Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Cost-effectiveness and budget impact of the fixed-dose dual bronchodilator combination tiotropium–olodaterol for patients with COPD in the Netherlands
Authors van Boven J , Kocks J , Postma MJ
Received 10 June 2016
Accepted for publication 4 August 2016
Published 19 September 2016 Volume 2016:11(1) Pages 2191—2201
DOI https://doi.org/10.2147/COPD.S114738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Job FM van Boven,1,2 Janwillem WH Kocks,2 Maarten J Postma1,3,4
1Department of Pharmacy, Unit of PharmacoEpidemiology & PharmacoEconomics, 2Department of General Practice, Groningen Research Institute for Asthma and COPD (GRIAC), 3Institute of Science in Healthy Aging & healthcaRE (SHARE), 4Department of Epidemiology, University Medical Center Groningen (UMCG), University of Groningen, Groningen, the Netherlands
Purpose: The fixed-dose dual bronchodilator combination (FDC) of tiotropium and olodaterol showed increased effectiveness regarding lung function and health-related quality of life in patients with chronic obstructive pulmonary disease (COPD) compared with the use of its mono-components. Yet, while effectiveness and safety have been shown, the health economic implication of this treatment is still unknown. The aim of this study was to assess the cost–utility and budget impact of tiotropium–olodaterol FDC in patients with moderate to very severe COPD in the Netherlands.
Patients and methods: A cost–utility study was performed, using an individual-level Markov model. To populate the model, individual patient-level data (age, height, sex, COPD duration, baseline forced expiratory volume in 1 second) were obtained from the tiotropium–olodaterol TOnado trial. In the model, forced expiratory volume in 1 second and patient-level data were extrapolated to utility and survival, and treatment with tiotropium–olodaterol FDC was compared with tiotropium. Cost–utility analysis was performed from the Dutch health care payer’s perspective using a 15-year time horizon in the base-case analysis. The standard Dutch discount rates were applied (costs: 4.0%; effects: 1.5%). Both univariate and probabilistic sensitivity analyses were performed. Budget impact was annually assessed over a 5-year time horizon, taking into account different levels of medication adherence.
Results: As a result of cost increases, combined with quality-adjusted life-year (QALY) gains, results showed that tiotropium–olodaterol FDC had an incremental cost-effectiveness ratio of €7,004/QALY. Without discounting, the incremental cost-effectiveness ratio was €5,981/QALY. Results were robust in univariate and probabilistic sensitivity analyses. Budget impact was estimated at €4.3 million over 5 years assuming 100% medication adherence. Scenarios with 40%, 60%, and 80% adherence resulted in lower 5-year incremental cost increases of €1.7, €2.6, and €3.4 million, respectively.
Conclusion: Tiotropium–olodaterol FDC can be considered a cost-effective treatment under current Dutch cost-effectiveness thresholds.
Keywords: COPD, cost-effectiveness, health economics, cost–utility, budget impact
Introduction
The Global initiative for chronic Obstructive Lung Disease (GOLD) defines chronic obstructive pulmonary disease (COPD) as a common preventable and treatable disease, characterized by persistent airflow limitation that is usually progressive and associated with enhanced chronic inflammatory airway response to noxious particles or gases.1 Exacerbations and comorbidities contribute to the overall severity of COPD. COPD has been projected to become the third largest cause of death by 2030, after cancer and cardiovascular disease.2 While smoking is widely recognized as the major risk factor for COPD, especially in developing countries, biomass smoke and indoor cooking result in an increasing prevalence of COPD.3
Besides nonpharmacologic treatments, several effective pharmacologic inhaled therapies for COPD are available. The main drug class to reduce symptoms and future risk in moderate to very severe COPD is the class of long-acting bronchodilators, that is, long-acting beta-agonists (LABA) and long-acting muscarinic antagonists (LAMA). In patients not sufficiently controlled with a single bronchodilator, a second bronchodilator of a different class may be added according to the GOLD guidelines. There seems a pharmacologic rationale for combining two classes of bronchodilators4 and some studies indeed showed the added value compared with the use of a single bronchodilator,5 but full synergistic effects have not been observed.
