Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
Cost Analysis and Rational Use of Anti-Glaucoma Therapy in a Tertiary Hospital in Ghana
Authors Ofei-Palm CNK , Tagoe NN, Jatoe D, Agyare A, Ankrah D
Received 1 April 2021
Accepted for publication 28 May 2021
Published 2 July 2021 Volume 2021:13 Pages 619—627
DOI https://doi.org/10.2147/CEOR.S311058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Charles Nii Kwade Ofei-Palm,1 Naa Naamuah Tagoe,2 Dong Jatoe,1 Angela Agyare,1 Daniel Ankrah3
1Lions International Eye Centre (LIEC), Pharmacy Unit, Korle Bu Teaching Hospital, Accra, Ghana; 2Lions International Eye Centre (LIEC), Korle Bu Teaching Hospital, Accra, Ghana; 3Pharmacy Department, Korle Bu Teaching Hospital, Accra, Ghana
Correspondence: Charles Nii Kwade Ofei-Palm
Lions International Eye Centre (LIEC), (Pharmacy Unit), Korle Bu Teaching Hospital, P. O. Box 77, Korle Bu, Ghana
Tel +233244661941
Email [email protected]
Introduction: Glaucoma is the leading cause of irreversible blindness worldwide. In Ghana, 19.4% of all blindness recorded is due to glaucoma. Reducing intraocular pressure medically (using eye drops) is the evidence-based therapeutic option.
Objective: To determine the rational use and undertake cost analysis of anti-glaucoma drugs among patients attending clinic at the Lions International Eye Centre (LIEC), Korle-Bu Teaching Hospital.
Methods: In this cross-sectional study, we reviewed all prescriptions presented to the pharmacy unit from 01/12/2015 to 31/03/2016. The dispensed drops were classified, and all anti-glaucoma drugs were identified. This was followed by cost analysis.
Results: A total of 588 prescriptions were captured, 27.3% (161/588) contained an anti-glaucoma medication. The mean number of anti-glaucoma medications was 1.71 of which 52.7% was prescribed to females. Prostaglandin analogs (PGA) were the most prescribed (37% (102/276)), followed by beta blockers (25.4% (70/276)), carbonic anhydrase group of medicines (16.3% (45/276)), combined beta blockers (11.2% (31/276)), alpha agonists (8.7% (24/276)) and miotics (1.4% (4/276)). The median (IQR) cost of anti-glaucoma therapy per prescription per month was GHC 65.00 (GHC38.5-GHC140) about [US$16.25 (US$ 9.6–US$35)]. Azopt (Brimonidine) was the most expensive with daily treatment cost of GHC 5.8 (about US$ 1.45), whilst the least expensive drug with a daily treatment cost of GHC 0.14 (about US$ 0.035) was timolol eye drops.
Conclusion: Prostaglandin analogs though expensive remain the most preferred treatment for managing glaucoma at the Korle-Bu Eye Centre in Ghana. This may adversely affect treatment among the poor since prostaglandins analogs are currently not covered by insurance.
Keywords: cost analysis, anti-glaucoma therapy, rational drug use, Korle-Bu, Ghana
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide. Glaucoma, however, presents an even greater public health challenge than cataracts: because it causes irreversible blindness.1
Currently, it is estimated that 80 million people in the world live with Open Angle Glaucoma (OAG) and Angle Closure Glaucoma (ACG) glaucoma of which 11 million are blind. This prevalence is an increase of about 20 million since 2010.2
The most prevalent glaucoma in Africa (primary open angle glaucoma) occurs at an earlier age. This is associated with a higher intraocular pressure, more rapid progression and late presentation.3
Efforts to understand more about the magnitude and distribution of glaucoma in Africa have usually been limited by reliance on clinic populations and inadequate definitions of glaucoma. Nonetheless, the surveys indicate that OAG is an important cause of blindness in Africa. The early stages of both types of glaucoma are often asymptomatic so patients often present late, particularly in developing countries. The challenges are with late presentation for diverse reasons one of which is low awareness and the silent nature of the disease.
