Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Cost-Effectiveness Analysis of COVID-19 Vaccination in Low- and Middle-Income Countries
Authors Utami AM , Rendrayani F , Khoiry QA, Alfiani F , Kusuma ASW, Suwantika AA
Received 23 April 2022
Accepted for publication 6 September 2022
Published 13 September 2022 Volume 2022:15 Pages 2067—2076
DOI https://doi.org/10.2147/JMDH.S372000
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Auliasari M Utami,1 Farida Rendrayani,1 Qisty A Khoiry,1 Fitri Alfiani,1,2 Arif SW Kusuma,3,4 Auliya A Suwantika1,4,5
1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, West Java, Indonesia; 2Faculty of Health Science, Universitas Muhammadiyah Cirebon, Cirebon, West Java, Indonesia; 3Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, West Java, Indonesia; 4Center of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, West Java, Indonesia; 5Center for Health Technology Assessment, Universitas Padjadjaran, Bandung, West Java, Indonesia
Correspondence: Auliya A Suwantika, Faculty of Pharmacy, Universitas Padjadjaran, Jl. Raya Bandung-Sumedang Km. 21, Jatinangor, Sumedang, 45363, Indonesia, Email [email protected]
Background: WHO reported that 5.5 million people died in the world because of COVID-19. One of the efforts to mitigate the pandemic is administrating the vaccines globally.
Objective: The objective of this study was to review cost-effectiveness analysis of COVID-19 vaccination in low- and middle-income countries (LMICs).
Methods: We searched PubMed and EBSCO for the eligible studies with inclusion criteria using cost-effectiveness analysis, free full text, low-middle-income countries, and the publication date since the last year. Four reviewers conducted the review independently.
Results: The review identified four articles meeting the eligibility criteria. The settings were LMICs. Different perspectives and economic modelling used by the countries confirmed a similar result. They all explained that vaccination could prevent the infection spread and mortality caused by COVID-19 and showed high cost-effectiveness values.
Conclusion: Administering COVID-19 vaccines was cost-effective and even cost-saving. The studies found that vaccination was more cost-effective in reducing the spread of the COVID-19 virus and the mortality it caused than no vaccination.
Keywords: economic evaluation, cost-effective, affordable, immunization, developing countries
Introduction
Coronavirus disease 2019 (COVID-19), the current global pandemic, is an acute respiratory syndrome caused by the SARS-CoV-2 virus.1,2 The virus first appeared in a cluster of patients in Wuhan, China, near the end of 2019, showing pneumonia-like symptoms.1 WHO has stated that weekly case incidence and deaths have increased by 6% compared to the last week.3 The cases have reached over 256 million confirmed cases and 5.5 million reported deaths in November 2021.3,4
Although the mortality is relatively minor to the incidence, the disease has put public health systems and the global economy under pressure.3–6 Workforce and productivity are declining due to mitigation measures such as increasing social distance, home office, quarantine, lockdown, school closure, and outdoor restrictions.3–6 The virus’ rapid and intensive transmission has become the primary cause.5,7 Nowadays, there is no established effective drug against coronavirus infection other than a reused drug without clear evidence of its efficacy and safety.7,8 Because of no effective antiviral treatment,7,8 preventive and control strategies have become of concern.8 These include isolation and quarantine, cleaning and disinfection, personal protective equipment, and physical distancing.8 Controlling the spread of infection and achieving a normalized post-pandemic situation can only be achieved if immunity is increased naturally or by a vaccine.8 Then, the most important movement is a safe and effective vaccination since it could lead to better and faster herd immunity achievement.4,6,9,10
While some COVID-19 vaccines seem safe and effective, only high-income countries have the resources to provide the appropriate amount.11 Many high-income and upper middle-income countries have signed bilateral agreements with manufacturers and have pre-ordered enough vaccines to cover their population several times.11 In contrast, the majority of low- and middle-income countries (LMICs) do not have access to sufficient quantities of vaccines due to cost, limited available capacity, and logistical problems in production, distribution, and storage.11 In addition, pharmaceutical industries in LMICs play a crucial role in addressing the unmet medical needs of rising middle-class population.12
Although WHO has published guidelines for prioritizing vaccines, most predictions about the effects of vaccines focus on high-income countries, with few including economic considerations.11 Thus, cost-effectiveness studies in LMICs have a critical role. The objective of this study was to review all published studies on cost-effectiveness analysis of COVID-19 vaccines in LMICs that can be a recommendation for the implementation of COVID-19 vaccination in LMICs. To the best of our knowledge, this is the first review to give an insightful cost-effectivity analysis of COVID-19 vaccines in LMICs’ settings. Hopefully, this study can fill the gap and be a basis for vaccine development considerations.