In addition to bronchodilators, inhaled corticosteroids (ICS) have been commonly used, often in a fixed-dose combination (FDC) with a bronchodilator. Although ICS are considered the cornerstone pharmacologic treatment in asthma patients, the use of ICS in patients with COPD is more controversial.6 ICS can have both systemic and local side effects7,8 and there seems limited added clinical value as compared with bronchodilators.9 Recently, the WISDOM study showed that ICS could be stepped down safely, that is, without increased exacerbation risk, as long as dual bronchodilators were continued.10
Spiolto Respimat® is a once-daily dual bronchodilator consisting of the FDC of the LAMA tiotropium and the LABA olodaterol delivered through the Respimat® Soft Mist inhaler. Tiotropium has been used for over a decade and is among the most commonly used therapies for patients with COPD. The effectiveness, safety, and cost-effectiveness of tiotropium have been evaluated in several large randomized controlled trials such as the UPLIFT and the POET.11–13 Due to its recent market introduction, so far only few studies have been performed with olodaterol; however, initial results seem beneficial with regard to its efficacy,14,15 and its safety profile is comparable with existing LABAs.1
Recently, the FDC of tiotropium and olodaterol showed increased effectiveness regarding lung function, physical functioning, and health-related quality of life compared with the use of its mono-components.16–18 Yet, while effectiveness and safety have been shown, the cost-effectiveness of the new FDC is still unknown. In times of an increasing burden of chronic diseases on governmental health care budgets, there is an obvious need for economic evaluation of newly available treatments. Therefore, the aim of this study was to assess the cost-effectiveness of tiotropium–olodaterol FDC in patients with moderate to very severe COPD.
Materials and methods
Study design
A cost–utility analysis was performed, using an individual-level Markov model. A cost–utility analysis is a special type of cost-effectiveness analysis that uses the quality-adjusted life-year (QALY) as outcome measure.
Type of model
A deterministic individual-level Markov model was used in which patients were tracked over time rather than placed in fixed health states (such as GOLD 1, 2, 3, and 4). To populate the model, individual patient-level data (age, height, sex, COPD duration, baseline forced expiratory volume in 1 second value [FEV1]) were obtained from the tiotropium–olodaterol clinical TOnado trial.16 As patient’s lung function declines, increased mortality risk, management costs, and exacerbation risk are seen, while quality of life decreases. These phenomena form the basis of the individual-based Markov model.19
Note that an advantage of the current model from typical cohort models is that it is not “memory-less”, a common feature of Markov models in which the risk of future events is not affected by what has occurred in previous cycles. For example, the risk of exacerbation in future cycles increases based on the incidence of an exacerbation in the previous year, in line with observations in clinical studies.20
Perspective
The cost–utility analysis was performed from the Dutch health care payer’s perspective. Applying a health care payer’s perspective implicates that only direct medical costs, such as medication, hospitalization, and primary care visits, were included. Indirect costs, such as work productivity losses,21 were neglected as differences in these costs were not measured. In addition, as the vast majority of COPD patients were older than the legal retirement age, the relative contribution of these costs seems limited in this population.
Comparison
In the Markov model, treatment with tiotropium–olodaterol FDC was compared with treatment with tiotropium. Tiotropium is one of the most widely used drugs for COPD and is considered a suitable comparator to represent usual care in the Netherlands.22 In addition, tiotropium has been used as comparator in a previous economic study that evaluated the cost-effectiveness of another FDC LABA–LAMA.23
Cycle length
The cycle length of the model was 1 month in order to minimize the probability of patients experiencing multiple exacerbations in a given cycle and to fully capture the impact of exacerbations on costs and outcomes. Using a longer cycle length would not allow modeling of those patients who may suffer from exacerbations in consecutive months, which could be the case for COPD patients with the frequent exacerbator phenotype.20 Shorter than 1-month cycle lengths would not be necessary in the current model as it is unlikely that a patient would experience more than one moderate or severe exacerbation within this time frame.24 The cycle length was 1 month, however, the cycles through 52 weeks corresponded to the clinical trial visits and ranged from 2 to 12 weeks in duration.16
Time horizon
The time horizon of the analysis was 15 years. As COPD is a chronic condition and according to the Dutch pharmacoeconomics guidelines, the model allowed patients to be followed over their lifetime, defined as a maximum period of 60 years, or a maximum age of 100 years given the minimum age of 40 years in the tiotropium–olodaterol FDC clinical trial. A 5-year time horizon may be considered the most comparable to published long-term trials in COPD considering that the studies providing clinical inputs (ie, lung function decline, exacerbation rates, mortality) all included follow-up times of <5 years. However, given the mean age of 64 years in the trial,16 and the high rates of mortality in COPD patients compared with the general population, a 15-year time horizon was considered most appropriate.
