Back to Journals » Risk Management and Healthcare Policy » Volume 15
Cost-Effectiveness Analysis of Gefitinib Alone and Combined with Chemotherapy as First-Line Treatment for Patients with Advanced Non-Small-Cell Lung Cancer
Authors Wang Y, Huang K, Sun S, Deng Y, Xie X
Received 7 December 2021
Accepted for publication 14 February 2022
Published 1 March 2022 Volume 2022:15 Pages 351—359
DOI https://doi.org/10.2147/RMHP.S352827
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Yao Wang,1,2 Kaiyu Huang,1,2 Sijia Sun,1,2 Yahong Deng,1,3 Xuefeng Xie1,2
1School of Pharmacy, Anhui Medical University, Hefei, 230032, People’s Republic of China; 2Inflammation and Immune-Mediated Diseases Laboratory of Anhui Province, Hefei, People’s Republic of China; 3Department of Pharmacy, First Affiliated Hospital of Anhui Medical University, Hefei, 230000, Anhui, People’s Republic of China
Correspondence: Xuefeng Xie, School of Pharmacy, Anhui Medical University, 81 Meishan Road, Hefei, 230032, People’s Republic of China, Tel +8613721098599, Fax +86551-65161040, Email [email protected]
Background: The rational choice of drugs for treating patients with advanced non-small-cell lung cancer (NSCLC) is significantly impacted by changes in modern drug policy, health insurance negotiation, and budget impact analyses. Here, we provide a basis for rational drug use decisions in clinical practice and promote the widespread use of pharmacoeconomic methods in clinical decision-making based on current drug policies in China and real-world data.
Methods: A Markov model was developed to evaluate the health and economic outcomes in patients with advanced NSCLC treated with first-line chemotherapy with gefitinib and gefitinib plus chemotherapy. Clinical data, cost, and utility data were extracted from published literature or real-world data; sensitivity analysis was performed to assess the uncertainty in the results. The results were summarized as QALYs and the ICER.
Results: The average cost and QALYs associated with gefitinib and gefitinib plus chemotherapy strategies were $62,882.83 and 1.70 and $84,509.30 and 1.93, respectively. The ICER for gefitinib plus chemotherapy versus gefitinib alone was $95,135.50. The one-way sensitivity analysis showed that the utility value of progressive disease (PD) had the greatest impact on the treatment outcome. Probabilistic sensitivity analysis showed that if China’s willingness to pay threshold was $33,300/QALY, the probability of superiority of the gefitinib plus chemotherapy regimen was 0.
Conclusion: The study suggests that, from the perspective of the Chinese health system, gefitinib plus chemotherapy is not a cost-effective option for NSCLC patients with EGFR mutations. These findings may help clinicians make the best treatment decisions for patients with NSCLC.
Keywords: gefitinib, Markov model, cost effectiveness, NSCLC
Introduction
Lung cancer is the most commonly occurring cancer and the leading cause of cancer death; it accounts for 18.0% of all cancer deaths.1 Non-small-cell lung cancer (NSCLC) comprises approximately 80–85% of all lung cancers.2 As the deadliest of the prevalent types of cancer, surgical resection is the single most successful curative option. However, nearly 70% of lung cancer patients have locally advanced or metastatic disease at the time of diagnosis. These patients are usually asymptomatic before onset.3,4 Until the advent of personalized medicine, treatment for advanced NSCLC was limited to chemotherapy, with response rates of typically 20–30% and a progression-free survival (PFS) of 3–5 months following first-line chemotherapy.5 Conventional chemotherapies remain the mainstay treatments for unselected NSCLC; however, the study of epidermal growth factor receptor (EGFR) gene activation mutations in NSCLC and the use of specific inhibitors targeting them have played a key role in treating patients at these stages of the disease. EGFR tyrosine kinase inhibitors (TKIs) are the standard first-line therapy in advanced NSCLC patients with EGFR mutations. Currently available TKIs such as erlotinib, gefitinib, and afatinib target EGFR and provide significant tumour responses in patients with NSCLC harbouring such mutations, with a response rate of 62–83% and a PFS of 9–13 months, significantly improving the quality of life (QOL) compared to that with chemotherapy.5,6
