Back to Journals » Risk Management and Healthcare Policy » Volume 17
Cost-Effectiveness of First-Line Atezolizumab versus Chemotherapy in Non-Small-Cell Lung Cancer Patients Ineligible for Platinum-Containing Regimens
Authors Li LF, Qi R , Wei TT, Feng L, Zhang X, Liu Q
Received 14 December 2023
Accepted for publication 13 March 2024
Published 12 April 2024 Volume 2024:17 Pages 927—933
DOI https://doi.org/10.2147/RMHP.S451846
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Lan-Fang Li,1 Ran Qi,1 Tian-Tian Wei,1 Lei Feng,1 Xin Zhang,1 Qiao Liu2
1Department of Clinical Pharmacy, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining, Shandong, 272029, People’s Republic of China; 2Department of Pharmacy, The Second Xiangya Hospital of Central South University, Changsha, Hunan, 410011, People’s Republic of China
Correspondence: Qiao Liu, Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, People’s Republic of China, Email [email protected]
Purpose: The IPSOS study provided evidence supporting the efficacy and tolerability of first-line atezolizumab compared to single-agent chemotherapy for non-small-cell lung cancer (NSCLC) patients ineligible for treatment with a platinum-containing regimen. This study aimed to assess the cost-effectiveness of atezolizumab specifically in this population, considering the perspective of the Chinese healthcare system.
Patients and Methods: In this analysis, a three-state Markov model was utilized. The survival data were derived from the IPSOS clinical trial. Direct medical costs and utility values were collected from national authoritative database and published literature. The primary outcomes were costs, quality-adjusted life-years (QALYs) and incremental cost-effectiveness ratio (ICER). To ensure the robustness of our model, both one-way and probabilistic sensitivity analyses were conducted.
Results: Atezolizumab monotherapy led to an increase in costs of $4139.23 compared to single-agent chemotherapy. Additionally, it resulted in a gain of 0.14 QALYs, leading to an ICER of $29,365.79 per QALY, which was below the willingness-to-pay threshold of $36,066 per QALY used in the model. One-way sensitivity analyses revealed cost of atezolizumab and utility of progressive disease (PD) as major influencing factors for ICER. Furthermore, probabilistic sensitivity analyses confirmed our base-case results.
Conclusion: From the perspective of the Chinese healthcare system, atezolizumab emerges as a cost-effective choice for the first-line treatment of NSCLC patients ineligible for platinum-based chemotherapy.
Keywords: atezolizumab, cost-effectiveness, NSCLC, IPSOS
Introduction
The Global Burden of Disease Study has highlighted the significant impact of lung cancer as a leading contributor to the burden of non-communicable diseases worldwide.1,2 In terms of both incidence and mortality, lung cancer takes precedence among all types of cancers, accounting for approximately 22% of cancer-related deaths in China.3–6 Non-small cell lung cancer (NSCLC) represents the majority, ranging 85% to 90%,7,8 of all lung cancers cases, with nearly 60% of NSCLC patient already diagnosed at an advanced stages.9–11 According to the Chinese Society of Clinical Oncology guidelines for NSCLC (2023 edition), platinum-based chemotherapy doublets remain the primary treatment strategy for advanced NSCLC patients who not possess epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase-positive (ALK) mutations.12 The guideline recommends this approach as the first-line option for patients with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–1 (Level I), while it may be considered as a secondary choice (Level II) for individuals with an ECOG PS score of 2. Unfortunately, for NSCLC patients who are ineligible for platinum-containing regimens, the available treatment options are quite limited.
In recent years, cancer immunotherapy, particularly with atezolizumab targeting PD-L1, has revolutionized treatment options for advanced NSCLC lacking driver mutations.13,14 Atezolizumab’s approval by the National Medical products Administation for first or second-line NSCLC treatment underscores its remarkable clinical efficacy.15 Notably, a Phase 3 study (IPSOS) compared atezolizumab monotherapy to standard single-agent chemotherapy (gemcitabine or vinorelsbine) in the first-line treatment of NSCLC patients ineligible for platinum-containing regimens, revealing improved overall survival (median OS 10.3 months vs 9.2 months; stratified hazard ratio 0.78 [0.63–0.97], p=0.028) and a favorable safety profile for atezolizumab.16 The study categorized patients based on PD-L1 expression levels into four tiers (TC <1%, TC ≥1%, TC 1–49%, and TC ≥50%), with varying percentages across these levels for both atezolizumab monotherapy and single-agent chemotherapy recipients. Notably, PD-L1 expression was not restricted by the nadir criteria of the IPSOS trial.