Methods
Search Method and Strategies
We searched in PubMed and EBSCO until April 30th, 2022. The search strategies developed by all the research teams are described in Table 1. Appropriate MeSH headings of these terms were used, and boolean operators were applied when necessary to cater to the different use of terms in the literature. Search results were not limited to English. The year of publication was determined from 2021 as COVID-19 vaccines have been recognized since this timeframe. In particular, we used the major selection criteria of study design should be complete economic evaluation, which were classified in one of the formal health-economic categories of cost minimization analysis (CMA), cost-effectiveness analysis (CEA), cost utility analysis (CUA) or cost benefit analysis (CBA), and conducted in LMICs’ settings. Retrieved abstracts were independently screened by the research team (in particular, AMU, FR, QAK and FA). We excluded non-relevant studies, systematic review studies, studies available in abstract only and studies which are not English.
 |
Table 1 Search Methods and Strategies |
Search Results
All search results were downloaded into the reference manager for screening purposes. The full articles were screened by four reviewers according to the eligibility criteria. All study designs were included (cross‐sectional studies, baseline data from experiments [ie, randomized or non‐randomized trials], retrospective studies, prospective cohort studies, and case-control studies). All published studies in English had the cost and effectiveness of COVID-19 vaccines from any of the LMICs from March 1st, 2021 to April 30th, 2022. The studies stated the mean age of participants to be ≥18 years. Figure 1 presents the article’s selection process.
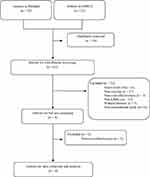 |
Figure 1 The article’s selection process. |
Data Processing
We extracted information from the articles regarding author(s) name, year of publication, country of study, type of COVID-19 vaccine, cost economic modelling, and result. The data extracted were then presented in tables. The articles reviewed by applying the question to the data: “how is the cost-effectiveness of Covid-19 vaccines in LMICs?” A descriptive-analytical approach was used in this review process. We used narrative summaries to present the findings. The analysis was conducted and discussed by all members of the review team. The results were compared, and the differences were resolved through discussions. Table 2 presents the most relevant aspects of these studies.
 |
Table 2 Study Characteristics |
Result
Selected Studies
The search identified 55 and 22 original research articles in PubMed and EBSCO, respectively; as many as 16 of them were duplicates. We selected nine studies by applying the inclusion and exclusion criteria. Most of the articles were non-vaccine and non-LMICs studies. Finally, we identified four studies eligible for the final review after excluding five non-cost-effectiveness studies. Figure 1 shows the detailed studies selection. The settings of the selected studies were South Africa, Pakistan. Turkey, and Thailand. All of them were novel studies in the cost-effectiveness analysis of COVID-19 vaccination conducted in 2021.
Study Design and Data Collection
All selected studies provided information on the economic analysis, specifically cost-effectiveness analysis (CEA) in LMICs. The CEA studies calculated the incremental cost-effectiveness ratio (ICER) by comparing a vaccination scenario to a no-vaccination scenario, allowing the cost and outcome differences for vaccination to be estimated.