Model assumptions
No adverse events related to treatment with bronchodilators were considered. This was justified given that side effects from bronchodilators are comparable across products and do not contribute significantly to either costs or outcomes of treatment. The model did not account for the possibility of differential rates of treatment switch. Differential add-on treatment and treatment discontinuation were not considered. All surviving patients died at an age of 100 years. These assumptions have been chosen as there is no indication from clinical trials that any of the available bronchodilator combinations differ in these aspects. Accounting for these aspects would therefore not directly bias the analysis in favor or against tiotropium–olodaterol FDC.
Model inputs
Lung function decline
Following treatment initiation, improvement in FEV1 was tracked in clinical visits in the clinical trial up to 52 weeks for the assessment of tiotropium–olodaterol FDC versus tiotropium.16 Classification of patients according to severity level was done using postbronchodilator trough FEV1 values so that patients were classified by their potential to achieve a given level of lung function. Patients were classified into GOLD stages based on FEV1 using the GOLD 2007 criteria (GOLD stage II: 50%≤ FEV1 predicted <80%, stage III: 30%≤ FEV1 predicted <50%, stage IV: FEV1 predicted <30%) (Table S1).
After the trial period of 52 weeks, a constant linear decline in FEV1 was applied over time, not differentiated by treatment, but by the GOLD severity stage. As of its long-term follow-up (4 years), the rates of decline by severity stage observed in the tiotropium arm of the UPLIFT trial are currently used in the model, with greater declines in the less severe stages (Table S2). These differential rates of decline obtained from the UPLIFT trial25 are consistent with the findings of a review that concluded that lung function decline is more prominent earlier in the disease.26 Given that UPLIFT is one of the longest recent clinical trials on tiotropium in COPD, with published rates of decline that vary by GOLD stage, this was chosen as the best data source of lung function decline.25 The study was exempted from IRB approval because of the use of anonymized data. Written informed consent was also therefore not sought.
Exacerbation risk
Exacerbation risk in the base case was estimated based on a random-effects logistic regression analysis of patient-level data of exacerbations from the 4-year UPLIFT trial comparing treatment with tiotropium to placebo. Using data from the tiotropium treatment group only, the risks regarding the dichotomous outcomes of moderate exacerbation (nonsevere) and hospitalization due to severe exacerbation within a month were estimated. The explanatory variables in the regression analyses (Tables S3 and S4) included exacerbations in previous year, FEV1% predicted, hospitalizations in previous year, and patients’ age in months. There was no direct effect of tiotropium–olodaterol on exacerbation risk modeled. However, exacerbation was indirectly influenced as a result of improved lung function in the tiotropium–olodaterol FDC arm in the first 52 weeks, compared with tiotropium.16
Mortality
Patients faced a mortality risk in every cycle in the model and the exact risk was calculated as a function of COPD severity stage. Excess mortality due to COPD was obtained by comparing mortality with GOLD stage in the clinical trial versus the general population mortality adjusted by age and sex. The mortality rate applied in each cycle was obtained from two sources. The first source was a study of a large cohort of COPD patients in which mortality was a primary outcome that was used to estimate the excess mortality in COPD patients.27,28
Mortality in the general population by age and sex was obtained from the Dutch Bureau of Statistics. Mortality risk was not impacted by the incidence of exacerbations given that it would be double counting the risk. Previous published models used similar approaches to calculate mortality, differing mainly in the data source used to obtain the mortality risk.29
Utilities
In order to reflect the impaired quality of life in patients with COPD, utility weights were assigned independently to each disease state, and to each exacerbation event.
In the base case, previously published utility values were used.30 Utility weights by disease state (Table S2) had been derived from a representative subset of patients enrolled in the UPLIFT trial.