Atezolizumab offers significant clinical advantages but comes with a high price that creates a substantial financial burder. Its annual cost in China exceeds the per capita GDP in China by 3.73 times.17 With 1060,600 new cases of advanced lung cancer diagnosed annually in China, approximately 328,000 individuals are not suitable candidates for platinum-containing regimens.18 This sizable population represents a significant group that could potentially benefit from atezolizumab treatment. Consequently, there is an urgent necessity for pharmacoeconomic evaluations of atezolizumab in the management of NSCLC patients ineligible for platinum-based chemotherapy. To address these concerns, this study aims to investigate the cost-effectiveness of atezolizumab monotherapy for NSCLC patients who are ineligible for platinum-based chemotherapy within the perspective of the Chinese healthcare system.
Materials and Methods
Overview
With the software Excel (version 2019) for mathematical modeling and R (version 4.0.4, http://www.r-project.org) for survival fitting, a economic evaluation was constructed to investigate the cost-effectiveness of atezolizumab and single-agent chemotherapy for patients who are ineligible for platinum-based chemotherapy NSCLC from the Chinese health-care perspective. This economic evaluation was conducted based on the IPSOS study (ClinicalTrials.gov identifier: NCT03191786), which sought compare the cost-effectiveness of atezolizumab monotherapy with single-agent chemotherapy (vinorelbine or gemcitabine) as a first-line treatment for patients with NSCLC who were not suitable for platinum-containing regimens. Since the data used in this study was obtained from published sources, the approval from ethical review was exempted by the Clinical Ethics Committee (EC) of affiliated hospital of Jining medical university according to the Measures for Ethical Review of Life Science and Medical Research Involving Humans (2023).19 This economic evaluation was guided by the Chinese guidelines for pharmacoeconomic evaluation (2020).20
Model Construction
This study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guidelines, which provide standardized guidelines for reporting health economic evaluations.21 In this study, a Markov model was constructed to evaluate the long-term costs and quality-adjusted life-years (QALYs) of atezolizumab and chemotherapy. The Markov model consisted of three mutually exclusive health states: progression-free survival (PFS), progressive disease (PD), and death. Patients in both treatment arms were assumed to be in the PFS health state at the beginning of the simulation and could either remain in this state or transition to another health state based on the calculated transition probability during each Markov cycle. The model structure is shown in Figure 1.
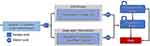 |
Figure 1 Model structure. |
The analysis was conducted from the perspective of the Chinese health-care system, using a lifetime horizon. The cycle length of the model was set to 21 days, which aligned with the medication plan used in the IPSOS trial. The primary outcomes assessed in the model included the costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratio (ICER). Costs and health outcomes were discounted at a rate of 5%, following the Chinese Pharmacoeconomic Evaluation Guidelines 2020.20
Clinical Effectiveness
This study utilized efficacy and safety data from the IPSOS trial. The transition probability from the progression-free survival (PFS) health state to death (pFTD) for each treatment was derived from the overall survival (OS) curve of the IPSOS trial. However, since PFS curves were not available, the probability of remaining in the PFS health state (pFTF) for both the atezolizumab and chemotherapy groups was estimated using median PFS time.
To extract data points from the OS curves, the GetData Graph Digitizer (version 2.26; http://www.getdata-graphdigitizer.com/index.php) was utilized to reconstruct individual patient data following Guyot et al’s method.22 Five commonly used parametric survival distributions (exponential, gamma, Weibull, log-logistic, and log-normal) were assessed for their goodness-of-fit in extrapolating long-term efficacy beyond the clinical trial follow-up period.23 The best-fit survival model, determined by smaller Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), as well as visual fitting comparisons of KM curves and models curves,24 led to the selection of the log-normal distribution for fitting the OS data in both the atezolizumab and chemotherapy groups. Supplementary Figure S1 illustrates the OS fitting curves for both the atezolizumab and chemotherapy groups, whereas Supplementary Table S1 provides a comprehensive description of the survival model selection, and Supplementary Table S2 presents the survival parameters used in the model.