In Ghana, 19.4% of blindness is due to glaucoma. The prevalence of blindness and severe visual impairment was 0.74% and 1.07%, respectively. In rural Ghana, one-third (34.1%) of all glaucoma patients report bilaterally blind while half are uniocularly blind.4 Reports indicate that at least half of eyes are already blind at presentation.5
At present, there are no therapies available that prevent the development of glaucoma. Similarly, no therapies are available to reverse glaucoma-induced vision loss. However, a reduction of the intraocular pressure (IOP) has been shown to protect against further damage to the optic nerve head.6 Reducing IOP is presently the evidence-based, most accepted, and most practiced therapeutic approach of glaucoma patients.7 Results of key landmark trials have shown that lowering intraocular pressure is the main stay of management.8
Innovations in diagnosis and therapy have made glaucoma management complex. Though surgical options are available for lowering intraocular pressure, medication remains the first line in most cases of glaucoma.9
Drug management of glaucoma commonly includes five classes of drugs: α-adrenergic agonists, β-adrenergic antagonists, cholinergic agonists, prostaglandin analogues, and carbonic anhydrase inhibitors. There are combinations of some these drugs.
In most developing countries like Ghana, most clients do not have the money to pay for the eye drops which they may need to use for the rest of their lives.4
The therapy for the glaucoma is now in a dynamic phase. The management of the disease has an enormous impact on our society in terms of patient’s morbidity, loss of productivity, number of ophthalmic consultations and health care costs. The increase in health care costs may be due to the lifelong management of the condition once a patient has been diagnosed with the disease.
As recently discovered pharmacological agents and other treatment modalities become available, ophthalmologists may be put in a dilemma about choosing a low cost but effective anti-glaucoma medication from the wide variety of options available.
In spite of all these, very few studies have been done in Ghana to look at the cost analysis of glaucoma therapy in general to provide patients and health care providers with calculated yearly costs of topical glaucoma medications.
We therefore conducted this study to determine the rational use and undertake cost analysis of anti-glaucoma drugs among patients attending clinic at the Lions International Eye Centre (LIEC) of the Korle-Bu Teaching hospital. We also sought to provide patients and healthcare providers with calculated yearly costs of topical anti-glaucoma medications prescribed.
Methodology
This was a cross-sectional study at LIEC, Korle-Bu Teaching Hospital based on adherence to the standard prescription form at the hospital. As a routine, all prescriptions submitted and dispensed at the pharmacy are detained. We reviewed prescriptions that were presented to the pharmacy unit from 1st October 2015 to 31st March 2016. All prescriptions that had at least one medicine served were captured at the unit. Medicines that were not available or dispensed (due to non-affordability of patients) were not captured as patients took those prescriptions away. A copy of the prescription was obtained with the help of a pre-inserted carbon, in a special format. A copy of the prescription was kept at the pharmacy unit together with a copy of the point of sales receipt which generates the names and cost of drugs purchased. Two (2) members of staff were trained to extract data from the prescription forms. A specialist pharmacist and a pharmacy technologist reviewed the classifications of all the medicines on the prescriptions captured.
All the prescriptions were analyzed for the type of anti-glaucoma medication prescribed, percentage of mono-therapy vs combination therapy prescribed, percentage of fixed dose combination prescribed, percentage medicines prescribed using generic names and cost analysis (monthly and yearly) for all the types of anti-glaucoma drugs including their dose, frequency, duration as well as route of administration. Each parameter was expressed as a percentage.
Cost Analysis of Different Anti-Glaucoma Medication
The average cost per milliliter (mL) of different drugs (eye drops) was determined by calculating cost per mL of the various brands. As size of a drop is between 25 and 50 ul, an average of about 27 drops constitutes 1 mL. For drugs used in both eyes, we then calculated the number of drops required per day (determined by using the manufacturer’s recommended daily dosing regimen) for each medication, and then we calculated the cost of therapy for a month and a year.
Data extracted was entered into Microsoft Excel and subsequently exported into SPSS Version 20 for analysis. Descriptive statistics of patients’ demographics and types of prescribed anti-glaucoma medications were presented as counts and proportions. Chi-square test was used to analyze the data where appropriate and values with P < 0.05 were considered statistically significant.
