Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Cost-Effectiveness and Budget Impact Analyses of Tisagenlecleucel in Pediatric and Young Adult Patients with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia from the Singapore Healthcare System Perspective
Authors Wang XJ, Wang YH, Ong MJC , Gkitzia C, Soh SY , Hwang WYK
Received 23 December 2021
Accepted for publication 7 April 2022
Published 3 May 2022 Volume 2022:14 Pages 333—355
DOI https://doi.org/10.2147/CEOR.S355557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Xiao Jun Wang,1 Yi-Ho Wang,2 Matthew Jian Chun Ong,1 Christina Gkitzia,3 Shui Yen Soh,4 William Ying Khee Hwang5– 7
1Novartis Singapore Pte Ltd, Singapore; 2Novartis Asia Pacific Pharmaceuticals Pte Ltd, Singapore; 3Novartis Pharma AG, Basel, Switzerland; 4KK Women’s & Children’s Hospital, Singapore; 5National Cancer Centre Singapore, Singapore; 6Singapore General Hospital, Singapore; 7Duke-NUS Medical School, Singapore
Correspondence: Xiao Jun Wang, Novartis Singapore Pte Ltd, 20 Pasir Panjang Road, #10-25/28 Mapletree Business City (West Tower), 117439, Singapore, Tel +65 67226010, Email [email protected]
Purpose: Children and young adults with relapsed or refractory (r/r) acute lymphoblastic leukemia (ALL) have poor survival due to ineffective therapy options. The newly approved chimeric antigen receptor T-cell (CAR-T) therapy, tisagenlecleucel, has demonstrated improved survival but at a high up-front cost. The study aims to evaluate the cost-effectiveness and budget impact of tisagenlecleucel versus salvage chemotherapy regimen (SCR) or blinatumomab (BLN) for the treatment of pediatric and young adult patients with relapsed/refractory B-cell ALL from the Singapore healthcare system perspective.
Patients and Methods: A three-health state partitioned survival model was constructed to analyze the cost-effectiveness of tisagenlecleucel vs SCR/BLN with/without allogenic hematopoietic stem cell transplantation (allo-HSCT) over a lifetime period. Clinical efficacy for tisagenlecleucel, SCR and BLN were based on pooled data from ELIANA, ENSIGN and B2101J trials, the study by von Stackelberg et al 2011, and MT103-205 respectively. Medical costs from pre-treatment until terminal care, including treatment, side effects, follow-up, subsequent allo-HSCT and relapse, were considered. Incremental cost-effectiveness ratios (ICERs) were estimated as the incremental costs per quality-adjusted life-year (QALY) gain. Additionally, the financial impact of tisagenlecleucel introduction in Singapore was estimated, comparing the present treatment scenario (without tisagenlecleucel) with a future scenario (with tisagenlecleucel), over 5 years.
Results: In the base-case analysis, tisagenlecleucel treatment demonstrated cost-effectiveness with an ICER of S$45,840 (US$34,762) per QALY (vs SCR) and S$51,978 (US$39,315) per QALY (vs BLN). The estimated budget ranges from S$477,857 (US$361,438) to S$1.4 million (US$1.05 million) annually for the initial 5 years.
Conclusion: Tisagenlecleucel is likely to be a cost-effective treatment option with limited budget implications while treating r/r ALL patients who have failed at least 2 lines of prior therapies.
Keywords: tisagenlecleucel, acute lymphocytic leukemia, partition survival model, cost-effectiveness, budget impact, Singapore
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy. It accounts for approximately 25% of all cancers and 75–80% of all leukemias among children1 and is the leading cause of cancer-related deaths among children.2 In about 80–85% children, ALL starts in precursor B-cells while in remaining 15%–20%, it starts in T-cells.3 In Singapore, the age-standardized incidence rate of childhood ALL is reported to be 3.78 per 100,000 person-years.4
Although the overall cure rate of ALL in children reaches 80% to 85% with conventional frontline chemotherapy,5,6 approximately 20% of patients who respond eventually relapse.7 In relapsed patients, overall response rate (ORR) to a second-line therapy is about 85% which decreases to <50% after subsequent therapeutic attempts.8 The 10-year survival probability for patients with multiple relapses is reported to be <10%.9 In addition 2 to 3% of patients are refractory and remain unresponsive to initial induction treatment. Effectively treating such relapsed or refractory (r/r) ALL patients can be challenging.10
Blinatumomab (BLN) and a salvage chemotherapy regimen (SCR) consisting of fludarabine, cytarabine, idarubicin and granulocyte colony-stimulating factor (FLAG-IDA) are the two treatment options approved by the Health Sciences Authority (HSA) for r/r ALL among pediatric and young adults in Singapore. However, outcomes with these treatments are sub-optimal and they are primarily used to bridge to allogeneic hematopoietic stem cell transplantation (allo-HSCT)11,12 contributing to high additional costs.13,14 Although allo-HSCT is a potentially curative treatment option for pediatric and young adults with r/r ALL, it is limited by eligibility requirements. About 50% of the patients do not receive allo-HSCT either because of non-availability of a matched donor or failure to achieve complete remission.15 The outcomes of allo-HSCT are sub-optimal [5-year overall survival (OS): 45–70%] and there is high non-relapse mortality (10–20%), and potentially lethal adverse events (AEs).16–19
In March 2021, Singapore approved tisagenlecleucel to treat pediatric and young adult patients from 2 years up to, and including, 25 years of age with B-cell ALL that is refractory, in relapse post-transplant or in second or later relapse.20 Tisagenlecleucel is a single-dosed chimeric antigen receptor T-cell (CAR-T) therapy which offers pediatric and young adult r/r ALL patients hope for a potential cure. Tisagenlecleucel achieved its primary endpoint in the pivotal ELIANA study with 82.3% ORR at 3 months after tisagenlecleucel infusion.21 With a follow-up of 38.8-month, tisagenlecleucel has demonstrated durable responses, with duration of response of 57.9% at 24 months and 52.2% at 36 months. OS at 24 months and 36 months was 67.7% and 62.8% respectively.21 The real-world evidence has supported the efficacy outcomes with patients experiencing lesser AEs than that demonstrated in the clinical studies.22–26
Despite promising clinical results, initial upfront costs may deter healthcare payers as cost-effectiveness and budget impact of tisagenlecleucel for this indication in Singapore is unknown. Previous cost-effectiveness and budget impact of tisagenlecleucel in Singapore has been limited to adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL)27 and cannot be extrapolated to the r/r ALL population.