The majority of patients with EGFR mutations and locally advanced or metastatic NSCLC treated with an EGFR TKI ultimately develop acquired resistance and generally experience disease progression within 1 year. The median survival of patients after the emergence of acquired resistance is generally less than 2 years.7 A Phase II (NEJ) 002 randomized trial in EGFR-mutant (EGFRm) advanced NSCLC demonstrated the superiority of first-line EGFR TKI treatment over platinum-based regimens in terms of PFS and QOL for patients with advanced NSCLC harbouring activating EGFR mutations.8 In another phase II (NEJ 005) randomized trial in EGFRm advanced NSCLC, compared with gefitinib alone, pemetrexed and carboplatin plus gefitinib prolonged PFS.9
In a Phase III (NEJ) 009 trial, the efficacy and safety of gefitinib combined with chemotherapy were compared with those of gefitinib alone in NSCLC patients with EGFR mutations.10 The results revealed that maintenance gefitinib plus chemotherapy notably prolonged the median PFS compared with gefitinib alone (20.9 vs 11.9 months; hazard ratio [HR] for disease progression or death, 0.490; [P<0.001]), and the median OS in the combination group was also significantly longer than that in the gefitinib group (50.9 vs 38.8 months; HR for death, 0.722; P = 0.021). The rate of grade ≥3 adverse drug events was higher in the combination group than in the gefitinib group (65.3% vs 31.0%). Thus, choice combination therapy appears to be an attractive option for the treatment of EGFRm NSCLC. However, the consideration of cost-effectiveness in health decision-making is critical for clinicians and policymakers when optimizing the allocation of limited health resources, and as modern drug policy, health insurance negotiations, and budget impact analysis change, rational drug selection is critical for patients with advanced NSCLC. The purpose of this analysis was to examine the cost-effectiveness of maintenance combination therapy versus monotherapy in NSCLC from a Chinese health system perspective.
Materials and Methods
Patients and Therapy
Patient and therapy data for this study were extracted from the NEJ009 trial. Patients with histologically or cytologically confirmed nonsquamous NSCLC of stage IIIB or IV or EGFR mutation recurrence, aged 20–75 years, with 0–1 presentation status in the Eastern Cooperative Oncology Group and proper organ function were included. The main exclusion criteria were a severe concomitant systemic disease, interstitial pneumonia, another primary malignancy, T790M mutation, symptomatic brain metastases, and pregnancy. The trial was designed to compare the benefits of gefitinib alone versus gefitinib plus chemotherapy in first-line treatment regimens.
Model Structure
A Markov model was established using TreeAge Pro 2019 software to evaluate the cost and health outcomes of gefitinib alone and gefitinib in combination with chemotherapy in advanced NSCLC. The model included three discrete health states reflecting different characteristics of the disease: PFS, progressive disease (PD), and death (Figure 1).11,12 Because the treatment schedules in the NEJ009 trial were arranged by using 3 weeks as the unit, the cycle length of the Markov model was set to 3 weeks.13 The assessment period was 10 years because the 5-year survival rate was lower than 40%, and the initial health state for all of the patients was PFS. During each three-week cycle, the patients either remained in their assigned health state or progressed to a new health state. It was assumed that patients cannot return to previous health states.
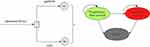 |
Figure 1 Schematics of the decision tree and the Markov state transition model. |
Model development and data analysis were performed in the R statistical environment (version 3.4.4). The main outcomes were quality-adjusted life-years (QALYs) and cost. The incremental cost-effectiveness ratio (ICER), which represents the cost of each additional QALY, is used to judge the cost-benefit. According to the China Pharmacoeconomic Evaluation Guide, the cost and utility values were discounted at a rate of 5% per year, and the cost-effectiveness threshold was 3 times China’s GDP per capita in 2020 ($33,300).
