Back to Journals » Eye and Brain » Volume 7
Cortical inputs to the middle temporal visual area in New World owl monkeys
Authors Cerkevich C, Collins C, Kaas J
Received 26 June 2014
Accepted for publication 26 August 2014
Published 23 December 2014 Volume 2015:7 Pages 1—15
DOI https://doi.org/10.2147/EB.S69713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Margaret Wong-Riley
Christina M Cerkevich,1 Christine E Collins,2 Jon H Kaas2
1Center for the Neural Basis of Cognition and Systems Neuroscience Institute, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; 2Department of Psychology, Vanderbilt University, Nashville, TN, USA
Abstract: We made eight retrograde tracer injections into the middle temporal visual area (MT) of three New World owl monkeys (Aotus nancymaae). These injections were placed across the representation of the retina in MT to allow us to compare the locations of labeled cells in other areas in order to provide evidence for any retinotopic organization in those areas. Four regions projected to MT: 1) early visual areas, including V1, V2, V3, the dorsolateral visual area, and the dorsomedial visual area, provided topographically organized inputs to MT; 2) all areas in the MT complex (the middle temporal crescent, the middle superior temporal area, and the fundal areas of the superior temporal sulcus) projected to MT. Somewhat variably across injections, neurons were labeled in other parts of the temporal lobe; 3) regions in the location of the medial visual area, the posterior parietal cortex, and the lateral sulcus provided other inputs to MT; 4) finally, projections from the frontal eye field, frontal visual field, and prefrontal cortex were also labeled by our injections. These results further establish the sources of input to MT, and provide direct evidence within and across cases for retinotopic patterns of projections from early visual areas to MT.
Keywords: middle temporal area, visual cortex, parietal cortex
Introduction
In the present study, we revealed the ipsilateral cortical connections of the middle temporal visual area (MT) of owl monkeys by making up to three injections of different tracers within the area. MT was the first of the extrastriate visual areas in primates, after the second visual area, V2, to be established by microelectrode mapping of its retinotopic organization in relation to cortical architecture.1–3 MT is now known to be involved in motion perception and4–9 directing pursuit eye movements10,11 and as a key area in the dorsal stream of visuomotor processing.12–14 MT proved to be particularly easy to identify because of its dense myelination and expression of cytochrome oxidase (CO) in comparison with adjoining cortex. As a result, the connections of MT with other areas of cortex have been extensively studied across a range of primate species, with considerable agreement across studies on the major MT connections with other cortical areas and regions.15–23 The connections revealed by injections of tracers in MT are generally consistent with those MT connections revealed by injections in other areas of cortex.24–27
We sought to add to this accumulation of evidence on the connections of MT in primates in several ways. First, interpretations of the organization of extrastriate visual cortex have continued to evolve, and the connection patterns can now be related to more recent understandings. For example, there is now considerable evidence for a single V3 with dorsal and ventral halves in primates, rather than a dorsal V3 and a ventral V3 (see Lyon and Connolly28), and the relation of MT to adjoining areas including the middle temporal crescent (MTc or V4t), the middle superior temporal area (MST), and the dorsal and ventral fundal areas of the superior temporal sulcus (FSTd and FSTv, respectively) is better understood.23,25 Furthermore, it is possible to inject several different retrograde tracers into different retinotopic locations in MT, thereby revealing the existence of retinotopic patterns of input connections, although this has not been done previously. Finally, strengths of MT connections have only been quantified in one previous study on marmosets,21 and quantifications of connections are needed for comparison with other primates.
In the present study, we investigated the possibility of retinotopic patterns of MT connections with other cortical areas by placing injections of two or three distinguishable tracers in separate locations in MT of three owl monkeys. The results were analyzed from brain sections cut parallel to the surface of manually flattened cortex in order to obtain the most accurate portrayal of connection patterns. These connection patterns were then related to cortical architecture and assigned to cortical fields, based on present understandings of the arrangement of cortical areas in owl monkeys and other primates. In particular, we include both a V3 and a dorsomedial visual area (DM), following Lyon and Connolly,28 although other models remain viable.29,30 Counts of labeled cortical neurons were compared across cortical areas and regions for each MT injection. The results help establish the connection pattern of MT in primates, and provide compelling evidence that the early visual areas V1, V2, V3, the dorsolateral visual area (DL or V4), and DM have retinotopically congruent connections with MT.
Methods
Three adult owl monkeys (Aotus nancymaae) were used in this study. The experimental procedures were approved by the Vanderbilt University Animal Care and Use Committee and adhered to National Institutes of Health guidelines. All surgical procedures were performed under aseptic conditions.