Survival benefit with tisagenlecleucel is greater among r/r ALL patients than r/r DLBCL patients (24 months: 40.0%, and 36 months: 37.6%). The younger population of r/r ALL patients is also expected to accrue greater benefits from the potentially curative therapy over a longer projected lifespan (88 years in r/r ALL vs 44 years in r/r DLBCL). Due to these differences in survival, cost-effectiveness is expected to differ significantly for each indication. Budget impact from public funding for ALL population is also anticipated to be much lesser as only 5–7 patients are likely to be eligible for tisagenlecleucel each year in comparison with 28–34 DLBCL patients.
At present, r/r ALL patients face dismal survival with currently available therapeutic options. The introduction of tisagenlecleucel with likely extended survival may add significant value for this patient group. Therefore, it is crucial for payers and clinicians to understand how these survival benefits translate into economic benefits to inform resource allocation decisions. This study aims to evaluate the cost-effectiveness and budget impact of tisagenlecleucel for the treatment of pediatric and young adult patients with B-cell ALL that is refractory, in relapse post-transplant or in second or later relapse from the Singapore healthcare system perspective.
Materials and Methods
Cost-Effectiveness Analysis
Model Outline
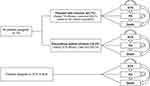
A cohort-based partitioned survival model (PSM) was developed in Microsoft Excel to analyze the cost-effectiveness of tisagenlecleucel vs SCR and BLN with or without allo-HSCT among pediatric and young adult patients with r/r ALL in Singapore. The PSM was selected as it facilitates the replication of within-trial data by allowing OS and progression-free survival (PFS) modelling based on study-observed events.28
In this model, the patients could be distributed across the three mutually exclusive health states: event-free survival (EFS), progressive disease (PD), and death (Figure 1). At model initiation, the whole cohort was assigned to the EFS state. During each cycle, patients were redistributed among the three health states. The probabilities of patients remaining in the EFS state were matched with the EFS curve for each treatment arm, while the probabilities of being in the PD state were estimated as the difference between the OS and EFS curves. Half cycle correction was not employed due to the relatively short monthly cycle of 30.44 days. Subsequent allo-HSCT was not considered as an independent health state as allo-HSCT associated health benefits are captured by PFS and OS for each treatment.
During the tisagenlecleucel trials,16.3% of enrolled patients did not receive tisagenlecleucel infusion due to several reasons (eg, manufacturing failure, withdrawal due to AEs, or death).21,29,30 To account for the possibility of infusion failure, in the tisagenlecleucel arm, a decision-tree was incorporated prior to PSM entry (Figure 1). In the comparator arms, patients proceeded directly to the PSM. The patients’ baseline characteristics (eg, age, gender distribution, average body weight and average body-surface area) were based on tisagenlecleucel infused patients in the ELIANA study.21 Taking reference from previous evaluations accepted by international HTA agencies,31–33 monthly cycles over a lifetime horizon (88 years) were modelled to comprehensively capture all health outcomes and costs over the patient’s remaining lifespan after the treatment initiation.
Only direct public healthcare costs with 3% annual discount were considered.34 In the tisagenlecleucel arm, 16.3% of the patients who failed to receive the infusion were assumed to eventually receive SCR. Hence, SCR associated costs were applied to this patient population in the tisagenlecleucel arm. Total costs were estimated distinctly for tisagenlecleucel, SCR and BLN as the aggregate of pre-treatment, drug, procedure, drug administration, hospital stay, AEs, EFS, relapse, subsequent allo-HSCT, and terminal care costs.
In the tisagenlecleucel arm, as efficacy was estimated from the time of infusion, an average time of 1.73 months from enrollment to tisagenlecleucel infusion was factored into life years (LYs) and quality-adjusted life years (QALYs) calculations. Total LYs and QALYs were summed up separately for the three treatment arms, and incremental cost-effectiveness ratios (ICERs) were determined as the total incremental costs per LY and per QALY gain.
Due to the absence of Singapore specific published willingness-to-pay (WTP) threshold, the World Health Organization (WHO) recommended thresholds were used to determine the cost-effectiveness of tisagenlecleucel.35 A treatment option was determined either as dominant (cost saving with QALY gain), highly cost-effective (ICER less than Singapore’s gross domestic product [GDP] per capita [S$88,991 in 2019]),36 cost-effective (ICER less than 3 times of GDP per capita [S$266,973]) or not cost-effective (ICER more than or equal to 3 times of GDP per capita). Using the currency conversion rate of US$1 = S$1.322137, final results are reported both in terms of S$ and US$.
Model Inputs
Efficacy Inputs
Efficacy determinants (OS and EFS) for tisagenlecleucel-infused patients were based on pooled data from the ELIANA (NCT02435849, data cut-off: 07/01/2019), ENSIGN (NCT02228096, data cut-off: 05/24/2019), and B2101J (NCT01626495, data cut-off: 05/07/2018) studies.21,29,30 Data pooling increased the sample size and reduced the uncertainty. Based on the infusion rate in the pooled trial data, 83.7% of tisagenlecleucel arm patients successfully received tisagenlecleucel infusion.21,29,30 The observed OS and EFS trial data, based on intent-to-treat (ITT), were used to model until year 3. In this model, a 3-year cure-point was adopted for this analysis based on published literature41 and validation from local clinical experts. Patients alive at the end of year 3 across all treatment groups were considered long-term ALL survivors with the same mortality risk. A common long-term death probability, derived from Singapore’s lifetable and published standard mortality ratio (SMR) for ALL survivors, was applied after year 3.42,43 (Figure 2).
 |
Figure 2 Estimated comparative OS and EFS. Abbreviations: BLN, blinatumomab; SCR, salvage chemotherapy regimen; TIS, tisagenlecleucel. |
SCR and BLN efficacy were determined from the study by von Stackelberg et al 2011 11 and MT103-205 (NCT01471782) 12,38 respectively. OS data were derived from the Kaplan-Meier curves published in the relevant SCR 11 and BLN.12 An established algorithm was then applied to obtain pseudo patient level data for extrapolation of OS.39 The number at risk and number of event information were incorporated into the reconstruction of individual patient data where available. OS extrapolation beyond the trial period and until year 3, was performed via parametric extrapolation using the weighted akaike information criterion (AIC) approach. As a single survival distribution might inadequately characterize the true efficacy of the treatment, a model averaging approach was used to account for the uncertainty associated with choosing one specific survival distribution. Weights were calculated based on AIC score using the following equation: Weight = Ak/(∑Ak), where Ak = e-(0.5×AIC). Since no published EFS data for SCR and BLN were available, EFS data until year 3 was derived from OS data by applying a constant cumulative hazard ratio (HR) between OS and EFS over time. The HR was estimated from the mitoxantrone arm in the UK ALL study.40 Similar to the tisagenlecleucel arm, no further progression events were considered after year 3 (EFS curve flattens up until it reaches the OS curve) and SMR adjusted mortality was applied beyond year 3 (Figure 2).