Medical Costs
In our cost-effectiveness analysis, we focused on direct medical costs, which included costs for drug acquisition, follow-up visits, second-line treatment, laboratory test and tumor-related examinations, managing adverse events (AEs), best supportive care and terminal care. The drug administration cycles aligned with those in the IPSOS trial. To determine the dosage of chemotherapy drugs, we assumed a typical patient had a body surface area (BSA) and weight of 1.72m2 and 65kg, respectively.25 For simplicity, we considered only the costs of grade 3–4 AEs. Upon disease progression, patients were recommended to receive second-line treatment based on the CSCO guidelines for NSCLC (2023 edition).26 In this analysis, patients in the atezolizumab group were selected for docetaxel maintenance therapy after disease progression, while patients in the chemotherapy group opted for nivolumab maintenance therapy. All costs were obtained from local bid-winning price and previously published literature.22–24,27,28 Additionally, all costs were adjusted to US dollars in 2023 (US $1 = CNY 7.128). All the related parameters are shown in Supplementary Table S3.
As there is no information available about the quality-of-life data from the IPSOS trial, utility values used in this study were obtained from previously published literature.29 For the PFS health state, a utility value of 0.804 was assigned, while for the PD health state, a utility value of 0.321 was assigned.
Sensitivity Analysis
To assess the uncertainty surrounding the model parameters, we conducted both one-way sensitivity analysis and probability sensitivity analysis (PSA) to evaluate the robustness of our base-case analysis findings. In the one-way sensitivity analysis, each model parameter was systematically tested within a predefined range of minimum and maximum values obtained from previously published literature. The results of the one-way sensitivity analysis are presented in a tornado diagram, which visually depicts the magnitude of the impact of each parameter on the results. For the PSA, all model parameters were simultaneously varied by sampling from predefined distributions. Costs were modeled using a Gamma distribution, while health state utility values, incidence of adverse events, and other parameters were sampled from a beta distribution. The PSA generated a cost-effectiveness scatter plot, illustrating the distribution of costs and effectiveness outcomes. Additionally, a cost-effectiveness acceptability curve (CEAC) was produced, demonstrating the probability that a particular intervention is considered cost-effective at various WTP thresholds.
Results
Base-Case Analysis
As displayed in Supplementary Figure S1, the OS imitated in our model is very similar with the cure OS in IPSOS trial, which means our model are credible.
From the perspective of the Chinese healthcare system, atezolizumab as a first-line treatment for patients with NSCLC resulted in an additional cost of $4139.23 and an increase of 0.14 QALYs compared to single-agent chemotherapy. The estimated ICER for atezolizumab in NSCLC patients is $29,365.79/QALY. (Supplementary Table S4) Considering a WTP threshold of $36,066/QALY, atezolizumab emerges as a cost-effective treatment option when compared to single-agent chemotherapy. The baseline characteristics that differ between the atezolizumab and chemotherapy groups are presented in Supplementary Table S5.
Sensitivity Analysis
In the one-way sensitivity analysis, the most influential parameters were found to be the utility of PD health state and price of atezolizumab. Other parameters, such as cost of nivolumab/cycle, cost of managing AEs in the chemotherapy group, and the discount rate, had a moderate impact on model results. The utility of PFS, second-line therapy, and the cost of routine follow-up had minimal effect on the model outcome. It is important to note that varying individual parameters did not result in any significant changes to the results. Additionally, the probability of atezolizumab being cost-effective at the current WTP threshold was 0%. For a visual representation of the one-way sensitivity, please refer to Supplementary Figure S2 depicting the tornado diagram.
The results of PSA were presented in Supplementary Figure S3 and Supplementary Figure S4, using a CEAC and cost-effectiveness scatter plot. The CEAC curve demonstrated the probability of atezolizumab being considered the optimal strategy under different WTP thresholds. Meanwhile, the probabilistic scatter plot visualized the outcomes of 1000 Monte Carlo simulation results. The CEAC revealed that at the WTP threshold of $36,066/QALY, there was a 59% probability of atezolizumab being cost-effective. Notably, when WTP threshold was increased to $90,000 the probability of atezolizumab being considered cost-effective rose to 97%. The cost-effectiveness scatter plot illustrated the results of 1000 iterations of Monte Carlo simulation. Importantly, 59% of the ICER values shown for 1000 iterations fall below the WTP values used in the model. These findings were consistent with the results obtained from the base-case analysis.