Ethical Statement
In this study, no patients were contacted. Collection of prescriptions of patients is a routine process in the hospital. In conducting the study all identifiable information of patients were de-identified from all prescriptions. The threat to patients was therefore minimal and according to the present Standard Operating Procedure of the Ghana Health Service Ethics Review Committee, ethical approval is not deemed necessary for this study.10
Results
A total number of 588 prescriptions were captured in the study because of the availability of the duplicate carbon prescription forms. Out of this number of prescriptions, 161 (27.3%) contained an anti-glaucoma medication. The total number of anti-glaucoma drugs in the 161 prescriptions was 276 with an average of 1.71 (about 2) per prescription.
Of 161 patients, 85 (52.7%) were prescribed to female clients and 76 (47.2%) to male clients. Mean age was 58.6±21.2 years with information on the ages not available for 68.7% of prescriptions captured.
Prostaglandin analogs (PGA) were the most prescribed (102/276 (37%)) medicines, followed by beta blockers (BB) (70/276 (25.4%)), with the Miotics (CM) (4/276 (1.4%)) being the least prescribed. See Figure 1.
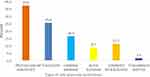 |
Figure 1 Prescribing patterns of anti-glaucoma medications. |
Latanoprost was the most prescribed (93/102 (91.2%)) among the prostaglandin analogue group with bimatoprost (Lumigan) (4/102 /3.9%)) being the least prescribed. See Figure 2.
 |
Figure 2 Distribution patterns for prostaglandin analogs. |
Timolol was the most prescribed (56/70 (80%)) beta blocker followed by levobunolol (Betagan) (8/70 (11.4%)) and betaxolol (Betoptic) (6/70 /8.6%)) in that order. See Figure 3.
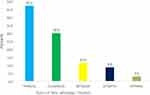 |
Figure 3 Distribution patterns for beta adrenergic blockers. |
Oral acetazolamide accounted for 72.3% (34/45) within the carbonic anhydrase group followed by brinzolamide (Azopt) (27.7% (13/45)).
With the combined beta blockers, Timolol+ brimonidine (Combigan) accounted for 64.5%, whilst Timolol + dorzolamide and Timolol+ Bimatoprost represented 32.3% and 3.2%, respectively.
Combination therapy or fixed dose combination therapy (FDC) constituted 55.1% of prescriptions whilst mono-therapy was 44.9%. Latanoprost was the most prescribed representing 45.2% of all the monotherapy treatment in the study, followed by timolol which recorded 17.3%. See Figure 4.
 |
Figure 4 Distribution patterns for monotherapy prescribed. |
Anti-glaucoma therapy prescribed as generics were 141 (51.1%) and proprietary brands accounted for 135 (48.9%).
Cost Analysis
Branded travoprost (Travatan) was the most expensive treatment amongst the prostaglandins with a daily treatment cost of GHC 4.90 (about US$1.20), followed closely by branded latanoprost (Xalatan) with a daily treatment cost of GHC 3.60 (about US$0.90). However, the generic latanoprost is about half the treatment cost of the branded counterpart. The least expensive drug with a daily treatment cost of GHC 0.14 (about US$ 0.035) was timolol eye drops whilst Brinzolamide (Azopt) was the most expensive with a daily treatment of GHC 5.80 (about US$1.45)
The median (IQR) cost of anti-glaucoma therapy per prescription per month was GHC 65.00 (GHC38.5-GHC140) about [US$16.25 (US$ 9.6–US$35)] See Table 1.
 |
Table 1 Cost Analysis of Various Anti-Glaucoma Medicines |
Discussion
In our study, the total number of drugs in the 161 prescriptions was 276. The average number of drugs per prescription was 1.71 (~2). This is well within the WHO recommended limit of 2 and falls within the rational use of drugs requirement as stipulated by the WHO. This recommended limit criteria set out by the WHO is an important tool for assessing rationality of prescriptions.11 Our results are comparable to other studies that had similar averages12 but higher in a study in India that reported average of 1.49 per prescription13 but lower averages compared to other similar studies.14
An increase in the number of average drugs per prescription is an important index. This index gives an indication for polypharmacy which is associated with an increased risk of drug interactions. This may lead to unwanted side effects and increase prescribing and dispensing errors.
Even though sex did not play any role in glaucoma as illustrated in these studies,2,15 our study revealed that prescriptions were given to more female (52.7%) than male (47.2%) clients. A similar result was reported in another study in Ghana and other studies in India.16,17 This calls for further and most probably larger studies to ascertain the role played by gender among Ghanaian patients with glaucoma.
