Back to Journals » Clinical Ophthalmology » Volume 14
Cosmetic Pterygium Surgery: Techniques and Long-Term Outcomes
Received 27 February 2020
Accepted for publication 14 May 2020
Published 18 June 2020 Volume 2020:14 Pages 1681—1687
DOI https://doi.org/10.2147/OPTH.S251555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Arun C Gulani, Aaishwariya A Gulani
Gulani Vision Institute, Jacksonville, FL, USA
Correspondence: Arun C Gulani
Gulani Vision Institute, 8075 Gate Parkway West Suite 102, Jacksonville, FL 32216, USA
Tel +1 904 296 7393
Fax +1888 650-0744
Email [email protected]
Background: To demonstrate the long-term results of enhanced cosmetic pterygium surgery with extensive Tenonectomy, adjunctive fibrin-glued amniotic membrane transplantation (AMT), and mitomycin C (MMC).
Methods: Retrospective chart review of patients who had pterygium surgery with AMT and MMC between January 2001 to July 2017 and had completed at least 6 months of follow-up. Early and long-term postoperative cosmetic outcomes, recurrence rate, and complications were analyzed. Cosmetic outcomes were evaluated based on patient and surgeon reported outcome measures.
Results: The study was conducted on a total of 603 eyes of 578 patients (316 males, 262 females) with an average age of 52.9 ± 15.1 years. At post-op day 1, patients reported no discomfort and could not tell which eye had surgery based on patient reported subjective grading scales. Over an average follow-up period of 23.1 ± 35 months (range: 6– 216 months), there was one pterygium recurrence (0.2%), eighteen granulomas (2.9%), one self-resolving scleral melt (0.2%), one correctable restricted ocular motility (0.2%), one pupil abnormality (0.2%), one dellen (0.2%) and one correctable upper lid abnormality (0.2%). Planned laser vision correction was used for residual corneal scar in eleven eyes (1.8%) as a staged refractive approach.
Conclusion: This study highlights an improved technique of an old concept of pterygium surgery that not only reduces the recurrence but also enhances cosmetic excellence and improves the quality of vision.
Keywords: amniotic membrane, mitomycin C, pterygium
Background
Pterygium is a common ocular surface pathology characterized by chronic inflammation, fibrovascular proliferation, and invasion. Its prevalence is common in areas with more exposure to ultraviolet radiation, which induces localized damage to the limbal stem cells and focal conjunctivalization of the cornea.1 Pterygium varies from small, atrophic lesions to large, aggressive fibrovascular growths.2 However, vision is usually compromised in the majority of cases. Moreover, pterygium patients are often embarrassed due to constant ocular redness and disfiguring growth appearance. Surgical intervention is usually indicated for cosmetic reasons or to correct visual disturbances due to chronic irritation, irregular astigmatism, blockage of the optical axis, or restriction of ocular motility. Surgery, however, is associated with an unpredictable rate of postoperative recurrence, which can also be worse than the primary lesion.
Over the years, ophthalmic surgeons have explored various techniques in an attempt to reduce pterygium recurrence.3–8 However, there is no single procedure that prevents recurrence entirely. While some reported techniques have clinically appropriate outcome and low recurrence rate, there is still a significant sector of unhappy patients due to either residual visual disturbances or suboptimal cosmesis, or both. It is therefore imperative to develop a novel treatment strategy to reduce the recurrence, achieve an outstanding cosmesis and meet patient expectations.
We herein report our long-term experience in improving the surgical technique toward better visual outcome, excellent aesthetic appearance and overall patient satisfaction.
Methods
Study Design and Participants
This retrospective study was approved by Western Institutional Review Board (Puyallup, WA) and conducted according to the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). The medical records of all patients who underwent pterygium surgery at the Gulani Vision Institute (Jacksonville, FL) between January 2001 and July 2017 were reviewed. Patients who completed at least 6 months follow-up were included in data analysis. Data collected included demographics, medical history, preoperative evaluation, surgical technique, postoperative course, visual outcome, aesthetic appearance and complications. In addition, we focused on early and longterm patient satisfaction, for which we obtained a consent to share potential identifying information, photographs or testimonials. Tables 1 and 2 illustrate subjective scoring system used by patients to evaluate their postoperative outcomes.
 |
Table 1 Postoperative Subjective Grading of the Appearance on Day One |
 |
Table 2 Postoperative Subjective Grading of Discomfort Day One |
Tables 3–5 are the scoring systems used by the surgeon to evaluate the patient outcomes.
 |
Table 3 Grading of the Postoperative Site on the Basis of External and Slit Lamp Examination |
 |
Table 4 Grading of the Amniotic Graft Placement Postoperatively on Day One |
 |
Table 5 Postoperative Grading of the Cornea on the Basis of Slit Lamp Examination |
We did not involve patients or the public in our work.