Discussion
Lung cancer is one of the most common malignant tumours in the world and ranks first among all types of cancer in terms of incidence and mortality in China.30 Lung cancer accounts for nearly 20% of cancer-related medical expenditure, according to China’s National Health Commission.31 Data shows that in 2020, China’s new cases of lung cancer were 820,000, of which 40% of NSCLC patients have contraindications to platinum-doublet chemotherapy, and the health demands of this part of the patient population remain unmet.5 The IPSOS trial was the first clinical study to demonstrated that atezolizumab significantly improves survival in patients with platinum-based chemotherapy-intolerant advanced NSCLC. Considering the higher price of atezolizumab compared to single-agent chemotherapy, oncologists and patients face the challenge of evaluating its cost-effectiveness when choosing a treatment regimen.
In this analysis, we developed a Markov model to evaluate the cost-effectiveness of atezolizumab monotherapy as a first-line treatment for NSCLC patients ineligible for platinum-containing regimens, considering the perspective of the Chinese health-care system. Our results reveal that first-line atezolizumab monotherapy yielded an additional 0.14 QALYs (0.61 QALYs vs 0.47 QALYs) compared to first-line single-agent chemotherapy, incurring an incremental cost of $4139.23 ($50,209.10 vs $46,069.87). This led to in an ICER of $29,365.79 per QALY gained. Using a WTP threshold of three times of GDP per QALY ($36,066/QALY), our analysis indicates that first-line atezolizumab monotherapy is an cost-effective option compared to single-agent chemotherapy in the context ofChina. One-way sensitivity analysis and PSA analyses confirmed the reliability of our base-case results. Recently, a similar analysis was conducted from the perspective of the UK healthcare system.32 This study revealed that at a WTP threshold of €36,000, atezolizumab was associated with a cost increase of €26,206 and an ICER of €94,873 compared to chemotherapy. Despite gaining an additional 0.28 QALYs, atezolizumab was not found to be economically viable for patients with advanced NSCLC who are ineligible platinum-containing regimens in the United Kingdom. However, there is still limited understanding regarding the economic aspects of atezolizumab for this patient population in China. Our present analysis aimed to address this knowledge gap and provide oncologists and healthcare decision makers in China with alternatives therapeutic options and pricing guidance for atezolizumab in advanced NSCLC.
While PD-L1 expression level plays a crucial role as a predictor of immunotherapy response, typically associated with improved efficacy,33 it is noteworthy that the IPSOS study categorized patients based on PD-L1 expression levels into four tiers (TC<1%, TC ≥ 1%, TC 1–49%, and TC ≥ 50%), demonstrating varying distributions among recipients of atezolizumab monotherapy and single-agent chemotherapy. Given the specific focus of this study on NSCLC patients ineligible for platinum-based chemotherapy, the significance of PD-L1 as an indicator may be limited within this defined patient population. Moreover, the IPSOS trial did not elucidate the variations in efficacy and safety of atezolizumab monotherapy across different PD-L1 expression levels, resulting in a lack of corresponding cost-effectiveness data for PD-L1 subgroups in our analysis. We are dedicated to updating our analysis with any novel discoveries”.
Our research possesses noteworthy strengths that deserve emphasis. Firstly, we utilized modeling techniques to estimate the lifetime costs and clinical outcomes associated with both the atezolizumab group and the single-agent chemotherapy group. By employing a Markov model, we were able to achieve superior accuracy and reliability in predicting long-term costs and health outcomes compared to traditional meta-analysis techniques. Moreover, our analysis benefitted from the utilization of clinical data, safety data, and medication cycles that were derived from the IPSOS trial. This ensured the robustness and validity of our findings, enhancing the credibility of our research. Furthermore, to enhance the alignment of our study with clinical practice, we diligently ensured that the drugs and doses used for second-line treatment after disease progression in our model were consistent with the recommendations outlined in the CSCO guidelines for NSCLC. This intentional alignment enables greater relevance and applicability of our results within the medical community, reinforcing the practical implications of our research.