Clinical Data
The probability of disease progression or death was derived from published literature. PFS and overall survival (OS) curves of monotherapy and combination therapy were obtained from the NEJ009 trial, and GetData Graph Digitizer14 software was used to collect data points from published PFS and OS curves. The lifetime and duration data acquired were cleaned up and converted into a data format suitable for survival analysis. Then, the Weibull survival model was used to fit the Kaplan-Meier survival curve of gefitinib and gefitinib combined with chemotherapy because Weibull distributions are flexible and widely used in cancer survival analyses,15 and the Weibull functions parameters λ PFS and λ OS of PFS and OS and the shape parameters γ PFS and γ OS of PFS and OS were obtained within the time range of the model. In addition, the similarity between the new fitting curve and the original curve was compared. R2 indicated the best goodness of fit for Kaplan-Meier survival data. The estimated Weibull scale (λ) and shape (γ) parameters are shown in Table 1. The survival probability at the time was calculated using the following formula: S(t) = P(T ≥ t) = exp(−λtγ). The probability of transition from PFS to PS at a given cycle was calculated by using the following formula: P(t)=1−exp[λ(t−u)γ−λtγ], where λ and γ are the Weibull parameters of PFS16 and u represents the cycle period. The estimated parameters of the Weibull survival function are shown in Table 1. The difference between the OS and PFS estimated from the parametric survival models was used to calculate the probability from PD to death.17
 |
Table 1 Key Model Input Parameter |
Cost and Utility Data
The analysis was conducted from China’s health system perspective. Direct medical costs, including those related to drug costs, best supportive care costs, salvage chemotherapy costs after disease progression, palliative care cost in terminal patients, management cost of treatment-related serious adverse events (AEs) (grade ≥3) during treatment, and follow-up costs per cycle, were included in the model.18 Direct nonmedical costs were ignored because individual differences are difficult to estimate. The adverse drug reaction (ADR) costs were calculated based on the cost of the drug used to treat the event. All costs were adjusted to 2020 prices based on the local consumer price index and are shown in US dollars (1 US dollar = CNY 6.90).
We assumed that patients with EGFR mutations received 250 mg gefitinib daily on a monotherapy regimen until disease progression. The assumptions for combination therapy were as follows: gefitinib 250 mg orally, qd, combined carboplatin area under curve 5, pemetrexed 500 mg/m2, 3 weeks as a cycle, up to 6 cycles, followed by both gefitinib and pemetrexed maintenance until disease progression, second-line treatment with a platinum regimen. We assumed that a typical patient weighed 65 kg, was 1.64 m tall, and had a body surface area of 1.72 m2 to calculate the dose of the drug. As the disease progressed, rescue treatment was given.19
QALY was measured as an effect parameter, which represented a combination of survival and utility. Utility is the reflection of a patient’s QOL, which ranges from 0 (death) to 1 (perfect health). Since the NEJ009 trial showed similar EORTC Core Quality of Life Questionnaire (QLQ-C30) scores between patients in the gefitinib and combination group, there was no significant difference in the QOL between the two groups, but the incidence of side effects varied widely between the two groups, and negative effects should be considered. We hypothesized that health utility preferences are related to disease conditions and AEs. The mean health utility scores for PFS, PD, and AE states were derived from published literature.20
Sensitivity Analyses
One-way and probabilistic sensitivity analyses (PSAs) were used to test the uncertainty in the model. In the one-way sensitivity analyses, to identify key model input parameters that had a substantial impact on the model outcome, relevant parameters were adjusted one by one to their respective low and high values, which are listed and illustrated in Tables 1 and 2. The ranges of the parameters used in the one-way sensitivity analyses were obtained from the published literature; when reported data were not available, a range of ±25% of the base-case value was used. The results of the one-way sensitivity analyses are presented in a Tornado diagram. The gamma distribution was selected for the cost parameters, and the beta distribution was used for the transition probability, proportion, and preference value parameters.21 For the PSA, parameters were sampled using the Monte Carlo method to run 1000 replicated outcomes. Based on the data, a cost-effectiveness acceptability curve was created to represent the likelihood that maintenance monotherapy or gefitinib plus chemotherapy would be considered cost-effective at various willingness to pay (WTP) levels for health gains (QALYs).
 |
Table 2 Base-Case Cost Estimates and Utilities |
Results
During Weibull fitting, the PFS values of gefitinib and gefitinib combined with chemotherapy were 11.9 and 22 months, respectively, and the OS values were 39.5 and 52 months, respectively. The simulated PFS and OS curves are consistent with the original data, and the fit was reasonable and acceptable.