Surgical procedure and tracer injection
Each animal was anesthetized with an initial injection of ketamine hydrochloride (10–50 mg/kg, intramuscularly), intubated, and maintained on 2% isoflurane anesthesia for the duration of the surgical procedure. Heart rate, blood oxygen levels, and temperature were monitored throughout the procedure. Each monkey was placed on a heating pad or under a heating lamp to maintain the body temperature at 37°C. A local anesthetic, lidocaine hydrochloride, was applied to the ears and subcutaneous skin before the animal was placed into a stereotaxic frame. The skin was incised to expose the skull. A craniotomy was performed to expose the caudal tip of the superior temporal sulcus and surrounding cortex. Once the dura was dissected, the exposed cortex was kept moist with the application of sterile saline.
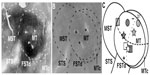
The location of MT was estimated relative to the location of the caudal tip of the superior temporal sulcus,1,31 and later confirmed anatomically through tissue processing for myelinated fibers and CO and reconstruction. Each injection of anatomical tracer was made using a Hamilton syringe mounted on a stereotaxic arm with glass micropipette tip attached to the syringe with epoxy. In each case, up to three distinguishable tracers, including 0.5–0.6 μL of cholera toxin subunit B (CTB) (1% CTB in distilled water; Sigma-Aldrich Co, St Louis, MO, USA); 0.25–0.5 μL of diamidino yellow (DY) (2% DY in 0.1 M phosphate buffer; Sigma-Aldrich Co); and 0.5–0.6 μL of Fluoro-Ruby (FR) (10% FR in distilled water; Molecular Probes®; Thermo Fisher Scientific, Waltham, MA, USA), were pressure-injected 0.5–1 mm below the cortical surface. Injection site locations were chosen with the goal to place the different tracers across the full extent of MT, thus covering different regions of the retinotopic map in this area (Figure 1).
Following the injections, gel film was inserted to replace the opened dura, the craniotomy closed with dental cement, and the skin sutured shut. The animal was then recovered from anesthesia, treated with prophylactic antibiotics and analgesics, and returned to its home cage for the duration of the survival period. Seven days after the tracer injections, the monkeys were deeply anesthetized with an injection of sodium pentobarbital (80 mg/kg), and, once areflexive, perfused through the heart with phosphate-buffered saline (pH 7.4) followed by 2% paraformaldehyde in phosphate buffer and 2% paraformaldehyde with 10% sucrose in phosphate buffer.
The cortex was separated from the rest of the brain and flattened as previously described.20,32 Each flattened cortex was held between two glass slides and stored overnight for cryoprotection in 30% sucrose at 4°C.
Tissue processing
The cortex of the injected hemisphere was cut tangential to the surface at a thickness of 40 μm on a freezing microtome. In all cases, alternate series of sections were mounted unstained for fluorescence microscopy, processed to reveal myelinated fibers with modified silver staining,33 and reacted with standard methods to reveal the distribution of CO.34 When appropriate, a series of sections was processed with CTB immunohistochemistry to reveal CTB injection sites and labeled cells.35,36
Data analysis
Locations and extent of injection sites and distributions of neurons filled by each tracer injection were plotted for the injected hemisphere with a fluorescent/bright-field Leitz microscope coupled to an X, Y encoder (Leica Microsystems, Wetzlar, Germany) and a Macintosh G3 computer (Apple Inc., Cupertino, CA, USA) running IGOR Pro™ software (WaveMetrics, Inc., Portland, OR, USA). Blood vessels and other landmarks were also marked on the plots for later use during alignment and reconstruction. Digital photomicrographs of sections stained to reveal cortical architecture, ie, those stained for myelinated fibers and CO, were taken using a Nikon DXM1200 camera mounted on a Nikon E800 microscope (Nikon Instruments, Melville, NY, USA). These digital images were then adjusted for contrast, saturation, lightness, and curves with Adobe Photoshop CS2™ (Adobe Systems Incorporated, San Jose, CA, USA), but were not otherwise altered.