Consistent with previous cost-effectiveness models of bronchodilator treatment in COPD, patients experienced a temporary decrement in utility during an exacerbation, lasting the duration of one cycle before returning to their previous utility level, assigned by severity stage.31 The magnitude of the utility decrement varied by whether the patient experienced a severe or nonsevere exacerbation. The utility decrement multipliers for each type of exacerbation obtained from the published literature were applied in the same way regardless of which data source for baseline utility values was used. Utility decrements following nonsevere and severe exacerbation were 0.85 and 0.50, respectively.32,33
Costs
Since the perspective adopted for the base-case analysis was that of the Dutch health care payer, the model reflected direct medical expenditures associated with bronchodilator therapy and other COPD treatments. All health care costs were expressed in 2014 Euros. An overview of the costs is provided in Table 1.
  | Table 1 Cost parameters |
Discounting
For costs occurring after 1 year, a discount rate of 4% was applied in line with the Dutch guidelines. For effects occurring after 1 year, a discount rate of 1.5% was applied in line with the Dutch guidelines.34
Outcomes
Clinical outcomes included life years, exacerbation free months per patient per year, annual nonsevere exacerbations, and annual severe exacerbations. In addition, an incremental cost-effectiveness ratio (ICER) was calculated with the formula Δcosts/Δeffects, with effects expressed as QALYs gained.
Model validation
The model has been used in a previous study and has been compared with existing COPD models.19 In addition, the model has been validated against results from the TOnado trial. It was found that total exacerbations were similar to the numbers observed in the trial, while modeled mortality was slightly higher.19
Sensitivity analyses
Both univariate and probabilistic sensitivity analyses were conducted.
Univariate sensitivity analysis allows the investigator to determine the impact of uncertainty around one model input on the cost-effectiveness results, while probabilistic analysis is conducted by taking values from statistical distributions (often based on confidence intervals or fixed deviations from the best estimate) for several parameters at the same time. When confidence intervals were not specified in a given data source, it was by authors’ consensus decided to modify inputs relating to costs and utilities by ±15%, in line with a previous COPD cost-effectiveness study.35
The probabilistic sensitivity analysis (PSA) was based on a Monte Carlo simulation, where the model was run a large number of times (ie, 100 times) with different sets of inputs simulated according to statistical distributions (Table S5) that were assigned for all parameters, surrounded by uncertainty likely to have a significant impact on the cost-effectiveness results. The choice of statistical distribution used for each parameter included in the PSA was based on published recommendations for economic evaluation in health care.36
For each simulation, inputs were randomly selected from their statistical distributions, and pairs of data points for costs and effectiveness for each treatment were obtained. At the end of the simulation process, the joint statistical distribution for costs and effectiveness was represented as a cloud of points on a cost-effectiveness plane. Cost-effectiveness acceptability curves were generated, representing the probability of each treatment being the most cost-effective for a range of cost-effectiveness thresholds.
Budget impact
Analytical framework
In a linked model, budget impact was assessed using a COPD patient-level model approach in Microsoft Excel. A Dutch health care payer’s perspective was adopted. Treatment-specific lung function improvements after 2 weeks were applied to baseline FEV1 distributions as found in the Netherlands and tracked over 5 years assuming a fixed 30 mL annual decline. Calculations were performed for an incident and a prevalent cohort, including only direct health care costs (Table 1). Given that health insurance bodies work with yearly budgets, budget impact was calculated per year with a maximum time horizon of 5 years. All inputs and assumptions were similar to the cost-effectiveness model. Costs are provided in their undiscounted form and no mortality was assumed.
Size of the eligible population
The COPD incidence and prevalence were based on the Dutch national data sources.37 The GOLD distributions for the incident38 and prevalent39 COPD population were based on the Dutch and, in case of absent Dutch data, international population estimates.
Current mix of treatments and alternative scenarios
Medication distributions over 5 years were based on the manufacturer’s projections of market share within a group of (combined) bronchodilators, including tiotropium, indacaterol–glycopyrronium, umeclidinium–vilanterol, aclidinium–formoterol, tiotropium–salmeterol (separate inhalers), vilanterol–fluticasone furoate (Table S6). Scenarios with and without tiotropium–olodaterol FDC over 1, 2, 3, 4, and 5 years, respectively were compared. Costs of these treatments were based on the Dutch costs prices (www.medicijnkosten.nl).
Uncertainty and scenario analyses
To account for lower medication uptake as seen in real-life practice, shown in the Dutch national adherence monitor,40 scenarios with different extents of medication adherence were assessed, including scenarios with 40%, 60%, 80%, and 100% medication adherence.