In China, the average cost and QALYs associated with gefitinib and gefitinib plus chemotherapy strategies were $62,882.83 and 1.70 and $84,509.30 and 1.93, respectively. The ICER for gefitinib plus chemotherapy versus gefitinib alone was $95,135.50. The predicted average costs and QALYs for the two treatment regimens are shown in Table 3. The life years associated with each strategy are listed for comparison.
 |
Table 3 Summary of Cost and Outcome Results from a Base-Case Analysis |
One-way sensitivity analysis was used to test the robustness of the model results when comparing gefitinib chemotherapy strategies with gefitinib plus chemotherapy strategies, and then the results were summarized into tornado maps using TreeAge 2019 software. The Tornado diagram summarizes the results of one-way sensitivity analysis, and the influencing parameters are arranged in descending order according to the influence degree of parameter value changes on ICER. Figure 2 shows that the utility value of PD had the highest impact on the outcome, followed by that of the cost of supportive care and PFS. Changes in individual parameters slightly altered the overall value associated with treatment, but they did not change the ICER-based conclusions regarding gefitinib and gefitinib plus chemotherapy regimens.
 |
Figure 2 Unidirectional sensitivity analysis tornado plot comparing the first-line gefitinib strategy with the gefitinib plus chemotherapy strategy in an entire population with EGFR mutations. |
To reflect the influence of all model input parameters on the research results, a PSA with 1000 simulations was performed by setting different distributions for each parameter. The results of PSA showed that gefitinib combined with chemotherapy was less cost-effective than gefitinib alone. The acceptable cost-effectiveness curve is shown in Figure 3. If the WTP threshold in China is below $33,300/QALY, then the probability of dominance of the gefitinib plus chemotherapy regimen is 0.
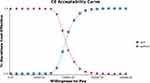 |
Figure 3 Cost-effectiveness acceptability curves for gefitinib alone versus the gefitinib combined with chemotherapy in China. |
Discussion
This study investigated the cost-effectiveness of two competitive first-line treatment options for NSCLC recommended by current National Comprehensive Cancer Network (NCCN) guidelines. Gefitinib, an EGFR tyrosinase inhibitor, is the first-line treatment for patients with locally advanced or metastatic NSCLC with EGFR sensitivity mutations. In the NEJ009 trial, gefitinib plus chemotherapy significantly extended progression-free survival and overall survival in patients with advanced EGFR mutation-positive NSCLC compared to monotherapy, but the combination was also associated with increased toxicity. However, it is undeniable that gefitinib combined with pemetrexed plus carboplatin may be a new choice for the first-line treatment of EGFR-positive advanced NSCLC. Long-term clinical trials have shown that gefitinib is more effective in Asians than in non-Asians.22 The main finding of the current analysis was that the gefitinib plus chemotherapy regimen provided better health outcomes for advanced NSCLC and EGFR mutations than the gefitinib alone strategy. In this study, the total cost and efficacy were $62,882.83 and 1.70 QALYs in the gefitinib group and $84,509.30 and 1.93 QALYs in the gefitinib combined with chemotherapy group. This demonstrated a benefit of 0.23 QALYs in the gefitinib combined with chemotherapy group and an incremental cost of $21,626.48. The ICER was $95,135.50 per QALY, much higher than the WTP threshold in China ($33,300.00). Therefore, the use of a gefitinib plus chemotherapy regimen in first-line therapy is not a favourable option for Chinese people.