Relating labeled neurons to cortical areas
To the extent possible, the boundaries of cortical areas were determined architectonically from individual brain sections as shown in Figure 1, and compiled and compared from sections processed for CO or myelin across the depth of cortex. These results and the location of labeled neurons in plotted sections were related to a single favorable section by drawing a reference section with a projection microscope, outlining cortex and including areal borders, sulcal patterns, and local landmarks such as blood vessels and injection holes (Figure 1). These landmarks were used to locally align results from adjacent sections and others to the reference section. The architectonic results allowed us to identify borders of MT, primary visual cortex (V1), and primary somatosensory cortex (area 3b) with a high degree of certainty. The borders of other areas of the MT complex (MST, MTc, and FST) were all outside the clear MT border and at least partly confirmed by architecture, as well as being consistent with previous estimates.25 The border of V2 with V1 was always obvious, and the outer border was variably apparent, but otherwise placed at estimated width of V2.20 The architectonic borders of V3 and DM were less apparent, and largely estimated from previous reports.32,37,38 DL borders were limited by location between MTc and V3, and upper and lower borders were estimated from previous reports.39–41 The approximate locations of other areas do not include boundaries, but conform to previously identified locations.20,42,43 Reconstructions of areal borders and locations, together with plots of labeled neurons, were created with Adobe Illustrator™ CS2 and CS5™ (Adobe Systems Incorporated). The numbers of labeled cells in each area was determined using the counting function in Adobe Illustrator.
Results
Three different tracers were placed in different locations in MT of two owl monkeys, and two were placed in MT of a third owl monkey. Cortex was flattened, and cut parallel to the surface so that labeled neurons could be related to locations in and around cortical areas. The collective results identify a number of areas or regions with neurons projecting to MT, and provide evidence about the visuotopic organization of visual areas.
Case 1
Injections of three distinct tracers were placed in different regions of MT in case 1 (Figure 1C [stars]). The injection locations were identified in the processed brain sections by the slight damage made by the injection syringe. DY was injected medially in MT, into the central representation of the lower field (Figure 2A). The diffusion zone around the core of this DY injection did not extend to either the horizontal meridian (HM) or vertical meridian (VM). An injection of FR was placed rostral to the DY injection in the lower field representation near MT’s representation of the HM (Figure 2B). This FR injection was the only injection in the peripheral visual representation in case 1. Finally, a CTB injection was placed in the representation of the far central upper visual field, just within 20° of visual space. While this injection was centered closer to the representation of the HM, the core of the injection was ovoid in shape, and stretched across much of the central visual representation from the HM toward the representation of the VM that lies along the lateral border of MT. This injection may have involved the white matter.
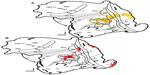
In case 1, DY was injected medial and caudal to the other injections, centering it in the representation of the central lower field (Figure 2A). Early visual areas, including V1, V2, V3, DL or V4, and DM, all contained labeled cells following the injection in MT. The neurons filled with DY were concentrated in lower field representations in areas with known retinotopic maps, thus matching the retinotopic location of the DY injection in MT. Many cells were labeled within MT (Table 1), as well as in associated areas within the MT complex, including MTc or V4t, MST, and FST. In FST, DY-labeled neurons were concentrated in the dorsal subdivision (FSTd). The lower visual field DY injection revealed projections from regions around the inferior occipital sulcus and rostral tip of the superior temporal sulcus to MT in the inferior temporal cortex (IT). In the posterior parietal cortex (PPC), DY-labeled cells sat caudally along the border with DM, in a patchy field that extended from the medial visual area (M) to the tip of the lateral sulcus. This region of the owl monkey PPC may correspond to the lateral intraparietal area (LIP) or ventral intraparietal area (VIP), or possibly a region, with known inputs to MT in macaques.17,26 A few DY-labeled cells were also found in a region of frontal cortex that included the frontal eye field (FEF) and frontal visual area (FV).
While the FR injection in case 1 was centered in the peripheral upper field near the MT representation of the HM, it spread rostrodorsally to include more of the temporal visual space, and slightly ventrally, ultimately crossing the HM (Figure 2B [red]). Cells filled as a result of this injection were found across V1, V2, V3, DL, MT, MTc, MST, and FST in a manner similar to those resulting from the DY and CTB injections in case 1. As with the other injections, FR-filled cells in areas with known retinotopic maps revealed organized projections to area MT. For example, FR-labeled neurons were found in both the upper and lower peripheral visual field representations in V1. Furthermore, they sat caudally away from the representation of the VM along the V1/V2 border. In addition to the early visual areas and divisions of the MT complex, FR-labeled cells indicated projections from a region near the inferior occipital sulcus, M, and the PPC. The FR injection was the smallest of the three injections in case 1, and may not have included the layers receiving the strongest projections from other cortical areas. This likely explains the overall lower number of labeled cells resulting from the FR injection (Table 1), as well as the more confined distribution of backfilled cells that did not include the frontal cortex.