The surgical technique was similar in all patients and performed by the same surgeon (AG) using the basic three steps: pterygium excision, mitomycin-C application, and amniotic membrane transplantation.
Pterygium Excision
(“Iceberg Concept Technique”)9 Following topical anesthesia (tetracaine hydrochloride, Tetravisc 0.5% Ocusoft, Rosenberg, TX) and preoperative topical antibiotic drops, patient was brought to the operative suite and laryngeal mask airway anesthesia was induced. After confirming anesthesia, a traction suture was placed at the opposite limbus using 7-0 black silk. The extent of the pterygium outside the visible inter-palpebral area was marked using a sterile marking pen. Intralesional 2% lidocaine with epinephrine was used for anesthesia and to further delineate the extent of pterygium in recurrent cases. The pterygium head was dissected off the cornea using Gulani Pterygium cross-action spreader in a single centrifugal movement. Dissection continued until we reached the bare sclera to separate the body of the pterygium from the healthy conjunctiva on top and the episclera underneath. Careful dissection allowed us to maintain a single dissection plane all the way to the fornices, which helps avoid button-holing the conjunctiva, invading the orbital septum, or injury to the rectus muscle underneath. For the latter, a modified muscle hook (Gulani muscle manipulator, Bausch and Lomb, Rochester, NY) was used to ascertain the anatomy. The entire pterygium mass was removed as one piece including its fan-like spread at the fornix. The single dissection plan and avoiding abrupt cutting help maintain bloodless field throughout the procedure. In addition, a weck-cell sponge (Beaver-Visitec, Waltham, MA) soaked in 1:1000 epinephrine was placed at the cut stump with gentle pressure for hemostasis while simultaneously creating a space for placement of amniotic membrane graft.
A specially designed 64-gage blunt blade was used to smoothen the corneal surface and the limbal area in a superficial, fast-moving pattern. Corneal scars were addressed with resistance-guided lamellar peel as needed.
MMC Application
Weck cell sponge pieces soaked in 0.04% MMC were placed at the truncated tissue under the conjunctival edge for 30 s, while protecting the remaining bare sclera from exposure to MMC by a dry Weck-cel. After removing the MMC sponges, the area was flushed with balanced salt solution.
AMT
The amniotic membrane graft (Amniograft®, Bio-Tissue®, Miami, FL) (AG) was cut to size, about 4 mm larger than the defect, and applied onto the cornea which acted like an illuminated “Receiving table” due to retro-illumination through a dilated pupil from topical epinephrine impact. AG was then slid onto the bare sclera and placed under the medial, superior and inferior conjunctiva with a no-touch “tyre–tool” technique. The AG was secured with fibrin glue (Tisseel® Baxter, Glendale, CA). The two components were applied separately beneath AG, and a Weck-cel sponge was used to place the graft on the sclera and also to swipe the fornices for any excess glue to avoid the possibility of postoperative keratitis/foreign body sensation. The 64-gauge blade was then used to trim the excess AG flush with the limbus in a single, circumlinear controlled motion. In cases with corneal scar, we considered extending AG beyond the limbus to enhance healing of the corneal surface. Alternatively, we applied sutureless amniotic membrane (ProKera® Bio-Tissue, Inc. Miami, FL) the day after the surgery. In some cases, where we identified scleral thinning after dissection, AG was also used in layers to reinforce the sclera while creating normal anatomical relations of the ocular surface as part of fornix reconstruction.
Postoperative Care
Postoperative treatment included antibiotic drops four times daily for 2 weeks, steroid drops four-time daily for the first week and then tapered down every week by one drop for 1 month, followed by non-steroidal drops once a day for 2 weeks.