A recent economic assessment23 determined the cost-effectiveness of gefitinib or gefitinib combined with carboplatin and pemetrexed chemotherapy for first-line treatment in patients with advanced EGFR mutation-positive NSCLC in China. The study found that compared with gefitinib, gefitinib combined with a chemotherapy regimen had an incremental cost of $37,795.92, an incremental QALY of −0.14, and an ICER of −277,121.22/QALY. Their results showed that gefitinib plus chemotherapy was not a relatively good treatment regimen in China, and the results were the same as ours. That article showed that the QALYs of the gefitinib plus chemotherapy regimen were less than those of the gefitinib monotherapy group, which was different from the results of our study. The main reason for the difference is that the sources of utility value were different. In our study, the negative utility of adverse reactions was calculated more accurately, and the drug cost in our study was closer to the real-world cost. Another recently published study24 reported an incremental cost of $7,078.59, an incremental QALY of 0.62, and an ICER of $11,499.98/QALY for the combination regimen versus gefitinib alone. Their results suggested that gefitinib combined with chemotherapy should be recommended in China, which is completely contrary to our results. The large difference in results may lie in the utility of the two schemes. Our study considered the utility of side effects. The incidence of side effects in the gefitinib plus chemotherapy group was much higher than that in the monotherapy group, and the NEJ009 trial showed no significant difference in QOL between the two groups, so the same health utility values were not used. With the widespread use of gefitinib, the sharp increase in medical costs has aroused concern among clinicians and administrators. In recent years, Chinese enterprises that develop gefitinib and pemetrexed generic drugs have gradually increased their dominance in the market, and China’s drug procurement through unified bidding has greatly reduced drug costs. However, previous pharmacoeconomic studies were performed before the drug costs of gefitinib and pemetrexed were controlled. The cost of drugs is very important for the study of pharmacoeconomics, and the cost of generic drugs was determined from our drug data after investigation.
As a first-line treatment for advanced NSCLC, gefitinib in combination with chemotherapy has the potential to improve survival and is a major determinant of clinical and economic outcomes. One-way sensitivity analysis showed that the utility of the PD state, the cost of optimal support treatment per cycle, and the utility of PFS were the three most important parameters affecting the robustness of the model. As shown in Figure 2, when the upper or lower limit is increased or decreased, the freezing point is significantly increased or decreased without affecting the final result.
However, this study has several limitations. First, due to a lack of data, we did not calculate the cost of pemetrexed and gefitinib maintenance therapy ourselves but obtained the optimal cost of supporting therapy directly from other published studies. Second, the utility of PFS and PD is derived from the published literature. Since health utility values may vary from region to region, sensitivity analyses were performed to assess the impact of these parameters. However, tornado maps showed that the utility of PD, the cost of optimal support treatment per cycle, and the utility of PFS were the three most important factors in this study. Third, some key clinical inputs, such as the exclusion of the cost of genetic testing due to individual differences, do not change the ICER results based on other cost-benefit analyses.19 Finally, the utility value of all serious AEs has not been fully considered; one study has reported the utility value for NSCLC, but the description of the utility value of each AE is not comprehensive, which may lead to biased results.
Conclusions
This study suggests that, from the perspective of the Chinese health system, gefitinib plus chemotherapy is not a cost-effective option for NSCLC patients with EGFR mutations. These findings may help clinicians make the best treatment decisions for patients with NSCLC. Due to current methodological deficiencies, more high-quality clinical and economic reality data are needed in this area, and a study with this focus will provide more reliable evidence as a framework to determine the value of different treatment options for advanced NSCLC.
Abbreviations
NSCLC, non-small-cell lung cancer; PFS, progression-free survival; EGFR, epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors; QOL, quality of life; EGFRm, EGFR-mutant; PD, progressive disease; ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life-years; AEs, adverse events; ADR, adverse drug reaction; QLQ-C30, Quality of Life Questionnaire; PSAs, probabilistic sensitivity analyses; WTP, willingness to pay; NCCN, National Comprehensive Cancer Network.