Neurons filled as a result of the case 1 central upper field CTB injection were distributed across visual areas, as well as in parietal and frontal cortex (not shown). While the increased number of cells labeled by the case 1 CTB injection and the scattered nature of the resulting filled neurons suggested that this injection may have included passing fibers in the white matter, the overall distribution of CTB cells was otherwise similar to those labeled after the case 1 DY and FR injections (Table 1). This included projections from V1, V2, V3, DL, and DM, where the concentration of CTB-labeled neurons sat more ventrally when compared to the more dorsal distribution of backfilled DY cells. A lower number of CTB-labeled cells were found in the lower field representations in each of these areas, likely due to diffusion of the tracer to and slightly across the HM in MT. Again, there were many intrinsic connections within MT. Backfilled CTB cells were also found in MTc, MST, and FST. Neurons filled with CTB as a result of the case 1 injection were distributed broadly throughout the rest of the temporal cortex with denser foci around the inferior occipital sulcus, within the superior temporal sulcus, and throughout IT, particularly near the polar division of this region (ITp) rostral to the tip of the superior temporal sulcus. In the parietal lobe, CTB-labeled cells were concentrated in M and much of the rest of the PPC. Foci of backfilled neurons were found in both the lower and upper banks of the lateral sulcus, though they tended to be collected caudally. While CTB-labeled neurons were distributed throughout the frontal cortex, they were concentrated in the FEF/FV region. There was also a focus of filled cells in the opercular prefrontal frontal cortex (PFC), in the region that may encompass the precentral opercular cortex (area PrCO), area 12/45, and area 47 of similar New World marmoset monkeys.44–46
Case 2
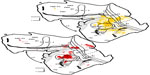
In case 2, three injections were placed across area MT (Figures 1C [squares] and 3). CTB was injected near the HM, just outside of the representation of the central upper field (Figure 3A). A small injection of FR was placed caudal to the case 2 CTB injection in the MT central upper field representation. Though this FR injection was centered at approximately the same mediolateral level as the CTB injection, it spread more toward the HM, while the weight of the CTB injection was balanced toward the VM (Figure 3B [red]). Case 2 contained the most rostral injection of all three cases, with an injection of DY on the border of areas MT and MST (Figure 3B [yellow]). Because the DY injection was made on the border between the two areas, cells labeled by it are likely to reflect projections to both MT and MST. The part of the injection core and surrounding diffusion zone that was in MT was contained in the representation of the extreme temporal periphery of the lower field.
The CTB injection into the peripheral upper field representation in case 2 labeled cells in a pattern similar to that revealed in the case 1 central upper field injection of CTB (Figure 3). Again, while some cells were labeled in the lower field representations, CTB-labeled cells were found mostly in the upper field representations of the early visual areas. Here, they tended to lie slightly more laterally compared to the previous case in these areas, reflecting the shift from an injection site just within the central vision in case 1 to one more rostral in the representation of the case 2 periphery. The apparent lines of labeled cells in unfolded V1, V2, and V3 were likely the result of uneven flattening of the cortex. Strong intrinsic connectedness within MT was indicated by heavy CTB labeling in this area (Table 1). MTc, MST, and FST also held CTB-filled neurons. Once again, the rest of the temporal cortex contained many labeled cells, with concentrations near the inferior occipital sulcus, in the superior temporal sulcus rostral to FST, and in IT, particularly near ITp. Patches of CTB-backfilled cells were also in the lateral sulcus. Caudally, the largest of these foci were continuous with the concentration of labeled cells in DM. Projections from M and the PPC were also revealed by this CTB injection into the upper visual field of MT. In frontal cortex, there were foci of labeled cells in the FEF, FV, and extreme ventrolateral PFC on the crest of the rostral lateral sulcus.
The FR injection in case 2 was placed at about the same distance from the HM as the CTB injection, but slightly more caudal into the representation of central vision. The pattern of labeled cells revealed by this injection was more restricted than that revealed by the CTB injection, but generally similar (Figure 3B [red]). V1, V2, V3, DL, and DM all contained filled neurons, as did MT and its surrounding areas. In areas with known retinotopy, the bulk of FR-labeled cells overlapped the regions with projections revealed by the more rostral, and thus peripheral, CTB injection. The number of FR-labeled cells in temporal cortex outside of the MT complex was low. Small but dense foci of backfilled FR neurons were also found in regions of the PPC and FEF that overlapped the locations of cells labeled by the case 2 CTB injection.