Drug management of glaucoma commonly includes five classes of drugs: α-adrenergic agonists, β-adrenergic antagonists, cholinergic agonists, prostaglandin analogs and carbonic anhydrase inhibitors. Combinations of these groups of drugs is also an available option for therapy.18 In our study, prostaglandin analogs were the most prescribed anti-glaucoma medicine representing 37% of all medications prescribed. Latanoprost was the most prescribed prostaglandin analog. Beta blockers represented 25.4% of the total category of anti-glaucoma medications prescribed in the study. Amongst the beta blockers, timolol was the most prescribed representing 80% of the total number of beta blockers found in the study.
Prostaglandin analogs as first line and most prescribed glaucoma medication in this study is corroborated by other studies.19,20 In contrast to other studies that were carried out in India, beta blockers especially timolol was most used.17,21 The reasons we attribute to the PGA usage as a first line choice is that it is the drug of choice recommended by Ghana Standard Treatment Guidelines22 for the treatment of Glaucoma in Ghana. It is also part of the Ghana Essential Drug List23 but unfortunately not included in the medicines list of the National Health Insurance Scheme (NHIS).24 The NHIS provides reduced healthcare cost, sometimes free (including cost of medications) to patients on the scheme. A look at the cost analysis proved that prostaglandins were far more expensive than beta blockers. This indicates that all those patients with a prostaglandin analog prescription will have to pay full payment for their medication by cash. This will bring an increased economic burden to clients especially those with low socio-economic status. It may even lead to non-adherence to treatment which may have a profound effect on disease prognosis.
Prostaglandin analogs have superseded beta adrenergic blockers as the primary mode of treatment for primary open angle glaucoma because of better patient compliance. This is because the prostaglandin analogs are used once a day whereas the beta blockers are used 2–3 times daily. The prostaglandin analogs also have lesser adverse effects compared to the beta blockers.25 Furthermore, Prostaglandin analogs compared to β-blockers have greater efficacy in lowering diurnal and nocturnal IOP with lesser systemic adverse effects.26
With the carbonic anhydrase inhibitors, apart from being the third most prescribed medications, they are the only group with an oral preparation. Oral acetazolamide accounted for 72.3% of the total number drugs prescribed from this group and gutt brinzolamide (Azopt) accounted for only 27.7%.
It is not surprising to see acetazolamide as the only used oral anti-glaucoma. Topical drugs have proved more efficacious than oral medications. The rationale for the preference of topical over oral preparations is to minimize systemic side effects. It is a routine practice in this hospital to prescribe oral acetazolamide between a week to three months after which it is discontinued and replaced with topical carbonic anhydrase inhibitors where necessary. Acetazolamide is prescribed a lot because it is affordable and covered under National Health Insurance Scheme. Most of the patients put on it cannot afford prostaglandins analogs so despite its side effects it still remains useful. It also attains faster reduction of IOP. It is used for a short period of time so that the known (not documented in our population) risk of acidosis, bone marrow depression, renal stones, gastro-intestinal disturbances, tinnitus and hypokalemia could be minimized.21
In our study, topical carbonic anhydrase inhibitor brinzolamide was prescribed in (4.0%) of the patient. It was prescribed less frequently as mono therapy. This may be due to higher frequency of instillation required (3 times a day) compared to prostaglandin analogs which are instilled once at night and β-blockers mainly timolol prescribed for instillation twice daily. It is more expensive compared to the costs of prostaglandin analogs and β-blockers. These factors are important in considering the compliance in patients with glaucoma. Compliance plays an important role in control and prevention of progression of the disease. Brimonidine was used less frequently in this study because of its cost.
Prescriptions for combination therapy were more prevalent compared to prescriptions for monotherapy. This is in line with other studies that indicated that combined therapy yields additive and better results than monotherapy in higher intra ocular pressure (IOP) reduction.27 However, combination therapy is given only when the patient requires more than one anti-glaucoma medication. Combination therapy can be with fixed drug combinations (FDC) or concurrent use of more than one anti-glaucoma medication. Most FDC contains a beta blocker. FDC therapy leads to improved compliance and enhance patient convenience, compliance, cost effectiveness and safety.28 Our results are comparable to other studies but demonstrated higher usage of FDC therapies.17,21
In our study, FDC with beta blockers accounted for 11.2% of the medications. Timolol + brimonidine (combigan) mainly prescribed as a proprietary or branded drug accounted for 64.5% of the study. Apart from lowering the IOP, brimonidine also has neuro protective effect29 and also constituted 8.7% of anti-glaucoma medications in this study. The neuro-protective effect of brimonidine might have contributed to the prescribers’ choice even though it is expensive. This is in line with other studies that suggested the same drug as the most frequently prescribed FDC.21 Other studies have reported timolol plus dorzolamide17 and timolol plus bimatoprost as the most used FDC. In our study, they were second and third most frequently used FDC respectively.