A cost-effectiveness study of a COVID-19 vaccine was conducted by Reddy et al comparing two scenarios (with vaccination and without vaccination) by using a microsimulation model to estimate cost-effectiveness values, assessing evaluation of cost and clinical outcome in each scenario, and considering cost per year of life saved (YLS) in South Africa.13 Pearson et al estimated cost-effectiveness by comparing vaccinated and unvaccinated in a specific population group older (+65 years), considering cost per disability-adjusted life years (DALYs) in Sindh Province, Pakistan.14 A study by Hagens et al evaluated the cost-effectiveness of strategies for COVID-19 vaccinations for Turkey by using mathematical modelling in various potential scenarios, and compared the vaccination group with the no vaccination group by calculating cost per quality-adjusted life year (QALY) gained.15 Suphanchaimat et al conducted a study that determined which policy was the most cost-effective in selecting the target population (migrants and Thais) for the administration of COVID-19 vaccines by using ICER/one case averted, using ICER/one life saved, and ICER/one baht GDP.16 More information about the study design and data collection of the selected studies is presented in Table 3.
 |
Table 3 Methodological Characteristics |
Perspective, Time Horizon, and Modeling
CEA should state its analytic perspective and cost elements to provide thorough considerations the decision-maker could make.17–19 Decision context becomes very important because each perspective has limitations.18,19 Understanding the perspective used prompts decision-makers to fill the gap between them.18,19 One of the CEA studies applied the policymaker perspective,13 while another employed both healthcare and partial societal,14 and the rest utilized the provider perspective.15,16
Besides the study perspective, the time horizon determines the CEA value assessment.20 Because vaccination is a long-term intervention, the cost-to-benefit ratio has to consider more than 1-year time horizon.20 Only one selected study followed this consideration. It applied a 10-year time horizon.14 Two selected studies used a 1-year time,13,15 whereas the rest reported a shorter time.16 Discounting rate must be applied to adjust the health effect and cost if the time horizon is more than 1-year.20 The 10-year study followed WHO guidelines in determining the discount rate.14 Future expenses and annual capital investments were discounted at 3%, while health outcomes were at 3% (base case) or 0% (scenario range).14
There is a difference between the two 1-year studies. The study by Reddy et al stated no discounting in this time horizon,13 though Hagens et al set a 3% discount rate for both costs and health outcomes.15 The last selected study did not report the discount rate because the time for analysis was short.16
All of the selected studies had different economic modelling choices. Reddy et al took a Clinical and Economic Analysis of COVID-19 Interventions (CEACOV).13 It is a dynamic microsimulation model used to evaluate clinical outcomes and cost-effectiveness.21 The study compared the program between vaccination and no vaccination from the policymaker perspective in South Africa.13 Another model was used in Pakistan by Pearson et al,14 which developed Bayesian inference via the Markov Chain Monte Carlo model from the healthcare and partial societal perspective.14 The model fitted the introduction date, the primary reproduction number, a time-varying ascertainment rate, and the standard deviation of reported data points around the model.14 Hagens et al compared vaccination with no vaccination program in Turkey using a dynamic transmission model.15 They assumed homogeneity among various aspects over all age groups and vaccinated and non-vaccinated persons.15 In Thailand, Suphanchaimat et al used a compartmental susceptible-exposed-infected-recovered (SEIR) combined with a system dynamics (SD) model as a base.16 The model divided people into SEIR categories.16 The differences between this model and the traditional one are the subgroups of the infectious category (before and after isolation) and the separation of the population (Thais, registered migrants, and unregistered or undocumented migrants).16 These differences were related to quarantine and vaccination payer.16 Table 3 presents the evaluation characteristics of each selected study.