Results
Cost-effectiveness
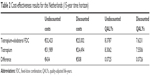
Base-case analyses, using our best estimates for each input variable, were performed first. In Table 2, the per-patient results of the base-case cost-effectiveness analysis are shown, based on the 15-year time horizon. Patients on tiotropium–olodaterol FDC gained 8.38 QALYs at an expense of €32,423. Patients on tiotropium gained 8.31 QALYs at an expense of €31,989.
  | Table 2 Cost-effectiveness results for the Netherlands (15-year time horizon) |
Taking into account discounting over this 15-year time horizon, an ICER of €7,004/QALY results; without discounting the ICER is €5,981/QALY.
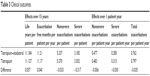
In Table 3, the clinical outcomes are shown. Over a 15-year time horizon, treatment with tiotropium–olodaterol FDC resulted in slightly better clinical outcomes including life-years gained and prevented exacerbations of all types. Annually, treatment with tiotropium–olodaterol FDC resulted in the prevention of 0.035 exacerbations (0.03 severe and 0.006 nonsevere) per patient.
  | Table 3 Clinical outcomes |
Sensitivity analyses
In Table 4, univariate sensitivity analyses are shown for extreme values of the model. Results seem robust in several sensitivity analyses.
In Figure 1, the PSA is shown. Treatment with tiotropium–olodaterol, as well as tiotropium alone, resulted in both cost increases and QALY gains, although differences were marginal. Additionally, a cost-effectiveness acceptability curve was constructed (Figure 2).
  | Figure 1 Probabilistic sensitivity analysis (100 iterations). |
  | Figure 2 Cost-effectiveness acceptability curves. |
As shown, tiotropium–olodaterol FDC is cost-effective at the lowest – so far – suggested Dutch cost-effectiveness threshold of €20,000.41 The probability of being cost-effective at that threshold is 61.4%. The probability would slightly increase to 65.3% at higher thresholds such as at €30,000,22 but remains stable despite higher thresholds.
Budget impact
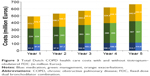
In Figure 3, the total yearly national Dutch costs of COPD treatment over a time horizon up to 5 years is presented for a scenario without (left part of each column) and with (right part of each column) tiotropium–olodaterol, assuming 60% medication adherence. Mainly due to expected rise in number of COPD patients, total COPD costs are increasing from €489.1 million without tiotropium–olodaterol (€489.2 with) in 2015 to €663.2 without tiotropium–olodaterol (€664.1 with) million in 2019. As shown, introducing tiotropium–olodaterol will be responsible for less than 1% of the total increase in COPD expenses.
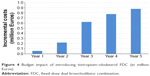
In Figure 4, the incremental budget impact of introducing tiotropium–olodaterol FDC over a time horizon of 5 years is presented. Due to expected rise in number of COPD patients and increased market share projections (ranging from 0.6% of COPD patients that require long-acting bronchodilators on tiotropium–olodaterol in year 1 to 7.7% in year 5, see Table S4), incremental costs are increasing from €55,288 in 2015 to €877,641 in 2019 (summing up to €2.6 million over 5 years).
  | Figure 4 Budget impact of introducing tiotropium–olodaterol FDC (in million Euros). |
Scenarios with 40%, 80%, and 100% medication adherence resulted in lower or higher 5-year incremental cost increases of €1.7, €3.4, and €4.3 million, respectively.
Discussion
As a result of cost increases, combined with QALY gains, results showed that tiotropium–olodaterol FDC had an ICER of €7,004 per QALY. Without discounting, the ICER was €5,981 per QALY. Results were robust in univariate and probabilistic sensitivity analyses. Budget impact was estimated at €2.6 million over 5 years, assuming 60% adherence. Scenarios with 40%, 80%, and 100% adherence resulted in lower or higher 5-year incremental cost increases of €1.7, €3.4, and €4.3 million, respectively.