Several potential limitations must be discussed in this study. Firstly, since the IPSOS trial did not provide specific information on the types of adverse events (AEs), we relied on a recently published pharmacoeconomic evaluation to estimate the cost associated with managing these AEs in our study. While the absence of AE data from the IPSOS trial introduces some potential bias into our analysis, the results of our one-way sensitivity analysis support the finding that the cost of dealing with AEs does not significantly impact the model’s outcomes. Secondly, regarding the selection of drugs in the chemotherapy group, the IPSOS trial has not yet disclosed the specific proportion of gemcitabine and vinorelbine usage. Consequently, we assumed an equal distribution of 50% for both drugs in this study. Although this assumption may introduce some degree of deviation in calculating chemotherapy costs, it is worth noting that the cost per cycle of gemcitabine and vinorelbine is nearly identical (gemcitabine cycle cost is $126.85, vinorelbine cycle cost is $119.31). Therefore, as a conservative approach, we believe this assumption will have minimal impact on the overall results. Thirdly, one of the potential limitations that should be discussed in this study is the lack of consideration for the potential impact of widely used concomitant drugs on immunological agents.34 The failure to account for these factors in the modeling process represents a limitation of the current analysis, highlighting the need for further research to address these gaps and enhance the comprehensiveness of future studies.
Conclusion
In conclusion, from the perspective of the Chinese health-care system, atezolizumab has a high probability of being a cost-effective choice for the first-line treatment of NSCLC patients ineligible for platinum-based chemotherapy.
Data Sharing Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
All authors contributed to this publication were listed.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Research Foundation of the Affiliated Hospital of Jining Medical University [grant number 2020-BS-005].
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1859–1922. doi:10.1016/S0140-6736(18)32335-3
2. Liu G, Kang S, Wang X, Shang F. Cost-effectiveness analysis of atezolizumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer with different PD-L1 expression status. Front Oncol. 2021;11:669195 doi:10.3389/fonc.2021.669195
3. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018. Global Cancer Statistics?. Cancer Commun. 2019;39(1):22 doi:10.1186/s40880-019-0368-6
4. Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016. JAMA Oncol. 2018;4(11):1553. doi:10.1001/jamaoncol.2018.2706
5. Cao W, Chen H, Yu Y, Li N, Chen W. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chinese Med J-Peking. 2021;134(7):783–791. doi:10.1097/CM9.0000000000001474
6. Xiang G, Gu L, Chen X, et al. Economic evaluation of first-line camrelizumab for advanced non-small-cell lung cancer in China. Front Public Health. 2021;9:743558. doi:10.3389/fpubh.2021.743558
7. Zhou D, Luo X, Zhou Z, et al. Cost-effectiveness analysis of tislelizumab, nivolumab and docetaxel as second- and third-line for advanced or metastatic non-small cell lung cancer in China. Front Pharmacol. 2022;13:880280 doi:10.3389/fphar.2022.880280
8. Zhang X, Zhang H, Li L, Feng L, Liu Q. Cost-effectiveness analysis of pembrolizumab plus chemotherapy in squamous non–small-cell lung cancer in China. Risk Manage Healthc Policy. 2023;16:1849–1857. doi:10.2147/RMHP.S429394
9. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi:10.3322/caac.21565
10. Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527. doi:10.1200/JCO.19.00934
11. Wu B, Lu S. The effect of PD-L1 categories-directed pembrolizumab plus chemotherapy for newly diagnosed metastatic non-small-cell lung cancer: a cost-effectiveness analysis. Translational Lung Cancer Res. 2020;9(5):1770–1784. doi:10.21037/tlcr-19-605
12. Han J, Tian K, Yang J, Gong Y. Durvalumab vs placebo consolidation therapy after chemoradiotherapy in stage III non-small-cell lung cancer: an updated PACIFIC trial-based cost-effectiveness analysis. Lung Cancer. 2020;146:42–49. doi:10.1016/j.lungcan.2020.05.011
