Society. Treating MS. Available from: https://www.nationalmssociety.org/Treating-MS.
3. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2 Suppl):S15–S20.
4. Hersh C, Fox R. Multiple sclerosis. Available from: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/neurology/multiple_sclerosis/.
5. Nazareth TA, Rava AR, Polyakov JL, et al. Relapse prevalence, symptoms, and health care engagement: patient insights from the Multiple Sclerosis in America 2017 survey. Mult Scler Relat Disord. 2018;26:219–234. doi:10.1016/j.msard.2018.09.002
6. Mowry EM, Beheshtian A, Waubant E, et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology. 2009;72(20):1760–1765. doi:10.1212/WNL.0b013e3181a609f8
7. Berkovich R. Treatment of acute relapses in multiple sclerosis. Neurotherapeutics. 2013;10(1):97–105. doi:10.1007/s13311-012-0160-7
8. Nazareth T, Datar M, Yu TC. Treatment effectiveness for resolution of multiple sclerosis relapse in a US health plan population. Neurol Ther. 2019;8(2):383–395. doi:10.1007/s40120-019-00156-5
9. Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76(3):294–300. doi:10.1212/WNL.0b013e318207b1f6
10. Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46(6):878–886. doi:10.1002/1531-8249(199912)46:6<878::aid-ana10>3.0.co;2-q
11. Lehmann HC, Hartung HP, Hetzel GR, Stuve O, Kieseier BC. Plasma exchange in neuroimmunological disorders: part 1: rationale and treatment of inflammatory central nervous system disorders. Arch Neurol. 2006;63(7):930–935. doi:10.1001/archneur.63.7.930
12. Elovaara I, Kuusisto H, Wu X, Rinta S, Dastidar P, Reipert B. Intravenous immunoglobulins are a therapeutic option in the treatment of multiple sclerosis relapse. Clin Neuropharmacol. 2011;34(2):84–89. doi:10.1097/WNF.0b013e31820a17f3
13. Soelberg Sorensen P, Haas J, Sellebjerg F, Olsson T, Ravnborg M. IV immunoglobulins as add-on treatment to methylprednisolone for acute relapses in MS. Neurology. 2004;63(11):2028–2033. doi:10.1212/01.wnl.0000145798.61383.39
14. Bayry J, Hartung HP, Kaveri SV. IVIg for relapsing-remitting multiple sclerosis: promises and uncertainties. Trends Pharmacol Sci. 2015;36(7):419–421. doi:10.1016/j.tips.2015.04.012
15. Mallinckrodt Pharmaceuticals. Acthar® Gel prescribing information; 2021. Available from: https://www.acthar.com/pdf/Acthar-PI.pdf.
16. Costello J, Njue A, Lyall M, et al. Efficacy, safety, and quality-of-life of treatments for acute relapses of multiple sclerosis: results from a literature review of randomized controlled trials. Degener Neurol Neuromuscul Dis. 2019;9:55–78. doi:10.2147/DNND.S208815
17. Kaplan J, Miller T, Baker M, Due B, Zhao E. Topline results of a prospective observational registry of repository corticotropin injection for the treatment of multiple sclerosis relapse. Multiple Sclerosis J. 2020;26:48–49.
18. Nazareth T, Datar M, Sheer R, Yu T, Schwab P. Multiple sclerosis relapse resolution with corticosteroid alternatives: a retrospective claims analysis of us health plan data. Multiple Sclerosis J. 2018;24:900–901.
19. Gold LS, Suh K, Schepman PB, Damal K, Hansen RN. Healthcare costs and resource utilization in patients with multiple sclerosis relapses treated with H.P. Acthar Gel®. Adv Ther. 2016;33(8):1279–1292. doi:10.1007/s12325-016-0363-0
20. Wan GJ, Chopra I, Niewoehner J, Hunter SF. Cost per response analysis of repository corticotropin injection versus other alternative treatments for acute exacerbations of multiple sclerosis. Drugs Context. 2020;9:1–7. doi:10.7573/dic.2020-9-4
21. Oleen-Burkey M, Castelli-Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse: the patient’s perspective. Patient. 2012;5(1):57–69. doi:10.2165/11592160-000000000-00000
22. Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II-an ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–172. doi:10.1016/j.jval.2015.02.001
23. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi:10.1001/jama.2016.12195
24. Sormani MP, Bonzano L, Roccatagliata L, Mancardi GL, Uccelli A, Bruzzi P. Surrogate endpoints for EDSS worsening in multiple sclerosis. A meta-analytic approach. Neurology. 2010;75(4):302–309. doi:10.1212/WNL.0b013e3181ea15aa
25. National Multiple Sclerosis Society. Managing multiple sclerosis: relapse management. Available from: https://www.nationalmssociety.org/For-Professionals/Clinical-Care/Managing-MS/Relapse-Management.
26. Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ. 2010;13(4):618–625. doi:10.3111/13696998.2010.523670
27. Heatwole C, Johnson N, Holloway R, Noyes K. Plasma exchange versus intravenous immunoglobulin for myasthenia gravis crisis: an acute hospital cost comparison study. J Clin Neuromuscul Dis. 2011;13(2):85–94. doi:10.1097/CND.0b013e31822c34dd
28. Mauskopf J, Fay M, Iyer R, Sarda S, Livingston T. Cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing forms of multiple sclerosis in the United States. J Med Econ. 2016;19(4):432–442. doi:10.3111/13696998.2015.1135805
29. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(7):1914–1929. doi:10.1093/brain/awq118
30. Multiple Sclerosis Trust. Expanded Disability Status Scale (EDSS). Available from: https://www.mstrust.org.uk/a-z/expanded-disability-status-scale-edss.