In a previous study, the cost-effectiveness of the mono-component tiotropium was favorable.12 In addition, a more recent economic evaluation of another fixed-dose LABA–LAMA (umeclidinium–vilanterol), with tiotropium as comparator, concluded that the new treatment was considered cost-effective in the Spanish setting with an estimated ICER of €21,475.22 This ICER was higher than in our study, but note that a shorter time horizon of 3 years was used and indeed shorter time horizons may not capture the full economic benefits, as shown in our sensitivity analyses. In a cost-effectiveness analysis of another dual bronchodilator, indacaterol–glycopyrronium FDC was not compared with tiotropium, but with both its mono-components and with salmeterol–fluticasone, and was found cost-saving in the Swedish health care setting.42 Yet, due to the choice of these different comparators, which may not be considered usual care, and the inclusion of pneumonia as side effect of ICS use, results are not directly comparable to our study.
In 2007, the Dutch report of the National Institute of Public Health (RIVM)43 reported that €415 million was spent on COPD treatment and 36% comprised medication. The estimated total burden of COPD in our model was €550 million in 2014 and 28% comprised medication. Hence, our burden estimate was slightly higher, while the part due to medication was somewhat lower. Differences in total expenses are likely due to methodological differences and the year of calculation. Indeed, the RIVM expects an increase in number of COPD patients from about 350,000 currently to 600,000 in 2032 and then our expenses are in line with future projections.43 In addition, RIVM calculations were based on prescription data, while up to 10%–15% of respiratory medication was actually never dispensed.44 Notably, when real-world medication adherence measures were applied in our study, medication expenses were considerably lower. The assumption of 60% adherence is well in line with adherence reported in Dutch national data over the period 2007–2014 (60%–64%),40 confirming the validity of our base-case budget impact assumption on adherence.
This is the first full economic evaluation of tiotropium–olodaterol FDC for the Netherlands. It is based on the best currently available evidence, a validated model, and includes extensive uncertainty analyses. In addition, exacerbation risk, a main cost driver, was calculated using a real-life approach. While some previously published models in COPD have estimated the risk of both severe and moderate exacerbations by treatment as well as by GOLD stage,29,45 other economic evaluations have estimated overall exacerbation risk by treatment only46 or estimated the proportion of severe to nonsevere exacerbations by GOLD stage.47 These approaches have limitations in that the probability of exacerbations is not affected by having experienced a previous exacerbation, while a history of exacerbations has been shown to be the most reliable predictor for future exacerbations.19,23 Yet, some potential limitations have to be noted. In the cost-effectiveness analyses, adverse events from bronchodilators were not incorporated, although these side effects are considered of only minor importance and likely to be equally prevalent among comparators. In addition, differential rates of treatment switch or add-on treatments were not considered; however, these were assumed equally plausible. The same goes for the extent of treatment discontinuation. Finally, relatively long-term projections of 15 years are naturally surrounded by uncertainty regarding new treatments and health care policies.
Based on current Dutch thresholds, the cost-effectiveness ratio of tiotropium–olodaterol FDC in the Netherlands is generally favorable. However, other factors than solely cost-effectiveness ratios may play a role in recommendations regarding reimbursement and implementation in clinical guidelines. From a clinical perspective, one should take into account that once-daily therapies are associated with increased adherence48,49 and that nonadherence to COPD medication is associated with decreased clinical and economic outcomes.50 Moreover, when two inhaled drugs are indicated, the use of a single inhaler is recommended in order to avoid confusion and inhalation technique errors.51 In choosing the optimal inhaler, patient’s needs and preferences should not be overlooked.
Regarding future research, it is recommended to assess the effectiveness and cost-effectiveness of tiotropium–olodaterol and other FDC LAMA–LABAs in real-life studies, including their effects on patient-reported outcomes.
Conclusion
Tiotropium–olodaterol FDC can be considered a cost-effective treatment under currently suggested Dutch cost-effectiveness thresholds.
Disclosure
This study was funded by Boehringer Ingelheim. The institutions of JVB, JWK, and MJP (University of Groningen) has received research grants from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, and Novartis, all manufacturers of respiratory drugs. JVB received consultancy fees from AstraZeneca and travel support from the Respiratory Effectiveness Group and the European COPD Coalition. JWK received several research grants and has been paid for consultancy services by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. MJP received grants and honoraria from various pharmaceutical companies, including those developing, producing, and marketing COPD drugs. The authors report no other conflicts of interest in this work.