The patients were scheduled for postoperative follow-up visits at 1 day, 1 week, 1 month, 3 months, 6 months, and yearly thereafter as needed. Best efforts were utilized for out of town/state/country patients in contacting them and/or their eye doctors with policy to call or come in anytime if able to make it back to town. On day one, all patients were asked to walk up to the mirror and exclaim how they felt. The patients were then examined thoroughly and the course of the recovery and healing is recorded. Complications such as postoperative inflammation, pyogenic granuloma, inclusion cysts, and Dellen formation were also recorded. Recurrence time and extent were also recorded as conjunctival if the fibrovascular re-growth is limited to the conjunctiva and as corneal if the tissue growth extends to the cornea across the limbus.12
Results
A total of 578 patients (316 males, 262 females) with an average age of 52.9 ± 15.1 years were included in the study (34 patients excluded). Of these cases, there were a total of 603 eyes with pterygium (570 primary and 33 recurrent) that underwent extensive Tenonectomy with adjunctive intraoperative MMC and AMT. The surgery was uneventful in all cases, except one who had extensive double head pterygium and developed recurrence 1 month after surgery.
Patients were asked to walk up to the mirror on postoperative day one, and exclaim how they felt (Figure 1).10 Patients were asked to look carefully at their eyes and provide verbal subjective exclamation which was documented as the postoperative patient subjective assessment of appearance (see Grading Scale in Table 1). Most patients reported Grade 1, denoting that they could not tell which eye had surgery based on visual observation. Patients were also asked to report their level of discomfort (Grading Scale in Table 2) and all patients had no discomfort (Grade 1) within 24 hrs.
Slit lamp and visual examination was performed by the surgeon and revealed normality at the operative site (Grade 1, Table 3) for all patients on postoperative day 1 (Figure 2). All patients also had successful AG placement (Grade 1, Table 4) at day one, however there was one case that flew back to his referring physician who accidentally rubbed the amniotic graft off at 1 week (Grade 3). After examination of the cornea, examination revealed a clear cornea (Grade 1) in 588 eyes and 15 eyes had residual corneal scar (Table 5).
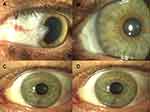 |
Figure 2 Representative surgical outcome. (A) Pre-op, (B) day one postoperative; (C) week one postoperative; (D) year one postoperative. |
For the follow-up period of 23.1 ± 35 months (range 6–216 months), there was only 1 case (0.2%) of recurrence. Of note, only 202 (33.5%) of the cases had a follow-up of 12 months or greater. As stated above, this particular recurrent case (50-year-old female) had a complex surgical intervention due to extensive preoperative surface involvement. Other postoperative complications from the study included sub-amniotic hemorrhage (0.3%), granuloma (2.0%), scleral melt (0.2%), and strabismus (0.2%). The two cases with sub-amniotic hemorrhage were due to accidental trauma and resolved spontaneously over 2 weeks. A total of 18 patients who developed pyogenic granulomas (mean: 3.5 months postop) had also resolved spontaneously on topical steroids after 1 month of treatment, while the remaining three cases required cautery excision and healed completely within 2 weeks with no recurrence since. All pyogenic granulomas resolved in 2.13 months (mean). There was one case of scleral melt (at 4 weeks) resolved over 3 months after the use of artificial tears and lubricants. In cases of simultaneous bitemporal surgery, one patient needed a correctable muscle alignment surgery (5 weeks postop), and in another instance, a patient developed a localized pupil distortion of unknown cause with no quantitative impact on vision while in one case the patient developed correctable ptosis of the upper lid and one patient developed a dellen-like bluish discoloration of sclera improving over time. All of these cases had undergone simultaneous, bi-temporal surgery as a common factor. Due to the low recurrence rate (0.2%), there was no difference in the recurrence rate based on age, race or gender.
In 11 out of 15 eyes with residual corneal scars, we applied planned Laser Corneoplastique principles to use the Excimer Laser in a refractive mode to bring these patients to emmetropic vision.11 These patients went on to achieve Grade 1 cosmesis (Figure 3) even though these patients were pre-disposed to limited lifestyle options and cosmetically poor appearing eyes that required glasses or contact lenses. Additionally, 8 eyes underwent planned Premium Cataract surgery to achieve the visual outcome of 20/20.