Healthcare Resource Utilization and Costs
Healthcare costs considered include pre-treatment, treatment, AEs, follow-up (event-free and relapse), subsequent allo-HSCT, and terminal-care costs (Tables 1–3). Drug and administration costs were retrieved from data published by the Singapore government.34 Remaining costs were attained either from private hospitals or clinical experts. Costs obtained from private hospitals were converted to their expected costs in public healthcare institutes using a conversion factor of 2.18 that was derived from the ratio of available procedures/treatments costs in public and private settings.
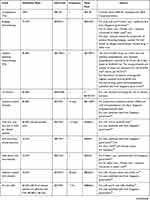 |  |  |
Table 1 Cost and Resource Utilization Model Inputs for TIS |
 |  |
Table 2 Cost and Resource Utilization Model Inputs for BLN |
 |
Table 3 Cost and Resource Utilization Model Inputs for SCR |
Patients in the tisagenlecleucel arm incurred leukapheresis, bridging chemotherapy (BC), and lymphodepleting chemotherapy (LDC) as the pre-treatment costs in the first monthly cycle. All the tisagenlecleucel arm patients were presumed to incur leukapheresis costs, irrespective of the infusion status. 90.5% of the tisagenlecleucel-infused patients were considered for BC (based on the pooled data from tisagenlecleucel trials).21,29,30 All the successfully tisagenlecleucel infused patients (83.7%) were considered to incur the cost for LDC. Leukapheresis cost was calculated as per clinician advice, while BC cost was considered as the sum of drug and its administration cost for single cycle of SCR. The LDC included drug, administration and inpatient hospitalization costs. The dosing schedule and number of doses were based on the ELIANA trial and NCCN guidelines.21,44 Inpatient hospitalization was required by 67.1% patients for an average 20.15 days based on the ELIANA trial data.21 Patients who did not receive tisagenlecleucel infusion were assumed to be managed with SCR and did not receive any BC.
Treatment costs were based on year 2020 and included costs associated with drug acquisition or procedure, drug administration, hospital and intensive care unit (ICU) stay. One-time cost for tisagenlecleucel preparation and infusion was charged to the Singapore government. The proportion of patients requiring non-CRS-related ICU stay, and their respective length of stay (LOS) after LDC until 60 days post tisagenlecleucel infusion were based on the ELIANA trial and Singapore’s tisagenlecleucel label.21 The SCR cost was estimated based on the salvage regimen cytarabine, fludarabine, idarubicin and G-CSF (FLAG-IDA). The dosage and treatment duration of the four chemotherapeutic agents in FLAG-IDA were based on NHS Network Site Specific Group protocol45 and further validated by local clinicians. Patients were assumed to receive FLAG-IDA in the inpatient setting with 28 days average LOS, based on the advice of Singapore’s clinicians and Gaynon et al, 2006.46,47 The BLN (drug and administration) costs were estimated based on the dosing schedule and treatment duration reported in the HSA Blincyto Product Information.48 The model assumed that all patients would complete 5 cycles (out of 9 recommended cycles) and accrue administration and hospitalization costs. The daily inpatient stay cost for SCR/BLN was assumed to be the same as that for tisagenlecleucel.
For both tisagenlecleucel and BLN treated patients, grade 3 and 4 CRS management cost was further estimated as the aggregate of the ICU stay, tocilizumab acquisition and its administration cost. The incidence of CRS among tisagenlecleucel and BLN treated patients were obtained from the ELIANA trial and von Stackelberg et al 2016, respectively.12,21 The average ICU LOS (11.10 days) and tocilizumab dosing (1.24 dose) were taken from the ELIANA trial21 and applied to both tisagenlecleucel and BLN treated patients.
Similarly, the cost associated with management of low immunoglobulin levels due to B-cell aplasia was estimated for both tisagenlecleucel and BLN treated patients. The IVIG utilization rate was obtained from the ELIANA study and von Stackelberg et al 2016 for tisagenlecleucel and BLN, respectively.12,21 The average number of IVIG doses was obtained from the ELIANA study while the monthly dose of 400mg/kg, amounting to 6 vials of 3000 mg each, was based on local clinicians’ advice. Other grade 3 and 4 AEs with occurrence rate of ≥5% in any of the arms were included as one-time costs. The AE rates were obtained from the ELIANA trial for tisagenlecleucel, Raetz et al 2008 for SCR and von Stackelberg et al 2016 for BLN.12,21,49
Further, subsequent allo-HSCT within one year of follow-up was considered for patients who subsequently needed it. The subsequent allo-HSCT rate was derived from the pooled ELIANA, B2101J, and ENSIGN,21,29,30 von Stackelber et al 2011,11 and Gore et al 2018 38 for tisagenlecleucel, SCR and BLN, respectively. The subsequent allo-HSCT rate among tisagenlecleucel infused patients was estimated to be 17.5%, with SD of 0.0269 and 95% CI of 17.1% to 17.9%. Patients who subsequently went for allo-HSCT incurred costs from procedures, including stem cell harvesting, 12 months follow-up after transplant, and associated AEs. The AE rates were determined as per local clinicians’ advice.