References
Global strategy for the diagnosis, management and prevention of COPD, Global Initiative for chronic Obstructive Lung Disease (GOLD) 2015. Available from: http://www.goldcopd.org/. Accessed December 22, 2015. | ||
Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet. 1997;349(9064):1498–1504. | ||
van Gemert F, Kirenga B, Chavannes N, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health. 2015;3(1):e44–e51. | ||
Dale PR, Cernecka H, Schmidt M, et al. The pharmacological rationale for combining muscarinic receptor antagonists and beta-adrenoceptor agonists in the treatment of airway and bladder disease. Curr Opin Pharmacol. 2014;16:31–42. | ||
ZuWallack R, Allen L, Hernandez G, Ting N, Abrahams R. Efficacy and safety of combining olodaterol Respimat((R)) and tiotropium HandiHaler((R)) in patients with COPD: results of two randomized, double-blind, active-controlled studies. Int J Chron Obstruct Pulmon Dis. 2014;9:1133–1144. | ||
Barnes PJ. Inhaled corticosteroids in COPD: a controversy. Respiration. 2010;80(2):89–95. | ||
Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829. | ||
van Boven JF, de Jong-van den Berg LT, Vegter S. Inhaled corticosteroids and the occurrence of oral candidiasis: a prescription sequence symmetry analysis. Drug Saf. 2013;36(4):231–236. | ||
Price D, Yawn B, Brusselle G, Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22(1):92–100. | ||
Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. | ||
Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP; UPLIFT investigators. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374(9696):1171–1178. | ||
Hoogendoorn M, Al MJ, Beeh KM, et al. Cost-effectiveness of tiotropium versus salmeterol: the POET-COPD trial. Eur Respir J. 2013;41(3):556–564. | ||
Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. | ||
Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat(R) versus placebo and formoterol twice daily in patients with GOLD 2–4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:697–714. | ||
Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat(R) in patients with GOLD 2–4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629–645. | ||
Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4). Eur Respir J. 2015;45(4):969–979. | ||
Sauer R, Hansel M, Buhl R, Rubin RA, Frey M, Glaab T. Impact of tiotropium + olodaterol on physical functioning in COPD: results of an open-label observational study. Int J Chron Obstruct Pulmon Dis. 2016;11:891–898. | ||
Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319. | ||
Selya-Hammer C, Gonzalez-Rojas Guix N, Baldwin M, et al. Development of an enhanced health-economic model and cost-effectiveness analysis of tiotropium + olodaterol Respimat® fixed-dose combination for chronic obstructive pulmonary disease patients in Italy. Ther Adv Respir Dis. Epub 2016 Jul 12. | ||
Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. | ||
van Boven JF, Vegter S, van der Molen T, Postma MJ. COPD in the working age population: the economic impact on both patients and government. COPD. 2013;10(6):629–639. | ||
Geijer RM, Tuut MK, in’t Veen JC, Broekhuizen BD, Chavannes NH, Smeele IJ. The NHG guidelines “Adult asthma” and “COPD”. Ned Tijdschr Geneeskd. 2015;159:A9076. | ||
Miravitlles M, Galdiz JB, Huerta A, Villacampa A, Carcedo D, Garcia-Rio F. Cost-effectiveness of combination therapy umeclidinium/vilanterol versus tiotropium in symptomatic COPD Spanish patients. Int J Chron Obstruct Pulmon Dis. 2016;11:123–132. | ||
Donaldson GC, Wedzicha JA. COPD exacerbations. 1. Epidemiology. Thorax. 2006;61(2):164–168. | ||
Tashkin DP, Li N, Halpin D, et al. Annual rates of change in pre- vs. post-bronchodilator FEV1 and FVC over 4 years in moderate to very severe COPD. Respir Med. 2013;107(12):1904–1911. | ||
Tashkin DP. Variations in FEV(1) decline over time in chronic obstructive pulmonary disease and its implications. Curr Opin Pulm Med. 2013;19(2):116–124. | ||
Wise RA, Anzueto A, Calverley P, et al. The Tiotropium Safety and Performance in Respimat Trial (TIOSPIR), a large scale, randomized, controlled, parallel-group trial-design and rationale. Respir Res. 2013;14:40. | ||
Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369(16):1491–1501. | ||
van Boven JF, Tommelein E, Boussery K, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respir Res. 2014;15:66. | ||