31. Kaplan J, Miller T, Baker M, Due B, Zhao E, Wan GJ. Patient-reported outcomes from a prospective observational registry of repository corticotropin injection for the treatment of multiple sclerosis relapse. J Manag Care Special Pharma. 2020;26(4–aSuppl):S42. doi:10.18553/jmcp.2020.26.4-a.s1
32. Grifols Therapeutics LLC. Gamunex®-C. Available from: https://www.gamunex-c.com/documents/27482625/27482925/Gamunex-C+Prescribing+Information.pdf/9258bd0f-4205-47e1-ab80-540304c1ff8e.
33. Pilutti LA, Dlugonski D, Pula JH, Motl RW. Weight status in persons with multiple sclerosis: implications for mobility outcomes. J Obes. 2012;2012:868256. doi:10.1155/2012/868256
34. Cleveland Clinic Mellen Center for Multiple Sclerosis Treatment and Research. Mellen center approaches: IVIG. Available from: https://my.clevelandclinic.org/departments/neurological/depts/multiple-sclerosis/ms-approaches/ivig.
35. van den Hout WB. The value of productivity: human-capital versus friction-cost method. Ann Rheum Dis. 2010;69(Suppl 1):i89–i91. doi:10.1136/ard.2009.117150
36. Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology. 2006;66(11):1696–1702. doi:10.1212/01.wnl.0000218309.01322.5c
37. Wallin MT, Culpepper WJ, Nichols E; Global Burden of Diseases Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269–285. doi:10.1016/S1474-4422(18)30443-5
38. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi:10.1056/NEJMp1011024
39. Dubois RW. Cost-effectiveness thresholds in the USA: are they coming? Are they already here? J Comp Eff Res. 2016;5(1):9–11. doi:10.2217/cer.15.50
40. Institute for Clinical and Economic Review. 2020–2023 value assessment framework; 2020. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_013120-4.pdf.
41. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi:10.1056/NEJMp1405158
42. Glanz BI, Degano IR, Rintell DJ, Chitnis T, Weiner HL, Healy BC. Work productivity in relapsing multiple sclerosis: associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value Health. 2012;15(8):1029–1035. doi:10.1016/j.jval.2012.07.010
43. Kohlmann T, Wang C, Lipinski J, et al. The impact of a patient support program for multiple sclerosis on patient satisfaction and subjective health status. J Neurosci Nurs. 2013;45(3):E3–E14. doi:10.1097/JNN.0b013e31828a4161
44. Massetti M, Aballea S, Videau Y, Remuzat C, Roiz J, Toumi M. A comparison of HAS & NICE guidelines for the economic evaluation of health technologies in the context of their respective national health care systems and cultural environments. J Mark Access Health Policy. 2015;3. doi:10.3402/jmahp.v3.24966.
45. Ernst R. Indirect costs and cost-effectiveness analysis. Value Health. 2006;9(4):253–261. doi:10.1111/j.1524-4733.2006.00114.x
46. Kaplan J, Miller T, Baker M, Due B, Zhao E. A prospective observational registry of repository corticotropin injection (Acthar® Gel) for the treatment of multiple sclerosis relapse. Front Neurol. 2020;11:598496. doi:10.3389/fneur.2020.598496
47. International Business Machines. IBM Micromedex® RED BOOK®. Available from: https://www.micromedexsolutions.com/home/dispatch.
48. Navarro-Martinez R, Cauli O. Therapeutic plasmapheresis with albumin replacement in Alzheimer’s disease and chronic progressive multiple sclerosis: a review. Pharmaceuticals (Basel). 2020;13(2):28. doi:10.3390/ph13020028
49. Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C
50. Centers for Medicare & Medicaid Services. Physician Fee Schedule. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched.
51. Katz U, Kishner I, Magalashvili D, Shoenfeld Y, Achiron A. Long term safety of IVIg therapy in multiple sclerosis: 10 years experience. Autoimmunity. 2006;39(6):513–517. doi:10.1080/08916930600825867
52. Strand V, Shah R, Atzinger C, et al. Economic burden of fatigue or morning stiffness among patients with rheumatoid arthritis: a retrospective analysis from real-world data. Curr Med Res Opin. 2020;36(1):161–168. doi:10.1080/03007995.2019.1658974
53. Hagiwara M, Borker R, Oster G. Economic burden of adverse events in patients with metastatic renal cell carcinoma. Clin Ther. 2013;35(12):1955–1963 e2. doi:10.1016/j.clinthera.2013.10.010
54. O’Brien JA, Ward AJ, Patrick AR, Caro J. Cost of managing an episode of relapse in multiple sclerosis in the United States. BMC Health Serv Res. 2003;3(1):17. doi:10.1186/1472-6963-3-17
55. O’Connell K, Kelly SB, Fogarty E, et al. Economic costs associated with an MS relapse. Mult Scler Relat Disord. 2014;3(6):678–683. doi:10.1016/j.msard.2014.09.002
56. Parisé H, Laliberte F, Lefebvre P, et al. Direct and indirect cost burden associated with multiple sclerosis relapses: excess costs of persons with MS and their spouse caregivers. J Neurol Sci. 2013;330(1–2):71–77. doi:10.1016/j.jns.2013.04.007
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.