Monthly follow-up costs varied according to the health state (EFS or PD), treatment type (tisagenlecleucel, SCR or BLN), and duration after the treatment completion. The follow-up frequency for tisagenlecleucel and SCR/BLN treated patients in the EFS state was derived from the ELIANA study protocol21 and Singapore clinicians’ advice, respectively. Monthly PD follow-up cost was considered following disease progression until death, except a month before death. Due to lack of published data about follow-up of relapsed ALL among children and young adults, the follow-up frequency for PD state was assumed to be double that for EFS state with SCR/BLN treatment for the first year and was assumed to be the same across all the comparator arms. However, the final cost estimation was validated by local Singapore clinicians. All patients who died were assumed to incur one-time terminal care cost, derived from Phua et al, 2020.50
Utility Inputs
The utilities for health states, and disutilities associated with treatment, specific AEs, as well as subsequent treatment modalities were retrieved from the published literature (Table 4). Utility values were based on health state rather than treatment arm. Health state utilities were derived from the ELIANA trial using a mapping algorithm for the South Korean population21,51 The model also considered additional age-related decrements as the population became older over the time-horizon.52
 |
Table 4 Utility Inputs |
The treatment disutilities were fetched from Sung et al,53 and were applied for the duration derived from Singapore’s tisagenlecleucel label, Raetz et al 2008 and HSA blincyto product information for tisagenlecleucel, SCR and BLN, respectively.48,49 These disutility estimates were assumed to capture the utility decrements for all short-term AEs except CRS. Additional disutilities associated with CRS related ICU stay were considered for patients with grades 3 or 4 CRS. The CRS rate was derived from the ELIANA trial21 and von Stackelberg et al, 201612 for tisagenlecleucel and BLN, respectively. For the tisagenlecleucel arm, an additional treatment disutility was also considered for ICU stays not due to CRS. A disutility of 0.85 (based on complete remission utility) for the duration of the CRS or non-CRS related ICU stay was derived from the ELIANA study.21 Patients with subsequent allo-HSCT were considered for an additional disutility, obtained from Sung et al. 2003.53 Because Sung et al, 2003 did not report duration associated with the reported disutility estimates, the disutility for allo-HSCT was assumed to last for one year after the treatment initiation in order to be consistent with the NICE mock appraisal.54
Sensitivity Analyses
To assess the sensitivity of the cost-effectiveness model to particular input uncertainties, deterministic sensitivity analyses (DSAs) were performed by changing one parameter at a time while holding all others unchanged. Clinical efficacy, subsequent allo-HSCT, utilities, and cost inputs were modified by the 95% confidence interval or the range, if reported. Otherwise, efficacy and utility inputs were varied by ±10%, and cost inputs by ±25% from the base case inputs.
Additionally, Monte-Carlo simulations with 1000 iterations was used to perform probabilistic sensitivity analysis (PSA). The simulation technique determined the cost-effectiveness probability of tisagenlecleucel vs SCR/BLN with or without allo-HSCT, against multiple willingness-to-pay (WTP) thresholds. PSA applied specific data distributions to simultaneously modify all important inputs influencing patient characteristics, efficacy, utility/disutility, subsequent allo-HSCT, and costs (Table 5). Results were illustrated as cost-effectiveness planes and acceptability curves comparing tisagenlecleucel against SCR/BLN with or without allo-HSCT. The base-case results were further validated by using efficacy inputs based on alternative parametric functions, different utility values, multiple alternative modeling scenarios and varied time horizon.
 |
Table 5 Probabilistic Sensitivity Analysis Inputs |
Budget Impact Analysis
A budget impact model (BIM) was also constructed to analyze the net budgetary impact of introducing tisagenlecleucel on Singapore’s healthcare system. The present treatment landscape (without tisagenlecleucel) was compared to a future scenario (with tisagenlecleucel) over a period of 5 years.
According to the clinicians’ advice, the estimated ALL (aged 2 to 25 years) incidence in Singapore is 48 (base year) and estimated to reach 60 cases in year 5 with 4.7% yearly growth rate. This yearly growth rate was estimated as an average of the yearly growth rate of any cancer from year 2011 to year 2015 (4.9%, 4.3%, 2.5%, and 7.08%).55 About 2.5% and 12.5% of these incidence cases will have refractory and relapse status following first-line therapy.10 Further, approximately 60% of them are expected to have r/r status after second-line therapy.56 Therefore, 5–7 new cases are anticipated to be annually eligible for tisagenlecleucel treatment during initial 5 years.
In the current situation without tisagenlecleucel, BLN market share was assumed to grow gradually from 25% in the base year to 35% in the fifth year while the remaining patients were allocated to the SCR. After tisagenlecleucel approval in Singapore, the tisagenlecleucel market share was assumed to increase gradually from 15% in the first year to 50% in the fifth year. The remaining patients were allocated to BLN and SCR treatment in the ratio of 1:3.
The budget impact of tisagenlecleucel was evaluated assuming substitution of SCR/BLN as standard of care. Key cost factors for budget impact comprised of drug acquisition and its administration, associated AEs, additional work-up and hospitalization, follow-up and subsequent allo-HSCT, which were additional to standard disease management. Discounting was not applied in the BIM. Scenario analyses were subsequently performed by varying market share distribution, coefficient for private vs public costs, number of BLN cycles, and no subsequent allo-HSCT.
Results
Cost-Effectiveness Analysis
Base-Case Analysis
In the base-case analysis, tisagenlecleucel treatment resulted in mean incremental gain of 11.78 Lys and 9.87 QALYs (vs SCR) and 8.70 LYs and 7.50 QALYs (vs BLN) over a lifetime horizon. The LY and QALY benefits were achieved at an incremental cost of S$452,317 (US$342,120) (vs SCR) and S$389,679 (US$294,742) (vs BLN). These translated into ICERs of S$33,454 (US$25,304) per LY and S$45,840 (US$34,672) per QALY (vs SCR), and S$38,468 (US$29,096) per LY and S$51,978 (US$39,315) per QALY (vs BLN) (Table 6).
 |
Table 6 Summary of Cost and Benefits TIS vs SCR and TIS vs BLN |
Despite the upfront costs, tisagenlecleucel proved to be a cost-effective treatment strategy and was associated with a greater QALYs gain compared to BLN or SCR (Figure 3). Notably, upfront costs of tisagenlecleucel were also partially offset by costs arising from more drug administrations, longer hospitalization, and greater need for subsequent allo-HSCT in BLN and SCR treated patients. Hospitalization costs associated with drug administration (without consideration of AEs) were lower for tisagenlecleucel due to one-time drug infusion; in comparison to SCR which required a cumulatively longer hospital stay due to multiple admissions for drug administration. AE costs including that for AE associated hospitalization were separately estimated and are higher for tisagenlecleucel treatment due to more severe AEs. Though cost offset was more prominent against BLN due to its higher drug and associated administration cost, the ICER was more favorable when tisagenlecleucel was compared with SCR rather than BLN due to the relatively higher incremental QALY gain.