The DY injection was on the MT/MST border. These two areas have overlapping inputs, complicating the interpretation of the results of this DY injection. However, it was clear that the distribution of DY-positive cells was similar to that revealed by other injections that were restricted to MT (Figure 3B [yellow]). Neurons backfilled with DY were found in representations of the far periphery in extreme medial V1, V2, V3, DL, and DM, matching the location of the injection in MT’s visual space. Both MT and MST were filled with DY-labeled cells, suggesting strong intrinsic connections within each region, and likely reflecting reciprocal interconnectedness between the two areas. MTc, FST, and IT each contained DY-filled neurons. Foci of DY-labeled cells were also found in M and PPC, in patches throughout the lateral sulcus, and in the FEF/FV. A small number of filled DY neurons indicated at least weak projections from other regions of frontal cortex to the MT/MST border region.
Case 3
Finally, we placed injections of two fluorescent tracers into the MT lower field representation in case 3 (Figure 1C [circles]). DY was injected into the central visual representation, near the medial border of MT and MTc (Figure 4A). This DY injection was near the representation of the VM in the central lower field of MT. An injection of FR was placed more rostrally in MT into the peripheral lower field representation of MT in case 3 (Figure 4B). It was further from the VM than the DY injection.
As with the fluorescent tracers in other cases, DY- and FR-labeled cells were plotted on the same sections in case 3. In Figure 4, the reconstructed plots of the two tracers are shown separately to provide a better view of projections revealed by each tracer from regions with a high degree of overlap. The distribution of cells filled by the DY injection resulted in labeled cells mostly in the central lower field representations of V1, V2, and V3, though each of these areas contained a few projecting neurons in the upper field representations of these areas. The DY injection near the lower field representation of the VM that sits along the MT/MTc border resulted in labeled cells clustered near the representations of the VM in V1, V2, and V3. The FR injection was placed rostral to the DY injection in case 3, thus in a representation of the lower periphery in MT. Resulting FR-labeled cells were also found in V1, V2, and V3, although these cells were spatially displaced into the more medial peripheral representations in these areas when compared to those regions containing DY-filled neurons. Furthermore, the FR-labeled cells tended to lie further away from the VM representations in V1, V2, and V3 than those cells labeled by the DY injection that was closer to MT’s representation of the VM. As in the other cases, DL contained a high percentage of neurons labeled with DY (Table 1). The DY cells were concentrated in the lower field representation, but the DL upper field also contained a number of cells. Only a few FR-labeled cells were found in DL, perhaps due to the more peripheral retinotopic location of this injection. DM also sent organized projection to MT, with peripherally projecting FR-labeled cells sitting more medially than those revealed by the more central DY injection. Backfilled DY and FR neurons were present throughout the MT complex, in MT, MTc, MST, and FST. The locations of these labeled cells tended to overlap, particularly the intrinsic projections that arose within MT around each injection. This included connections to each injection site from the region between the DY and FR injections. Outside of the MT complex, projections indicated by the DY central VM injection were concentrated caudally in IT, mainly in a region that stretched from near the MTc/ FST junction laterally around the inferior occipital sulcus and into the caudal IT division. A lower number of FR cells were labeled in more rostral divisions of IT, though the foci of labeled cells near the inferior occipital sulcus and ITp are in similar locations as those labeled in other cases. A dense but somewhat patchy field of DY-labeled cells covered cortex between MT and the lateral sulcus. This field was largely continuous with the labeled regions of DL and DM, and extended through the tip of the lateral sulcus into the PPC and M. Again, the overall number of neurons filled with FR was lower, but the locations of these FR-labeled cells overlapped those labeled by the DY injection. Other foci of projections to MT revealed by the case 3 DY and FR injections included patches of labeled cells found further rostrally in the lateral sulcus, in the FEF/FV, in the ventral premotor area, and in PFC.
Discussion
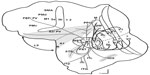
We placed eight injections into MT of three owl monkeys to reveal the cortical network of ipsilateral inputs to MT (Figure 5). V1, V2, V3, DL or V4, and DM all sent retinotopically organized projections to MT. A large number of labeled cells within MT following injection indicated high intrinsic interconnectedness. Areas surrounding MT, including MTc or V4t, MST, and FST, also provided inputs to MT. Many projections from outside the early visual areas and MT complex were also revealed by our injections, particularly from M, the PPC, the FEF, and the FV.