13. Yang Y, Wang Z, Fang J, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (oncology Program by innovent anti-PD-1-11). J Thorac Oncol. 2020;15(10):1636–1646. doi:10.1016/j.jtho.2020.07.014
14. Sun J, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi:10.1016/S0140-6736(21)01234-4
15. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X
16. Lee SM, Schulz C, Prabhash K, et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study. Lancet. 2023;402(10400):451–463. doi:10.1016/S0140-6736(23)00774-2
17. Yang Y, Xia Y, Su C, et al. Measuring the indirect cost associated with advanced non-small cell lung cancer: a nationwide cross-sectional study in China. J Cancer Res Clin. 2023;149(8):4205–4214. doi:10.1007/s00432-022-04258-w
18. Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. 2024. doi:10.1016/j.jncc.2024.01.006
19. Rui M, Wang Y, Fei Z, Zhang X, Shang Y, Li H. Will the markov model and partitioned survival model lead to different results? A review of recent economic evidence of cancer treatments. Expert Rev Pharm Out. 2021;21(3):373–380 doi:10.1080/14737167.2021.1893167
20. Cranmer H, Shields GE, Bullement A. A comparison of partitioned survival analysis and state transition multi-state modelling approaches using a case study in oncology. J Med Econ. 2020;23(10):1176–1185. doi:10.1080/13696998.2020.1796360
21. Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. doi:10.1016/j.jval.2021.11.1351
22. Kashiwa M, Matsushita R. Model-based cost-utility analysis of gemcitabine, cisplatin, and S-1 as triple therapy for advanced biliary tract cancer. Int J Clin Pharm-Net. 2023;45(4):875–883. doi:10.1007/s11096-023-01580-2
23. Liu S, Dou L, Wang K, et al. Cost-effectiveness analysis of nivolumab combination therapy in the first-line treatment for advanced esophageal squamous-cell carcinoma. Front Oncol. 2022;12:899966 doi:10.3389/fonc.2022.899966
24. Shang F, Zhang B, Kang S. Cost-effectiveness analysis of atezolizumab plus chemotherapy as first-line treatment for patients with advanced nonsquamous non-small-cell lung cancer in China. Expert Rev Pharmacoecon Outcomes Res. 2023;23(3):337–343. doi:10.1080/14737167.2023.2170877
25. Zheng Z, Lin J, Zhu H, Cai H. Cost-effectiveness analysis of pembrolizumab plus chemotherapy vs. chemotherapy alone as first-line treatment in patients with esophageal squamous cell carcinoma and PD-L1 CPS of 10 or more. Front Public Health. 2022;10:893387 doi:10.3389/fpubh.2022.893387
26. Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer-Am Cancer Soc. 2015;121(1):8–16 doi:10.1002/cncr.28914
27. Jiang Y, Li Y, Wang LXW. Cost-effectiveness analysis of nivolumab plus standard chemotherapy versus chemotherapy alone for the first-line treatment of unresectable advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. Int J Clin Pharm-Net. 2022;44(2):499–506. doi:10.1007/s11096-021-01372-6
28. Shao T, Ren Y, Zhao M, Tang W. Cost-effectiveness analysis of camrelizumab plus chemotherapy as first-line treatment for advanced squamous NSCLC in China. Front Public Health. 2022;10:912921 doi:10.3389/fpubh.2022.912921
29. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non–small cell lung cancer: an international study. Asia-Pac J Clin Onco. 2017;13(5):e195–203 doi:10.1111/ajco.12477
30. Holleman MS, van Tinteren H, Groen HJM, Al MJ, Uyl-de Groot CA. First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: a network meta-analysis. OncoTargets Therapy. 2019;12:1413–1421. doi:10.2147/OTT.S189438
31. Cai Y, Chen W, Wang X, et al. Contemporary trends on expenditure of hospital care on total cancer and its subtypes in China during 2008–2017. Chinese J Cancer Res. 2021;33(5):627–636. doi:10.21147/j.issn.1000-9604.2021.05.09
32. Jiang Y, Zhao M, Xi J, Li J, Tang W, Zheng X. Cost-effectiveness analysis of atezolizumab in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen: a United Kingdom health care perspective. Front Public Health. 2023;11:1282374 doi:10.3389/fpubh.2023.1282374
33. Mok TSK, Wu Y, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi:10.1016/S0140-6736(18)32409-7
34. Rossi G, Pezzuto A, Sini C, et al. Concomitant medications during immune checkpoint blockage in cancer patients: novel insights in this emerging clinical scenario. Crit Rev Oncol/Hematol. 2019;142:26–34. doi:10.1016/j.critrevonc.2019.07.005
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