Timolol remained the most used beta blocker accounting for 80% of usage in our study. This could be attributed to its low cost of GHC 0.14 per day as well as coverage under National Health Insurance Scheme. This prescription pattern is corroborated by other studies in which it was reported as the frequently prescribed drug.17
Similar patterns regarding beta-blocker usage have been reported.21
Only 1.4% patients were put on miotics or para-sympathomimetic in our study. This class of drugs are not preferred now for the management of glaucoma because of their side-effects like diminished night vision, reduced visual acuity, opacities, myopia and visual field contraction.16 Very few studies reported its usage.20 There is clear indication in our study that its usage is gradually diminishing in the treatment of glaucoma.
Many drugs were prescribed by their generic names (51.1%) in our study. Prescribing under generic name is considered economical and rational. The World Health Organization strongly advocates the practice of prescribing drugs by their generic names.30 The World Health Organization advocates use of only generic names from national essential medicines list (NEML) for better management.11 This study also revealed that the percentage of drugs prescribed from Ghana National Essential Drug List was very high almost 100%. The exceptions were the fixed dose combination therapies.
In our study, topical prostaglandin analogs have become a common first-choice glaucoma therapy, partly owing to their relatively consistent clinical efficacy, and also due to their lower frequency of adverse effects. However, topical prostaglandin analogs are expensive, ranging from generic GHC1.18 (US$0.26) to GhC1.93 (US$0.42) per day. Branded prostaglandin analogs ranged from GHC3.6 (US$0.80) to GHC4.9 (US$1.10) per day. The least expensive option for the medical therapy of glaucoma was generic timolol products. This study showed an average cost of GHC0.20 (US$0.04) per day compared to the branded timolol (Cusimolol-Alcon) with an average daily cost of GHC 0.59 (US$0.13).
Since Ghana is a developing country our cost per day is far lower than what is reported in the western world even as compared to the same branded product.31
For example, branded prostaglandin analog (Xalatan) that cost US$0.8 in our study, costs US$1.25 in the USA.31 In our study, the cost per year for branded brimonidine (Alphagan) and branded brinzolamide (Azopt) were US$223 and US$527 respectively but in a study conducted in the USA that evaluated yearly cost of glaucoma medications at a University-affiliated teaching hospital with its own health maintenance organization for a three-year period (1998 to 2000), the cost of branded brinzolamide (Azopt) was about half.32 This high cost makes the drug very unlikely to be prescribed by ophthalmologists in our setting even though it is a good option.
Finally, our study showed that the highest cost of glaucoma treatment per prescription per annum is US$ 420. This is similar to a study done in Ghana16 that looked amongst other things at the cost of glaucoma medications together with other non-direct medications cost associated with surgery.
The minimum wage in Ghana during the study period was GHC216 (US$54.00) monthly and GHC 2592 (US$648.00) per annum. The monthly cost of branded prostaglandin analog of GHC 65 (US$16.25), works out to 62% of a monthly salary of an average worker in Ghana, this cost could be considered too high. Again, the annual Real GDP Per Capita was (GHC) 1299.92 (US$ 325) with Growth in Real GDP Per Capita of 3.07% In Ghana, the minimum wage has consistently lagged behind comparison to per capita income.33
Our Prices were obtained from the hospital-based government facility where prices are heavily subsidized or reduced because of competitive tender processes and also low mark up as compared to private pharmacies and private hospitals where the average cost is likely to be higher.
Prostaglandin analogs remain the most preferred treatment for managing glaucoma by prescribers at the Lions International Eye Centre of the Korle-Bu Teaching Hospital followed by beta blockers. This is corroborated by most studies in developed countries.19,20 This outcome is also in line with standard treatment guidelines in Ghana.22 Non availability of PGA on the National Health Insurance Scheme may however adversely affect treatment among the poor who cannot afford to pay for these medications.