Sensitivity Analyses
DSA revealed that the base-case ICER (tisagenlecleucel vs SCR) was most sensitive to the cost of tisagenlecleucel treatment, time horizon, discount rate, utility value for the EFS health state, and subsequent allo-HSCT rate in the SCR arm (Figure 4A). At a deterministic ICER of S$45,840 (US$34,672), the ICER varied from S$34,871 (US$26,375) to S$56,809 (US$42,969) when the cost of tisagenlecleucel treatment was varied by ±25%. The ICER increased slightly to S$58, 850 (US$44,513) and S$74,868 (US$56,628) when the time horizon was shortened to 30 and 20 years respectively. The ICER also decreased to S$34,941 (US$26,428) and S$25,328 (US$19,157) with discount rates of 1.5% and 0% respectively. Variation in the utility for the EFS state within the reported 95% CI values also decreased the ICER from S$48,209 (US$36,464) to S$39,949 (US$30,216). The ICER was also moderately sensitive to subsequent allo-HSCT rate among SCR treated patients and ranged from S$47,891 (US$36,223) to S$43,816 (US$33,141) when the rate was varied within the reported 95% CI. The ICER was less sensitive to other variables. In essence, the model emerged to be most responsive to time related parameters, the cost of tisagenlecleucel treatment, and the subsequent allo-HSCT rate.
Likewise, in the case of tisagenlecleucel vs BLN, the ICER was most sensitive to the cost of tisagenlecleucel and BLN treatment, time horizon, discount rate and subsequent allo-HSCT rate in the BLN arm (Figure 4B). Compared to base-case ICER of S$51,978 (US$39,315), the ICER ranged between S$37,541 (US$28,395) and S$66,415 (US$50,234) and between S$48,886 (US$36,976) and S$55,070 (US$41,653) when tisagenlecleucel and BLN treatment cost were varied by ±25%, respectively. The ICER increased to S$66,799 (US$50,525) and S$85,029 (US$64,314) when the time horizon was shortened to 30 and 20 years respectively. The ICER also responded and decreased to S$39,386 (US$29,790) and S$28,218 (US$21,343) with discount rates of 1.5% and 0%, respectively. The ICER was also sensitive to subsequent allo-HSCT rate among BLN treated patients and ranged from S$49,873 (US$37,723) to S$54,112 (US$40,929) when the rate was varied within the reported 95% CI values. In summary, the model appeared most sensitive to the cost of both the treatment options compared, as well as time-dependent parameters.
The PSA results proved that ICERs are close to the base case deterministic result for both tisagenlecleucel vs SCR [S$45,394 (US$34,335)] and tisagenlecleucel vs BLN [S$52,106 (US$39,412)] (Figure 5A and B), with 0% likelihood of tisagenlecleucel dominating SCR or BLN. Tisagenlecleucel had 100% probability of being cost-effective against both SCR and BLN at WTP threshold of S$266,973/QALY (US$201,931/QALY), ie, 3 times of Singapore GDP S$88,991/capita (US$67,310/capita).36 Additionally, tisagenlecleucel was analyzed at WTP threshold of S$88,991/QALY (US$67,310/QALY) (1xGDP) and had 99.9% and 96.9% probability of being cost-effective against both SCR and BLN (Figure 5C and D). Since, none of the treatments were dominated by other treatments, a check for extended dominance was conducted. The cost-effectiveness frontier graph (Figure 5E) shows that none of the treatments fell into “extended dominance” category.
The base-case ICERs were further substantiated with the use of alternative input sources. This varied the base-case ICER from S$$43,368 (US$32,802) to S$74,868 (US$56,628) (tisagenlecleucel vs SCR) and from S$27,968 (US$21,154) to S$85,029 (US$64,314) (tisagenlecleucel vs BLN). As with DSA, findings were most responsive to shortened time horizons (Table 7).
 |
Table 7 Scenario Analysis Results for TIS vs SCR and TIS vs BLN |
Budget Impact Analysis
Following tisagenlecleucel launch, 5 to 7 patients are expected to be eligible for tisagenlecleucel treatment each year for the initial 5 years. With assumed distribution among different treatment options, only one patient is expected to receive tisagenlecleucel every year for the initial 3 years, increasing gradually to two and three patients at year 4 and 5 respectively. The budget impact was assessed to increase from S$477,857 (US$361,438) in year 1 to S$1,391,199 (US$1,052,265) in year 5 (Figure 6). Scenario analysis evaluated key elements of uncertainty and the results were close to the base-case in all the scenarios except when the market share was equally redistribution between tisagenlecleucel, SCR and BLN (Table 8).
 |
Table 8 Budget Impact Sensitivity Analyses Results |
 |
Figure 6 Net and total budget impact of TIS from healthcare system perspective. Abbreviation: TIS, tisagenlecleucel. |
Discussion
The launch of tisagenlecleucel in Singapore offers an effective new treatment strategy for r/r ALL among children and young adult patients who have failed at least 2 prior systemic therapies. However, the upfront cost of tisagenlecleucel, may possibly lead healthcare payers to question the cost-effectiveness of this therapy.
This cost-effectiveness analysis demonstrates that tisagenlecleucel treatment at a unit price of S$500K is a cost-effective option for r/r ALL among children and young adult patients when compared with SCR or BLN, from Singapore’s healthcare system perspective. DSA and PSA support model robustness and validate our results, with 100% likelihood of tisagenlecleucel being cost-effective at an assumed WTP threshold of three-times the GDP of Singapore.
Our study demonstrated that the upfront cost of tisagenlecleucel was offset by avoidance of higher drug administration and hospitalization costs, and subsequent allo-HSCT costs with both SCR and BLN treatments. Moreover, in addition to QALYs gained, tisagenlecleucel potentially reduces the long-term side effects from high dose chemotherapy and total body irradiation when employed in lieu of allo-HSCT. This suggests that public payers funding for tisagenlecleucel treatment can be considered “value for money” while enabling patients to gain access to a clinically effective lifesaving therapy.