Rutten-van Molken MP, Oostenbrink JB, Miravitlles M, Monz BU. Modelling the 5-year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ. 2007;8(2):123–135. | ||
Rutten-van Molken MP, Oostenbrink JB, Tashkin DP, Burkhart D, Monz BU. Does quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages? Chest. 2006;130(4):1117–1128. | ||
Spencer S, Jones PW; GLOBE Study Group. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax. 2003;58(7):589–593. | ||
Paterson C, Langan CE, McKaig GA, et al. Assessing patient outcomes in acute exacerbations of chronic bronchitis: the measure your medical outcome profile (MYMOP), medical outcomes study 6-item general health survey (MOS-6A) and EuroQol (EQ-5D). Qual Life Res. 2000;9(5):521–527. | ||
Health Care Insurance Board. Dutch Pharmacoeconomic Guidelines [in Dutch]. Available from: http://www.zorginstituutnederland.nl/binaries/content/documents/zinl-www/documenten/publicaties/publications-in-english/2006/0604-guidelines-for-pharmacoeconomic-research/0604-guidelines-for-pharmacoeconomic-research/Guidelines+for+pharmacoeconomic+research.pdf. Accessed July 24, 2015. | ||
Costa-Scharplatz M, Stallberg B, Goyal P, Asukai Y, Gruenberger JB, Price D. Cost-effectiveness of glycopyrronium bromide compared with tiotropium in patients with chronic obstructive pulmonary disease in Sweden. Appl Health Econ Health Policy. 2015;13(6):637–645. | ||
Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. New York: Oxford University Press; 2006. | ||
Dutch National Health Compass. Available from: http://www.nationaalkompas.nl/object_binary/o20019_RP-COPD-2013.xls. Accessed February 22, 2016. | ||
Minas M, Hatzoglou C, Karetsi E, et al. COPD prevalence and the differences between newly and previously diagnosed COPD patients in a spirometry program. Prim Care Respir J. 2010;19(4):363–370. | ||
Hoogendoorn M, Feenstra TL, Schermer TR, Hesselink AE, Rutten-van Molken MP. Severity distribution of chronic obstructive pulmonary disease (COPD) in Dutch general practice. Respir Med. 2006;100(1):83–86. | ||
Dutch medication adherence monitor. Available from: http://www.therapietrouwmonitor.nl/cijfers/astmacopd-7. Accessed January 26, 2016. | ||
Atthobari J, Asselbergs FW, Boersma C, et al. Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: A pharmacoeconomic analysis linked to the prevention of renal and vascular endstage disease (PREVEND) study and the prevention of renal and vascular endstage disease intervention trial (PREVEND IT). Clin Ther. 2006;28(3):432–444. | ||
Price D, Keininger D, Costa-Scharplatz M, et al. Cost-effectiveness of the LABA/LAMA dual bronchodilator indacaterol/glycopyrronium in a Swedish healthcare setting. Respir Med. 2014;108(12):1786–1793. | ||
Suijkerbuijk AW, de Wit GA, Wijga AH, et al. Societal costs of asthma, COPD and respiratory allergy. Ned Tijdschr Geneeskd. 2013;157(46):A6562. | ||
Ekedahl A, Mansson N. Unclaimed prescriptions after automated prescription transmittals to pharmacies. Pharm World Sci. 2004;26(1):26–31. | ||
Oostenbrink JB, Rutten-van Molken MP, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health. 2005;8(1):32–46. | ||
Price D, Gray A, Gale R, et al. Cost-utility analysis of indacaterol in Germany: a once-daily maintenance bronchodilator for patients with COPD. Respir Med. 2011;105(11):1635–1647. | ||
Price D, Asukai Y, Ananthapavan J, Malcolm B, Radwan A, Keyzor I. A UK-based cost-utility analysis of indacaterol, a once-daily maintenance bronchodilator for patients with COPD, using real world evidence on resource use. Appl Health Econ Health Policy. 2013;11(3): 259–274. | ||
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. | ||
Toy EL, Beaulieu NU, McHale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. | ||
van Boven JF, Chavannes NH, van der Molen T, Rutten-van Molken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103–113. | ||
van der Palen J, Klein JJ, van Herwaarden CL, Zielhuis GA, Seydel ER. Multiple inhalers confuse asthma patients. Eur Respir J. 1999;14(5):1034–1037. | ||
Hettle R, Wouters H, Ayres J, et al. Cost-utility analysis of tiotropium versus usual care in patients with COPD in the UK and Belgium. Respir Med. 2012;106(12):1722–1733. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.