The mean age was 58.6±21.2 years with 68.7% information on the ages not available. This percentage of missing values for the age made it difficult for us to compare our study with other age groups in other studies but this is quite similar to the prevalence study that was done at Tema in Ghana.34
The study did not account for patients or clients who presented prescriptions to the pharmacy but did not purchase the prescribed drug as well as those who presented prescriptions but could not be served due to unavailability of the drops at the pharmacy. Such clients would usually take their prescriptions away.
These findings cannot be generalized as prescribing preferences may vary from one hospital to another in the absence of a national treatment guideline for glaucoma diseases. However, being a national training center for most ophthalmologists in Ghana, the results may be a true representation of prescribing pattern for glaucoma medication in Ghana.
Further, the study did not provide the type of glaucoma treated as this was not available on the prescription.
Conclusion
We estimated in this study that each glaucoma patient will spend approximately between GHC 462 to GHC 1680 per annum. This will hopefully serve as a guide for patients and prescribers on how much is spent annually on the management of ophthalmic morbidities. Since glaucoma therapy is lifelong. There is the need to consider the cost-effectiveness and convenience in individual patients to ensure adherence to therapy to avoid irreversible blindness and decreased productivity.
The wide cost variation among various brands of the same drug our study could help the ophthalmologist to use all possible measures of pharmacoeconomics while prescribing an anti-glaucoma drug.
Recommendation
PGAs are recommended by the Ghana Standard and Treatment Guidelines as first line therapy for the management of glaucoma and are listed in the Essential Drug List of Ghana. They are however not covered by the National Health Insurance Scheme (NHIS) in Ghana. This study found PGA to be the most prescribed glaucoma medication though it was the most expensive of all glaucoma medications. We therefore advocate for the inclusion of PGA under the NHIS to make it available to all including the poor and vulnerable in our society.
Disclosure
The authors of this study have no conflicts of interest to declare.
References
1. Kutty GKV, Sambasivam N, Nagarajan M. A study on: drug prescribing pattern in Madurai city. Ind J Pharmacol. 2002;34(5):361–362.
2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
3. Cook C. Glaucoma in Africa: size of the problem and possible solutions. J Glaucoma. 2009;18(2):124–128. doi:10.1097/IJG.0b013e318189158c
4. Gyasi MK, Amoako M. Presentation patterns of primary open angle glaucomas in North eastern Ghana. Ghana Med J. 2010;1(44):25–30.
5. Lewallen S, Courtright P. Blindness in Africa: present situation and future needs. Br J Ophthalmol. 2001;85(8):897–903. doi:10.1136/bjo.85.8.897
6. Schulzer M, Drance SM, Douglas GR. A comparison of treated and untreated glaucoma suspects. Ophthalmology. 1991;98(3):301–307. doi:10.1016/S0161-6420(91)32296-6
7. Shaarawy T, Flammer J, Haefliger IO. Reducing intraocular pressure: is surgery better than drugs? Eye. 2004;18(12):1215–1224. doi:10.1038/sj.eye.6701374
8. Gordon MO, Beiser IA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi:10.1001/archopht.120.6.714
9. LeBlanc RP. Canadian glaucoma strategy. Can J Ophthalmol. 2007;42(1):60–65. doi:10.3129/canjophthalmol.06-100
10. Ghana Health Service. Ghana health service-ethics review committee: standard operating procedures; 2015. Available from: https://www.ghanahealthservice.org/downloads/Standard_Operating_Procedures.pdf.
11. World Health Organization. How to Investigate Drug Use in Health Facilities: Selected Drug Use Indicators. Geneva: World Health Organization. WHO/DAP; 1993:1–87.
12. Parween N, Gupta S, Kumar S, et al. Drug utilization study in primary glaucoma patients attending ophthalmology outpatient department of a Tertiary Care Hospital of Western Uttar Pradesh Delhi. J Ophthalmol. 2017;28(1):25–29. doi:10.7869/djo.286
13. Pradeep RJ, Vijay VM, Yeshwant AD. Drug utilization study in ophthalmology outpatients at a Tertiary Care Teaching Hospital. ISRN Pharmacol. 2013;768792. doi:10.1155/2013/768792
14. Jain VP, Shrivastava B, Agarwal M. Drug utilization pattern of drugs used along ophthalmic antiallergics formulations used in patients diagnosed with seasonal and perennial allergic conjunctivitis. Asian J Pharm Sci Res. 2011;1(5):15–20.