Modelling provides a necessary framework for synthesizing the available evidence and generating clinical efficacy and cost-effectiveness estimates.22,23 It consists of several health conditions representing the expected health effects of various treatments and predicts the relative effectiveness of techniques without direct comparison.23 The selected studies have used different modelling approaches. Thus, the decision-makers might not compare it directly because of different rationales, modelling assumptions, data strengths and uncertainty aspects of the parameters that were used in the models.22
Sensitivity Analysis
The various parameters used in economic assessment studies have uncertain aspects that could affect the results.13,20 Thus, the sensitivity analysis takes this into account.13,15,20 All of the selected studies conducted a sensitivity analysis,13–16 even one of them reported one-way and multi-way analysis.13 The standard parameters varied in the sensitivity analysis included vaccine cost, hospitalization cost, reproduction number, and vaccine effectiveness.13–16 Other parameters were distinctively associated with the study’s objectives,13–16 as seen in Pearson et al that was concerned about the duration of vaccination campaigns and natural immunity development.14
Cost Estimation
Economic costs are associated with the broader concept of resource consumption, whether or not such resources are exchanged.24 Due to limited resources, particularly LMICs, the cost is critical to be included in economic evaluation studies. All selected studies estimated the vaccination cost. Study in Thailand consisted of vaccine administration cost per person, which was free for Thais and registered migrants, but not for unregistered migrants, and vaccination cost per person [vial], Thais and registered migrants; meanwhile, unregistered migrants were being funded by the private sector or entrepreneurs.16 In South Africa and Pakistan, the study considers both the cost of vaccination doses and the cost of vaccine service delivery,13–16 whereas, in Turkey, the study considers only the cost of vaccine doses.15
All studies considered direct medical costs such as hospitalization, intensive care unit [ICU], diagnosis, treatment, and medication at home.13–16 In Turkey, however, only one study considered indirect medical costs like productivity losses owing to sick leave and premature death (see Table 4).15
 |
Table 4 Cost Elements |
Primary Result
All the results from the selected articles stated that promoting a vaccine program to reduce transmission and infection of COVID-19 was cost-effective and cost-saving (see Table 5). According to Reddy et al, in South Africa, the vaccination program could reduce the spread of infection and mortality with an ICER value of $520/YLS from several scenarios that have been tested and by assuming a conservative value of vaccine effectiveness.13
 |
Table 5 Primary Results |
Pearson et al affirmed that administering the vaccine in Pakistan could reduce the spread of infection with an ICER of $27.9 per DALY, considering the price of vaccine administration less than $10 and the value of the effectiveness above 70%.14 Prioritizing the administration of vaccines at the age above 65 years does not have a significant benefit compared to not giving priority.14
Study by Hagens et al in Turkey showed that giving vaccines could reduce transmission in the first scenario, with equal effectiveness on disease and transmission (90% effectiveness) with an ICER value of $511/QALY.15 In the second scenario, limited effectiveness in transmission (90% disease and 45% transmission effectiveness) with an ICER value of $1045/QALY.15 These two scenarios stated that administering vaccines was cost-effective and cost-saving.15
In Thailand, according to Suphanchaimat et al, the result showed that the cost-effective vaccination program in policy D (migrants centric) in all three categories was cost-effective.16 These three categories were cost-effectiveness analysis by different policy scenarios that yielded an ICER −1.0/one baht GDP, where a negative number means more benefits at a lower cost.16 The cost-effectiveness analysis by varying reproduction resulted in an ICER value of 14.2/one baht GDP.16 Another cost-effectiveness analysis, by varying vaccine effectiveness, resulted in an ICER of −1.2/one baht GDP.16
Discussion
Vaccination against COVID-19 has been proven to be more cost-effective than no vaccination at all. The clinical efficacy and economic value of the Covid-19 vaccination show superiority not only in high-income countries but also in LMICs. Unfortunately, the cost-effectiveness study towards COVID-19 vaccination, especially in LMICs, is finite. More researches are required to serve comprehensive confirmation about it.
All selected studies used a modelling approach to assess the cost-effectivity of the vaccination program.13–16 It also considered several alternatives evaluated in a sensitivity analysis, whether it is one way or multi-way.13–16 Although the economic modellings used by each selected study were different, they all confirmed the similar result that COVID-19 vaccination is cost-effective.