 |
Figure 3 Residual corneal scar. (A) Recurrent pterygium preoperative; (B) residual corneal scar 1 week postoperative; (C) 12 years post laser laser corneoplastique. |
Discussion
In this study, we achieved a significantly low recurrence rate (0.2%) by thoroughly removing all pterygium-associated tissue, controlling the use of MMC, and applying AMT with fibrin glue (ie, no stitches). This recurrence rate is dramatically lower than those previously published ranging from 3.3% to 42.3%, indicating that the surgical procedure described herein is valid and further verifies the notion that the surgical technique is probably the most critical factor influencing recurrence.12–16
To date, there is still a debate on the optimal extent of surgical excision necessary to prevent pterygium recurrence with many authors arguing the requirement of simple removal of the pterygium head while others argue extensive dissection and excision of the entire pterygium.17,18 A main concern with limited excision is the possibility of residual pterygium (especially for aggressive pterygia) that can lead to recurrence. Supporting this notion, Bai et al19 reported spatial differences in different parts of pterygium tissue wherein cells from the pterygia head and body showed higher colony forming units and was correlated to cell expression of p63α, a molecule involved in the regulation of epithelial maturation. Hence, the surgical procedure described in our study aims to remove the entire growth by using a lamellar corneal approach along with atraumatic yet complete pterygium removal. We also performed minimal dissection of the scar tissue until we found the bare sclera and identified a proper dissection plane. This is contrary to some techniques wherein surgeons chase the scar tissue from different approaches resulting in cutting into multiple planes and bleeding that may distort the anatomy and complicate subsequent surgical steps.
In our case series, AMT with fibrin glue helped restore the ocular anatomy, provide comfort, expedite healing and lend an elegant appearance of the eye. The use of AG has also been found to be effective in reducing the recurrence inhibiting myofibroblast transformation of normal ocular surface fibroblasts20 and pterygium body fibroblasts.21 As previously reported, 50% of the recurrence usually develop within the first 4 months and about 97% will happen within 12 months of excision,22 therefore we included patients who completed at least 6 months of follow-up. We were able to collect data up to 16 years of follow-up. Although there is a major concern about the influence of the race and ethnicity on the recurrence rate, our results have eliminated this concern as we included patients from all races and ethnic background from all over the world.23
Another critical component of pterygium surgery is setting proper expectations for the patient.24 We identify the risk factors, the timing and frequency of the recurrence, as well as other associated complications such as corneal and conjunctival scarring, fornix shortening symblepharon, ischemia or scleral thinning or melting. In addition, we also consider the need of planned postoperative laser vision correction for potential residual scarring. If predisposing conditions like rheumatoid arthritis, Sjogren's syndrome, dry eyes or collagen vascular diseases are present, we must involve the patient’s physician for a work up and management systemically.
No eye surgery is complete without addressing the patient’s vision. Besides aiming for a normal-looking eye, it is imperative to maintain their vision status as some patients may have had Lasik surgery, or in many cases, they can now become candidates for Lasik or premium cataract surgery. In many cases of corneal involvement, self-retaining amniotic membrane graft is placed postop day one to enhance the healing.
Additionally, in 11 cases with residual corneal scars (n=15), planned Laser Corneoplastique11 surgery following the pterygium surgery was performed using excimer laser in a refractive mode to bring these patients to emmetropic vision.
The importance of this technique goes beyond successfully treating pterygium and achieving white eyes 1 day postoperatively.25–27 In this study, all patients were instructed to look at their eye in a mirror on day one postoperatively. All patients reported no discomfort and could not tell which eye had surgery based on patient reported subjective grading scales. However, one cannot rule out the potential bias as the patient themselves knew which eye had the surgery. To our knowledge, no other study has evaluated patient-reported outcomes including patient concerns about cosmesis. Although some authors have provided ground work to emphasize the cosmetic appearance from a physician’s perspective.28–30 This exemplifies the notion for other surgeons to not only focus on restoring the ocular anatomy and fixing the complication but also to deliver an eye to emmetropic vision and a cosmetic outcome to meet or exceed patient expectation.
Conclusion
This study highlights an improved technique of an old concept of pterygium surgery that not only reduces the recurrence but also enhances cosmetic excellence and improves quality of vision.
Abbreviations
AG, amniotic membrane graft; AMT, amniotic membrane transplantation; MMC, mitomycin C.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by Western IRB (Puyallup, WA) #20181189. The IRB found that this research meets the requirements for a waiver of consent under 45 CFR 46.116(d).