Acknowledgments
The authors thank all the research assistants for their data collection. This work was supported by the The University Synergy Innovation Program of Anhui Province (No.GXXT-2021-068); Excellent Talents Program of Anhui Universities (No. Gxyq2018006).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi:10.1038/nature25183
3. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi:10.1016/S0025-6196(11)60735-0
4. Kazaz SN, Öztop İ. Treatment after first-generation epidermal growth factor receptor tyrosine kinase inhibitor resistance in non-small-cell lung cancer. Turk Thorac J. 2017;18(3):66–71. doi:10.5152/TurkThoracJ.2017.16042
5. Salgia R. Diagnostic challenges in non-small-cell lung cancer: an integrated medicine approach. Future Oncol. 2015;11(3):489–500. doi:10.2217/fon.14.275
6. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, Phase 3 study. Lancet Oncol. 2018;19(1):139–148. doi:10.1016/S1470-2045(17)30729-5
7. Mann H, Andersohn F, Bodnar C, et al. Adjusted indirect comparison using propensity score matching of osimertinib to platinum-based doublet chemotherapy in patients with EGFRm T790M NSCLC who have progressed after EGFR-TKI. Clin Drug Investig. 2018;38(4):319–331. doi:10.1007/s40261-017-0611-3
8. Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 2013;24(1):54–59. doi:10.1093/annonc/mds214
9. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124–136. doi:10.1200/JCO.19.01154
10. Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38(2):115–123. doi:10.1200/JCO.19.01488
11. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi:10.1016/S1470-2045(09)70364-X
12. Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. doi:10.1016/S1470-2045(16)30033-X
13. Elbasha EH, Chhatwal J. Theoretical foundations and practical applications of within-cycle correction methods. Med Decis Making. 2016;36(1):115–131. doi:10.1177/0272989X15585121
14. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi:10.1186/1471-2288-12-9
15. Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139. doi:10.1186/1471-2288-11-139
16. Wu B, Li T, Cai J, Xu Y, Zhao G. Cost-effectiveness analysis of adjuvant chemotherapies in patients presenting with gastric cancer after D2 gastrectomy. BMC Cancer. 2014;14:984. doi:10.1186/1471-2407-14-984
17. Chouaid C, Luciani L, LeLay K, et al. Cost-effectiveness analysis of Afatinib versus Gefitinib for first-line treatment of advanced EGFR-mutated advanced non-small cell lung cancers. J Thorac Oncol. 2017;12(10):1496–1502. doi:10.1016/j.jtho.2017.07.013
18. Wu B, Gu X, Zhang Q, Xie F. Cost-effectiveness of osimertinib in treating newly diagnosed, advanced EGFR-mutation-positive non-small cell lung cancer. Oncologist. 2019;24(3):349–357. doi:10.1634/theoncologist.2018-0150
19. Gu X, Zhang Q, Chu YB, et al. Cost-effectiveness of Afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer. 2019;127:84–89. doi:10.1016/j.lungcan.2018.11.029
20. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195–e203. doi:10.1111/ajco.12477
21. Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722–732. doi:10.1177/0272989X12458348
22. Chang AY. The role of gefitinib in the management of Asian patients with non-small cell lung cancer [published correction appears in Expert Opin Investig Drugs. 2008 May;17(5):825]. Expert Opin Investig Drugs. 2008;17(3):401–411. doi:10.1517/13543784.17.3.401
23. Li WQ, Li LY, Chai J, Cui JW. Cost-effectiveness analysis of first-line treatments for advanced epidermal growth factor receptor-mutant non-small cell lung cancer patients. Cancer Med. 2021;10(6):1964–1974. doi:10.1002/cam4.3733
24. Shu Y, Zhang Q, He X, Chen L. Cost-effectiveness analysis of gefitinib plus chemotherapy versus gefitinib alone for advanced non-small-cell lung cancer with EGFR mutations in China. Cancer Manag Res. 2021;13:8297–8306. doi:10.2147/CMAR.S334643
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