15. Klein BE, Klein R, Sponsel WE. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99(10):1499–1504. doi:10.1016/S0161-6420(92)31774-9
16. Ocansey S, Kyei S, Diafo A. Cost of medical management and prescription pattern for primary open angle Glaucoma (POAG) in Ghana- a retrospective cross sectional study from three referral facilities. BMC Health Serv. 2016;2016(16):1–8.
17. Yadav AK, Patel V. Drug use in primary open angle glaucoma: a prospective study at a tertiary care teaching hospital. Indian J Pharmacol. 2013;45(2):117–120. doi:10.4103/0253-7613.108279
18. Alward WL, Wood AJJ. Medical management of glaucoma. N Engl J Med. 1998;339(18):1298–1307. doi:10.1056/NEJM199810293391808
19. Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee; Canadian Ophthalmological Society. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. Can J Ophthalmol. 2009;44(1):S7–93.
20. American Academy of Ophthalmology, Glaucoma Panel. Preferred practice pattern® guidelines. primary open-angle glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2010. Available from: http://www.aao.org/ppp.
21. Rai S, Khilji S, Rao LG, et al. Prescribing pattern and adverse events of drugs used in patients with primary open-angle glaucoma (POAG) attending a tertiary care hospital: retrospective study. Natl J Physiol Pharm Pharmacol. 2017;7(2):189–193. doi:10.5455/njppp.2017.7.0822624082016
22. Ministry of Health, Ghana. Standard treatment guidelines; 2018. Available from: www.moh.gov.gh/wp-content/uploads/…/Standard-Treatment-Guideline-2010.pdf.
23. 7TH edition-Ministry of Health, Ghana. Essential medicines list; 2017. Available from: http://apps.who.int/medicinedocs/documents/s18014en/s18014en.pdf.
24. National Health insurance list; 2021. Available from: www.nhis.gov.gh/nhia.aspx.
25. Stewart WC, Konstas AG, Nelson LA, et al. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology. 2008;115(7):1117–1122. doi:10.1016/j.ophtha.2007.10.004
26. Liu JH, Medeiros FA, Slight JR, et al. Comparing diurnal and nocturnal effects of brinzolamide and timolol on intraocular pressure in patients receiving latanoprost monotherapy. Ophthalmology. 2009;116(3):449–454. doi:10.1016/j.ophtha.2008.09.054
27. Dutta LC, Maskati BT, Dutta KK. Current trends in drug therapy of POAG. Modern Ophthalmol. 2000;1:481–487.
28. Kulkarni SV, Damji KF, Buys YM. Medical management of primary open-angle glaucoma: best practices associated with enhanced patient compliance and persistency. Patient Prefer Adherence. 2008;2:303–314. doi:10.2147/PPA.S4163
29. Schuman SJ, Burke J, Cantor BL, et al. Innovations in the Medical Management of Glaucoma. Oxford institute for continuing Education; 2000.
30. World Health Organization. Action Programme on Essential Drugs and Vaccines .Essential drugs: Action for equity. World Health Organization. (1992). Available from: https://apps.who.int/iris./handle/10665/58957. Accessed June 27,2020
31. Fiscella RG, Green A, Patuszynski DH, et al. Medical therapy cost considerations for glaucoma. Am J Ophthalmol. 2003;136(1):18–25. doi:10.1016/S0002-9394(03)00102-8
32. Vold SD, Riggs WL, Jackimiec J. Cost analysis of glaucoma medications: a 3-year review. J Glaucoma. 2002;11(4):354–358. doi:10.1097/00061198-200208000-00013
33. Otoo KN. Minimum Wage Fixing in Ghana. 2018.
34. Budenz DL, Barton K, Whiteside-de Vos J, et al. Prevalence of glaucoma in an urban West African population: the Tema Eye Survey. JAMA Ophthalmol. 2013;131(5):651–658. doi:10.1001/jamaophthalmol.2013.1686
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