Previously published tisagenlecleucel CEA studies have demonstrated similar results. Two studies from North America have determined the cost-effectiveness of tisagenlecleucel to treat children and young adults with r/r ALL with an ICER of US$61,000 in comparison with BLN57 and CAD11,567 in comparison with SCR.58 While the US analysis is based on median follow-up of 13 months in the ELIANA trial, our CEA analysis is more robust in terms of use of longer-term data from the tisagenlecleucel trials. A separate US cost-effectiveness study demonstrated cost-effectiveness of tisagenlecleucel versus standard of care with an ICER value of US$64,000 against the WTP threshold of US$1,00,000.59 However, these aforesaid correlations need to be interpreted with caution due to methodological variations, selected model inputs and distinct characteristics of healthcare policies and cost structures. A recently published CEA from public healthcare perspective in Japan similarly demonstrated cost-effectiveness of tisagenlecleucel versus BLN with an ICER of ¥2,035,071 at WTP threshold of ¥7.5 million.60
Our analysis has certain distinct strengths. Most significantly, the utilization of 38.8-month long individual patient level follow-up data (longer than 3-year cure-point) has facilitated more accurate curve fitting while avoiding extrapolation associated uncertainties, hence, increasing the reliability of the analysis. Further, scenario analyses with efficacy estimates based on alternative parametric functions, alternative utility values, real-world tisagenlecleucel safety data, tisagenlecleucel hospitalization and length of stay, and various modelling scenarios did not significantly change our base-case findings, thus cemented the validity and robustness of our study.
In the current analysis, a 3-year cure-point was selected based on the literature41 and validated with the opinion from local clinical experts. The robustness of the 3-year cure-point was further supported by the marginal impact on ICER when the cure-point was prolonged to 5 years in the scenario analysis. As signified in the DSA, time-dependent parameters are the key drivers for ICER variations. The division of the lifetime horizon into two distinct time-periods, ie, initial 3 years (the cure-point) and beyond completion of 3 years, establish that the majority of QALY benefit is accumulated after the end of year 3 (Table 9). These findings are intuitive due to the potentially curative characteristics of tisagenlecleucel treatment, with health gains amassed over the remaining life span of the treated patients.
 |
Table 9 Segmentation Analysis of QALYs and LYs Gain Over ELIANA Study Time Horizon (3-Year Cure-Point) and Beyond 3-Years |
Due to the absence of Singapore-based mapping data, the use of a utility mapping from South Korean could be considered a close substitute for Singapore’s patient population based on the similarity of Asian ethnicity, and further bolsters our model framework. Furthermore, our study apprehended all the costs accrued over the entire lifespan of patients who relapsed after treatment. This comprehensive cost assessment could be considered crucial to fully appraise the appropriate economic value of innovative therapies like tisagenlecleucel. Lastly, PSM is a credible modelling approach regularly practiced while evaluating oncological medical technologies and has been recognized and endorsed by many HTA bodies while evaluating reimbursement dossiers for ALL.31–33 The modelling inputs selected were further verified by local clinical experts to accurately reflect current practices.
Our analysis also has a few drawbacks. Firstly, there is non-availability of head-to-head study data adding uncertainty to the comparative clinical outcomes of tisagenlecleucel vs SCR and vs BLN. As all tisagenlecleucel trials are single arm without any control group, it was difficult to determine the comparative efficacy and safety of tisagenlecleucel. The naïve indirect comparisons performed have inherent limitations due to possible confounding and selection biases. To account for aforementioned bias and confounding, we performed multiple scenario analyses for the survival estimation based on alternative parametric functions for all the treatment arms (Table 7). Secondly, uncertainty surrounding costs is of particular concern given our sensitivity analysis indicating that base case results are influenced by tisagenlecleucel treatment, hospitalization, as well as BLN treatment costs. Lack of Singapore specific relapse cost data meant that treatment costs after relapse relied on assumptions. Thirdly, the frequency of follow-up after relapse was assumed to be double that in the EFS state with chemotherapy during year 1 and was assumed to be similar irrespective of the treatment arm. Further, conversion of private costs to public costs and vice-versa using a conversion factor, may inaccurately estimate certain medical costs. We partially alleviated this shortcoming by seeking validation of our cost inputs with Singapore’s clinical experts. Finally, restriction of our budget impact evaluation to the first 5 years, fails to capture of the true economic value of tisagenlecleucel as it under-represents avoidance of life-long relapse costs.
Conclusion
Our analysis found that tisagenlecleucel is a cost-effective option against both SCR and BLN for treating ALL among children and young adult patients with ≥2 lines of prior treatment from Singapore’s healthcare system perspective, with projected budgetary implications ranging from S$0.48M in year 1 to S$1.4M in year 5 as the tisagenlecleucel’s market share advances from 15% to 50% during the initial 5 years. Based on the evidence presented, we demonstrated that tisagenlecleucel is a cost-effective treatment. Provision of coverage for tisagenlecleucel treatment costs is likely to represent good use of healthcare resources by facilitating the access of this effective treatment to patients in Singapore. A funding solution on this one-off therapy may require a multi-party partnership involving governments, insurers, hospitals, and pharmaceutical company, considering the multi-payer system in Singapore.
Acknowledgments
The authors would like to thank Dinesh Kumar and Nishkarsh Likhar for drafting the manuscript, Minal Jain for developing and performing economic analyses, and Kean Seng Lim for validating the manuscript content. All were employees of Novartis when they contributed to this manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Novartis Singapore Pte Ltd.
Disclosure
XJW, YHW, MJCO, and CG are employees of Novartis. WYKH has received personal fees as Advisory Board member from Novartis and Gilead, and grant from CordLife for a clinical study. SYS has no financial or other interests for declaration here. The authors report no other conflicts of interest in this work.
References
1. Coebergh JW, Reedijk AM, de Vries E, et al. Leukaemia incidence and survival in children and adolescents in Europe during 1978–1997. report from the automated childhood cancer information system project. Eur J Cancer. 2006;42(13):2019–2036. doi:10.1016/j.ejca.2006.06.005
2. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. doi:10.3322/caac.21219
3. CANCER.ORG. How is childhood leukemia classified?; 2020. Available from: https://www.cancer.org/cancer/leukemia-in-children/detection-diagnosis-staging/how-classified.html.
4. Katz AJ, Chia VM, Schoonen WM, Kelsh MA. Acute lymphoblastic leukemia: an assessment of international incidence, survival, and disease burden. Cancer Causes Control. 2015;26(11):1627–1642. doi:10.1007/s10552-015-0657-6
5. Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663–1669. doi:10.1200/JCO.2011.37.8018
6. Schrappe M, Reiter A, Ludwig WD, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM study group. Blood. 2000;95(11):3310–3322.
7. Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a children’s oncology group study. Leukemia. 2008;22(12):2142–2150. doi:10.1038/leu.2008.251
8. Ko RH, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic advances in childhood leukemia consortium study. J Clin Oncol. 2010;28(4):648–654. doi:10.1200/jco.2009.22.2950
9. Reismuller B, Peters C, Dworzak MN, et al. Outcome of children and adolescents with a second or third relapse of acute lymphoblastic leukemia (ALL): a population-based analysis of the Austrian ALL-BFM (Berlin-Frankfurt-Munster) study group. J Pediatr Hematol Oncol. 2013;35(5):e200–4. doi:10.1097/MPH.0b013e318290c3d6
10. Ceppi F, Duval M, Leclerc J-M, et al. Improvement of the outcome of relapsed or refractory acute lymphoblastic leukemia in children using a risk-based treatment strategy. PLoS One. 2016;11(9):e0160310. doi:10.1371/journal.pone.0160310
11. von Stackelberg A, Volzke E, Kuhl JS, et al. Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non-response to salvage protocol therapy: a retrospective analysis of the ALL-REZ BFM study group. Eur J Cancer. 2011;47(1):90–97. doi:10.1016/j.ejca.2010.09.020
12. von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase i/phase ii study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–4389. doi:10.1200/JCO.2016.67.3301
13. Maziarz RT, Guerin A, Gauthier G, et al. Five-year direct costs of acute lymphoblastic leukemia pediatric patients undergoing allogeneic stem cell transplant. Int J Hematol Oncol. 2016;5(2):63–75. doi:10.2217/ijh-2016-0001
14. Hanson SBaSG. 2014 U.S. organ and tissue transplant cost estimates and discussion; 2020. Available from: https://us.milliman.com/en/Insight/2014-usorgan-and-tissue-transplant-cost-estimates-and-discussion.
15. Nietfeld JJ, Pasquini MC, Logan BR, Verter F, Horowitz MM. Lifetime probabilities of hematopoietic stem cell transplantation in the U.S. Biol Blood Marrow Transplant. 2008;14(3):316–322. doi:10.1016/j.bbmt.2007.12.493
16. Dalle JH, Balduzzi A, Bader P, et al. Allogeneic stem cell transplantation from hla-mismatched donors for pediatric patients with acute lymphoblastic leukemia treated according to the 2003 bfm and 2007 international bfm studies: impact of disease risk on outcomes. Biol Blood Marrow Transpl. 2018;24(9):1848–1855. doi:10.1016/j.bbmt.2018.05.009
17. Dalle JH, Balduzzi A, Bader P, et al. The impact of donor type on the outcome of pediatric patients with very high risk acute lymphoblastic leukemia. A study of the ALL SCT 2003 BFM-SG and 2007-BFM-International SG. Bone Marrow Transpl. 2021;56(1):257–266. doi:10.1038/s41409-020-01014-x
18. Balduzzi A, Dalle JH, Wachowiak J, et al. Transplantation in children and adolescents with acute lymphoblastic leukemia from a matched donor versus an hla-identical sibling: is the outcome comparable? Results from the international bfm all sct 2007 study. Biol Blood Marrow Transpl. 2019;25(11):2197–2210. doi:10.1016/j.bbmt.2019.07.011
19. Peters C, Schrappe M, von Stackelberg A, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors-The ALL-SCT-BFM-2003 trial. J Clin Oncol. 2015;33(11):1265–1274. doi:10.1200/JCO.2014.58.9747
20. Novartis. Novartis receives approval for Kymriah® (tisagenlecleucel) by health sciences authority as Singapore’s first commercially approved CAR-T therapy. Available from: https://www.novartis.com.sg/news/media-releases/novartis-receives-approval-kymriah-tisagenlecleucel-health-sciences-authority.
21. Clinicaltrials.gov. Determine efficacy and safety of ctl019 in pediatric patients with relapsed and refractory b-cell all and high risk b-cell all at first relapse. Determine feasibility and safety of ctl019 therapy in pediatric patients with high risk b-cell all that relapsed < 6 months post all-hsCT. (ELIANA). Available from: https://clinicaltrials.gov/ct2/show/study/NCT02435849.
22. Dourthe M. Safety and efficacy of tisagenlecleucel (CLTl019) in B acute lymphoblastic leukemia in children and young adults: Rrobert Debré and Saint Louis hospitals experience.
23. Dourthe M. Safety and efficacy of tisagenlecleucel (CLTl019) in B-cell acute lymphoblastic leukemia in children and young adults: the French experience.
24. Bader A. Real life experience in the treatment of pediatric, adolescent and young adult ALL patients using commercially available CAR-T cells.
25. Grupp S, Hu Z-H, Zhang Y, et al. Tisagenlecleucel Chimeric Antigen Receptor (car) t-cell therapy for relapsed/refractory children and young adults with acute lymphoblastic leukemia (ALL): real World Experience from the Center for International Blood and Marrow Transplant Research (CIBMTR) and Cellular Therapy (CT) Registry.
26. Pasquini M, Hu ZH, Zhang Y. Real world experience of tisagenlecleucel Chimeric Antigen Receptor (CAR) T-cells targeting cd19 in patients with acute lymphoblastic leukemia (all) and diffuse large b-cell lymphoma (dlbcl) using the Center for International Blood and Marrow Transplant Research (CIBMTR) Cellular Therapy (CT) Registry.
27. Wang XJ, Wang YH, Li SCT, et al. Cost-effectiveness and budget impact analyses of tisagenlecleucel in adult patients with relapsed or refractory diffuse large B-cell lymphoma from Singapore’s private insurance payer’s perspective. J Med Econ. 2021;24(1):637–653. doi:10.1080/13696998.2021.1922066
28. Nicedsu.org.uk. Nice DSU technical support document 19: partitioned survival analysis for decision modelling in health care: a critical review. TSD 19 (nicedsu.org.uk); 2017.
29. Clinicaltrials.gov. Study of efficacy and safety of CTL019 in Pediatric ALL patients. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02228096.
30. Clinicaltrials.gov. Phase I/IIA study of cart19 cells for patients with chemotherapy resistant or refractory cd19+ leukemia and lymphoma (pedi cART19). Available from: https://clinicaltrials.gov/ct2/show/NCT01626495.
31. NICE. Single technology appraisal. Tisagenlecleucel for treating relapsed or refractory B-cell acute lymphoblastic leukaemia in people aged up to 25 years [ID1167], committee papers; 2018. Available from: https://www.nice.org.uk/guidance/ta554/documents/committee-papers.
32. CADTH. Canadian Agency for Drugs and Technologies in Health (CADTH) optimal use report. tisagenlecleucel for acute lymphoblastic leukemia: economic review report; 2020. Available from: https://cadth.ca/sites/default/files/pdf/car-t/op0538-tisagenlecleucel-economic-report-pALL-jan2019.pdf.