The modelling used were microsimulation, Markov model, dynamic transmission, and modified compartmental SEIR model.13–16 The differences made the decision-makers could not compare the effectiveness directly. They should understand the applying rationale, modeling assumptions, data’s strength used in populating the model, and uncertainty aspects of the parameters.22 It corresponded with the perspective and time horizon used. They all shaped the whole frame of the study.18,19,22,23
The objective of each study was a key point that determined the framing. The selected studies had a particular research question; thus they had a distinctive perspective and modelling. Nevertheless, they all compared a scenario between vaccination and no vaccination. In addition, they also conducted a sensitivity analysis to analyze the effect of various uncertain aspects of parameters. The standard parameters included vaccine cost, hospitalization cost, reproduction number, and vaccine effectiveness.13–16 Other parameters were distinctively associated with the objectives of the study itself,13–16 such as the vaccine acceptance,13 duration of vaccination campaigns,14 and non-productive period.15 All costs included in the analysis are direct medical costs such as vaccination costs, hospital treatment costs and hospital care costs for both those receiving regular treatment and those who need to be treated in the intensive care unit. For indirect cost, consider only the productivity loss due to COVID-19.
The minimum effectiveness of COVID-19 vaccines to become cost-effective is more than 50% to reduce transmission and mortality.14–16 Meanwhile, in South Africa regardless of the effectiveness value, the administration of COVID-19 vaccines is still cost-effective.13 The cost-effectiveness analysis methods used are ICER/YLS, which is defined as how many years a patient’s life can be saved by using the vaccine,13 ICER/DALY is defined as how many patients did not experience any disturbance in their productivity after vaccination,14 ICER/QALY was defined in terms of quality of life values obtained,15 and ICER/GDP was defined as the number of patients saved by measuring the GDP value used. In the calculation of the cost-effectiveness analysis, there were no scenarios of whether the virus would be re-infected after vaccination, so the results obtained were cost-effective and cost-saving.
This study is limited to the heterogeneity among the selected studies and the publication bias since all studies showed positive results in cost-effectiveness. Nevertheless, policymakers in LMICs can still consider the vaccination program due to the cost-effectiveness either in the high-income countries or in LMICs. As suggested in the study by Pearson et al, the affordability of the vaccine cost has to be highlighted by policymakers in planning the COVID-19 vaccination program.14
Conclusion
From the discussion above, we infer that the administration of the COVID-19 vaccine in various scenarios and economic models showed cost-effectiveness and even cost-saving results in LMICs. The government should focus on the policies to provide COVID-19 vaccines, especially in LMICs, to reduce the spread of the virus and the mortality from the COVID-19 virus. As countries with limited resources, more comprehensive studies are needed by LMICs to set the priority of various interventions for the pandemic mitigation.
Acknowledgment
This study was funded by Universitas Padjadjaran through AAS (Grant number: 1959/UN6.3.1/PT.00/2021).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Muralidar S, Ambi SV, Sekaran S, Krishnan UM. The emergence of COVID-19 as a global pandemic: understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie. 2020;179:85–100. doi:10.1016/j.biochi.2020.09.018
2. World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID-19; 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
3. World Health Organization. Weekly epidemiological update on COVID-19; 2021. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—23-november-2021.