Consent for Publication
Written informed consent was obtained from the patient in Figure 1 for publication of this Case Report and any accompanying images and videos. A copy of the written consents are available for review by the Editor of this journal.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001;119(5):695–706. doi:10.1001/archopht.119.5.695
2. Tan DTH, Chee S-P, Dear KBG, Lim ASM. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997;115:1235–1240. doi:10.1001/archopht.1997.01100160405001
3. Wong VA, Law FC. Use of mitomycin C with conjunctival autograft in pterygium surgery in Asian-Canadians. Ophthalmology. 1999;106(8):1512–1515. doi:10.1016/S0161-6420(99)90445-1
4. Al Fayez MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 2002;109(9):1752–1755. doi:10.1016/S0161-6420(02)01160-0
5. Lewallen S. A randomized trial of conjunctival autografting for pterygium in the tropics. Ophthalmology. 1989;96(11):1612–1614. doi:10.1016/S0161-6420(89)32667-4
6. McLean C. Pterygium excision with conjunctival autografting. Br J Ophthalmol. 1994;78(5):421. doi:10.1136/bjo.78.5.421
7. Ti SE, Chee SP, Dear KB, Tan DT. Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol. 2000;84(4):385–389. doi:10.1136/bjo.84.4.385
8. Liu J, Fu Y, Xu Y, Tseng SC. New grading system to improve the surgical outcome of multirecurrent pterygia. Arch Ophthalmol. 2012;130(1):39–49. doi:10.1001/archophthalmol.2011.328
9. Gulani A. Gulani iceberg technique. Cataract Refract Surg Today Eur. 2014.
10. Gulani A. No-stitch pterygium surgery: next day cosmetic outcomes. Video J Ophthalmol. 2009.
11. Gulani AC. Corneoplastique: art of vision surgery. Indian J Ophthalmol. 2014;62(1):3–11. doi:10.4103/0301-4738.126165
12. Shimazaki J, Shinozaki N, Tsubota K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br J Ophthalmol. 1998;82(3):235–240. doi:10.1136/bjo.82.3.235
13. Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104(6):974–985. doi:10.1016/S0161-6420(97)30197-3
14. Solomon A, Pires RTF, Tseng SCG. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001;108:449–460. doi:10.1016/S0161-6420(00)00567-4
15. Rosen R. Amniotic membrane grafts to reduce pterygium recurrence. Cornea. 2018;37(2):189–193. doi:10.1097/ICO.0000000000001407
16. Ma DH-K, See L-C, Liau S-B, Tsai RJ-F. Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol. 2000;84(9):973–978. doi:10.1136/bjo.84.9.973
17. Bahar I, Kaiserman I, Weisbrod M, McAllum P, Slomovic A. Extensive versus limited pterygium excision with conjunctival autograft: outcomes and recurrence rates. Curr Eye Res. 2008;33(5):435–440. doi:10.1080/02713680802045099
18. Hwang HS, Cho KJ, Rand G, Chuck RS, Kwon JW. Optimal size of pterygium excision for limbal conjunctival autograft using fibrin glue in primary pterygia. BMC Ophthalmol. 2018;18(1):135. doi:10.1186/s12886-018-0790-6
19. Bai H, Teng Y, Wong L, Jhanji V, Pang CP, Yam GH. Proliferative and migratory aptitude in pterygium. Histochem Cell Biol. 2010;134(5):527–535. doi:10.1007/s00418-010-0751-5
20. Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179(3):325–335. doi:10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X
21. Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20(4):325–334. doi:10.1076/0271-3683(200004)2041-5FT325
22. Hirst LW, Sebban A, Chant D. Pterygium recurrence time. Ophthalmology. 1994;101:755–758. doi:10.1016/S0161-6420(94)31270-X
23. Campagna G, Adams M, Wang L, Khandelwal S, Al-Mohtaseb Z. Comparison of pterygium recurrence rates among different races and ethnicities after primary pterygium excision by surgeons in training. Cornea. 2018;37(2):199–204. doi:10.1097/ICO.0000000000001453
24. Gulani A. A cornea-friendly pterygium procedure. Rev Ophthalmol. 2012:52–56.
25. Gulani A. Sutureless amniotic surgery for pterygium: cosmetic outcomes for ocular surface surgery. Tech Ophthalmol. 2008;6(2):41–44. doi:10.1097/ITO.0b013e31817dceb2
26. Gulani A, Dastur Y. Simultaneous pterygium and cataract surgery. J Postgrad Med. 1995;41(1):8–11.
27. Gulani AC. The Art of Pterygium Surgery. 1st ed. Stuttgart: Thieme Medical Publishers; 2019.
28. Hirst LW. Pterygium extended removal followed by extended conjunctival transplant: but on which eye? Cornea. 2013;32(6):799–802. doi:10.1097/ICO.0b013e31827e2a7f
29. Hirst LW. Cosmesis after pterygium extended removal followed by extended conjunctival transplant as assessed by a new, web-based grading system. Ophthalmology. 2011;118(9):1739–1746. doi:10.1016/j.ophtha.2011.01.045
30. Hirst LW, Smallcombe K. Double-headed pterygia treated with P.E.R.F.E.C.T for PTERYGIUM. Cornea. 2017;36(1):98–100. doi:10.1097/ICO.0000000000001036
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