33. MSAC. public summary document, tisagenlecleucel (CTL019) for treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL); 2019. Available from: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/A2B10F9A03293BC8CA2583CF001C7A4D/$File/1519.1%20Final%20updated%20PSD%20Nov%2019_redacted.pdf.
34. Agency for Care Effectiveness. ACE Clinical Guidance (ACG) process and methods; 2020. Available from: https://www.ace-hta.gov.sg/our-process-and-methods.html.
35. Hutubessy R, Chisholm D, Edejer TT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1(1):8. doi:10.1186/1478-7547-1-8
36. Department of Statistics Singapore. Department of Statistics Singapore; 2020. Avaliable from: https://www.singstat.gov.sg/modules/infographics/economy.
37. MAS. Monetary authority of Singapore. Exchange rates; 2021. Available from: https://eservices.mas.gov.sg/Statistics/msb/ExchangeRates.aspx.
38. Gore L, Locatelli F, Zugmaier G, et al. Survival after blinatumomab treatment in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood Cancer J. 2018;8(9):80. doi:10.1038/s41408-018-0117-0
39. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9. doi:10.1186/1471-2288-12-9
40. Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376(9757):2009–2017. doi:10.1016/S0140-6736(10)62002-8
41. Bassan R, Hoelzer D, Thomas X, et al. clinician concepts of cure in adult relapsed and refractory Philadelphia-negative b cell precursor acute lymphoblastic leukemia: a delphi study. Adv Ther. 2019;36(4):870–879. doi:10.1007/s12325-019-00910-z
42. Department of Statistics Singapore. complete life tables for Singapore resident population, 2017–2018. Avalibale from: https://www.singstat.gov.sg/publications/population/complete-life-table.
43. MacArthur AC, Spinelli JJ, Rogers PC, Goddard KJ, Abanto ZU, McBride ML. Mortality among 5-year survivors of cancer diagnosed during childhood or adolescence in British Columbia, Canada. Pediatr Blood Cancer. 2007;48(4):460–467. doi:10.1002/pbc.20922
44. NCCN Clinical Practice Guidelines in Oncology. pediatric acute lymphoblastic leukemia: version 2.2020; 2019. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
45. NHS Network Site Specific Group (NSSG). Haematology. myeloid group - FLA-IDA; 2020. Available from: http://nssg.oxford-haematology.org.uk/myeloid/protocols/ML-9-fla-ida.pdf.
46. Gaynon PS, Harris RE, Altman AJ, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: children’s oncology group study CCG-1941. J Clin Oncol. 2006;24(19):3150–3156. doi:10.1200/JCO.2005.04.5856
47. Svendsen AL, Feychting M, Klaeboe L, Langmark F, Schuz J. Time trends in the incidence of acute lymphoblastic leukemia among children 1976–2002: a population-based Nordic study. J Pediatr. 2007;151(5):548–550. doi:10.1016/j.jpeds.2007.07.006
48. HSA Product Information. Blincyto. Available from: https://myhealthbox.eu/en/view/3625248/8a63a97851d9c6b3780501cec3e7181a/leaflet.
49. Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: a children’s oncology group study[corrected]. J Clin Oncol. 2008;26(24):3971–3978. doi:10.1200/JCO.2008.16.1414
50. Phua L, Lee S, Ng K, Aziz M. Cost-effectiveness analysis of atezolizumab in advanced triple-negative breast cancer. BMC Health Serv Res. 2020;20(1). doi:10.21203/rs.2.22855/v1
51. Lee YK, Nam HS, Chuang LH, et al. South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value Health. 2009;12(8):1187–1193. doi:10.1111/j.1524-4733.2009.00579.x
52. Szende A, Janssen MF, Cabases JM. Self-reported population health: an international perspective based on EQ-5D; 2014: 1–196.
53. Sung L, Buckstein R, Doyle JJ, Crump M, Detsky AS. Treatment options for patients with acute myeloid leukemia with a matched sibling donor: a decision analysis. Cancer. 2003;97(3):592–600. doi:10.1002/cncr.11098
54. Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017;21(7):1–204. doi:10.3310/hta21070
55. National Cancer Centre Singapore. Cancer incidence in Singapore. Available from: https://www.nccs.com.sg/patient-care/cancer-types/cancer-statistics.
56. Locatelli F, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120(14):2807–2816. doi:10.1182/blood-2012-02-265884
57. Lin JK, Lerman BJ, Barnes JI, et al. cost effectiveness of chimeric antigen receptor t-cell therapy in relapsed or refractory pediatric b-cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36(32):3192–3202. doi:10.1200/JCO.2018.79.0642
58. Yang H, Zhang J, Hampe M. Cost-effectiveness of tisagenlecleucel in pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia: a Canadian societal perspective. Value Health. 2018; 21:S43.
59. Sarkar RR, Gloude NJ, Schiff D, Murphy JD. Cost-effectiveness of chimeric antigen receptor t-cell therapy in pediatric relapsed/refractory b-cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111(7):719–726. doi:10.1093/jnci/djy193
60. Wakase S, Teshima T, Zhang J, et al. cost effectiveness analysis of tisagenlecleucel for the treatment of adult patients with relapsed or refractory diffuse large b cell lymphoma in Japan. Cell Ther Transpl. 2021;27(6):506e1–506 e10. doi:10.1016/j.jtct.2021.03.005
61. The York Report. Exploring the assessment and appraisal of regenerative medicines and cell therapy products; 2016. Available from: http://docplayer.net/18693201-Exploring-the-assessment-and-appraisal-of-regenerative-medicines-and-cell-therapy-products.html.
62. Szende A, Janssen B, Cabases J Self-reported population health: an international perspective based on EQ-5D. Springer; 2014. Avaialbe from: https://link.springer.com/content/pdf/10.1007%2F978-94-007-7596-1.pdf.
63. Kelly MJ, Pauker SG, Parsons SK. Using nonrandomized studies to inform complex clinical decisions: the thorny issue of cranial radiation therapy for T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2015;62(5):790–797. doi:10.1002/pbc.25451
64. Kuhlen M, Willasch AM, Dalle JH, et al. Outcome of relapse after allogeneic HSCT in children with ALL enrolled in the ALL-SCT 2003/2007 trial. Br J Haematol. 2018;180(1):82–89. doi:10.1111/bjh.14965
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.