4. World Health Organization. WHO COVID-19 dashboard. Available from: https://covid19.who.int/region/euro/country/tr.
5. Su S, Du L, Jiang S. Learning from the past: development of safe and effective COVID-19 vaccines. Nat Rev Microbiol. 2021;19(3):211–219. doi:10.1038/s41579-020-00462-y
6. Ali I. Impact of COVID-19 on vaccination programs: adverse or positive? Hum Vaccin Immunother. 2020;16(11):2594–2600. doi:10.1080/21645515.2020.1787065
7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
8. Güner R, Hasanoğlu I, Aktaş F. COVID-19: prevention and control measures in community. Turk J Med Sci. 2020;50(SI–1):571–577. doi:10.3906/sag-2004-146
9. Solís Arce JS, Warren SS, Meriggi NF, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27(8):1385–1394. doi:10.1038/s41591-021-01454-y
10. Randolph HE, Barreiro LB. Herd Immunity: understanding COVID-19. Immunity. 2020;52(5):737–741. doi:10.1016/j.immuni.2020.04.012
11. Massinga Loembé M, Nkengasong JN. COVID-19 vaccine access in Africa: global distribution, vaccine platforms, and challenges ahead. Immunity. 2021;54(7):1353–1362. doi:10.1016/j.immuni.2021.06.017
12. Jakovljevic M, Liu Y, Cerda A, et al. The Global south political economy of health financing and spending landscape - history and presence. J Med Econ. 2021;24(sup1):25–33. doi:10.1080/13696998.2021.2007691
13. Reddy KP, Fitzmaurice KP, Scott JA, et al. Clinical outcomes and cost-effectiveness of COVID-19 vaccination in South Africa. medRxiv. 2021;
14. Pearson CAB, Bozzani F, Procter SR, et al.; CHiL COVID-19 Working Group; CMMID COVID-19 Working Group. COVID-19 vaccination in Sindh Province, Pakistan: a modelling study of health impact and cost-effectiveness. PLoS Med. 2021;18(10):e1003815. doi:10.1371/journal.pmed.1003815
15. Hagens A, Inkaya AÇ, Yildirak K, et al. COVID-19 vaccination scenarios: a cost-effectiveness analysis for Turkey. Vaccines. 2021;9(4):399. doi:10.3390/vaccines9040399
16. Suphanchaimat R, Tuangratananon T, Rajatanavin N, Phaiyarom M, Jaruwanno W, Uansri S. Prioritization of the target population for coronavirus disease 2019 (COVID-19) Vaccination Program in Thailand. Int J Environ Res Public Health. 2021;18(20):10803. doi:10.3390/ijerph182010803
17. Kim DD, Silver MC, Kunst N, Cohen JT, Ollendorf DA, Neumann PJ. Perspective and Costing in cost-effectiveness analysis, 1974–2018. Pharmacoeconomics. 2020;38(10):1135–1145. doi:10.1007/s40273-020-00942-2
18. Garrison LP Jr, Pauly MV, Willke RJ, Neumann PJ. An overview of value, perspective, and decision context-a health economics approach: an ISPOR special task force report [2]. Value Health. 2018;21(2):124–130. doi:10.1016/j.jval.2017.12.006
19. Franklin M, Lomas J, Walker S, Young T. An educational review about using cost data for the purpose of cost-effectiveness analysis. Pharmacoeconomics. 2019;37(5):631–643. doi:10.1007/s40273-019-00771-y
20. Supadmi W, Suwantika AA, Perwitasari DA, Abdulah R. Economic evaluations of dengue vaccination in the Southeast Asia region: evidence from a systematic review. Value Health Reg Issues. 2019;18:132–144. doi:10.1016/j.vhri.2019.02.004
21. Reddy KP, Shebl FM, Foote JHA, et al. Cost-effectiveness of public health strategies for COVID-19 epidemic control in South Africa: a microsimulation modelling study. Lancet Glob Health. 2021;9(2):e120–e129. doi:10.1016/S2214-109X(20)30452-6
22. Marshall DA, Grazziotin LR, Regier DA, et al. Addressing challenges of economic evaluation in precision medicine using dynamic simulation modeling. Value Health. 2020;23(5):566–573. doi:10.1016/j.jval.2020.01.016
23. Ministry of Health Malaysia. Pharmacoeconomic Guidelines for Malaysia. Available from: https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/pharmacoeconomic-guidelines-malaysia-malaysia-second-edition-2019-final-page-adjustment.pdf.
24. Lou J, Kc S, Toh KY, et al. Real-world data for health technology assessment for reimbursement decisions in Asia: current landscape and a way forward. Int J Technol Assess Health Care. 2020;36(5):474–480. doi:10.1017/S0266462320000628
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