Intrinsic connections of MT
Intrinsic connections from within MT were widespread, but at least somewhat organized. In general, intrinsic inputs arose for locations surrounding the core each injection. Both CTB injections resulted in labeling of cells across most or all of MT, perhaps due to the sensitivity of this particular tracer. Injections of fluorescent tracers DY and FR, however, resulted in more restricted uptake and a more refined pattern of filled neurons within MT. Following these injections, it can be seen that MT’s intrinsic connections tend to arise from adjacent parts of the visual representation, while more distant visual locations within MT contain fewer labeled cells (Figure 4).
Evidence for retinotopically congruent areal connections
Our injections revealed retinotopically organized projections from visual areas with known retinotopic maps to MT. This is particularly clear when comparing inputs to the upper and lower field representations in ventral and dorsal MT, respectively. As in MT of owl monkeys,1,2,47 the lower visual field is represented dorsally in V1, V2, and V3, while the upper visual field is represented ventrally.2,14,48–50 When injections were placed cleanly into either the upper or lower visual field representation in MT, projections arose from matched upper or lower field locations in V1 with very little input coming from the opposing representation. For example, cells labeled by the CTB injection into the more ventral MT upper field representation in case 1 were almost exclusively found in ventral V1, while those labeled by the case 1 lower field DY injection were found dorsally (Figure 2 [red and yellow]). When an injection of tracer was placed on the representation of the HM, such as the FR injection in case 1, resulting labeled cells were found in both the upper and lower field representations in dorsal and ventral V1 (Figure 2 [red]). Restricted projections to MT from the upper or lower field representations were found to arise from V2 and V3 as well. Topographic projections related to the VM were also indicated in these early visual areas. This is particularly clear in V1, where cells labeled by injections closer to the boundaries of MT, the location of the VM representation in MT,1 were in closer proximity to the representation of the VM along the V1/V2 border than injections located away from the boundaries of MT (eg, compare the locations of FR- and DY-labeled cells in cases 1 and 3 in Figures 2 and 4). Similar matched, though somewhat more diffuse, inputs to MT also arose from the upper and lower visual field representations in DL, where the lower and upper visual fields are represented dorsally and ventrally as in the other early visual areas.14,51 Cells labeled by injections into the MT lower field generally were in dorsocaudal DM when compared to those filled by the injections into the MT upper field representation, again matching the known visual map in DM.32,37,52
Tracers were injected across the rostral to caudal extent of MT, thus covering MT’s visual map from central to peripheral vision.1 Projections to MT were also organized along this dimension of visual space. The most caudal injections in each case resulted in cells labeled in the central visual representation in V1, V2, and V3.14,51 Neurons filled by tracers injected more rostrally in MT sat progressively further from the representation of the central visual field. Thus, when two injections were placed in the lower field representation, the most caudal MT injection resulted in labeled cells in central V1, V2, and V3, while the cells labeled by more rostral injections into the MT peripheral visual representation were displaced medially. For example, FR-filled neurons in both cases 1 and 3 were found in V1, V2, and V3 medial to those labeled by the DY injections into the central visual representation in MT in each case. A similar displacement was seen when two tracers were injected into the central and peripheral representations of the upper field, although, in this situation, cells labeled by the more rostral (ie, peripheral visual field) MT injection lay laterally in V1, V2, and V3 compared to those labeled by the injection into a location more central in the visual field (Figure 2 [yellow versus red]). Projections from DL and DM were similarly separated into central and peripheral representations in each area, respectively. Interestingly, projections organized along the central to peripheral visual dimension also arose from the FEF. Inputs from FEF to the central field representation in caudal MT arise laterally to those cells labeled by injections in more peripheral representations in rostral MT (Figures 3 and 4). This arrangement matches the topography of the FEF visual field previously demonstrated in a study of inputs from macaque visual cortex to the FEF.53
MT connections with higher order visual areas
The organization of other visual areas in the MT complex and many higher order visual areas remains unclear. For some of these regions, including a region of the PPC that may encompass the LIP and VIP, projections to MT suggest some organization. In others, for example IT, no retinotopically organized projections were indicated.
Overall, the pattern of connections of MT with other areas of cortex revealed in the present study is in agreement with previous results from owl monkeys,18,20,25,54 other New World monkeys,15,20–22,55 Old World macaques,17,19 and prosimian galagos.23 All studied primates appear to have neurons projecting to MT in visual areas V1, V2, and V3 (although V3 has not been consistently recognized), DM or a comparable region, parts of PPC that in macaques include LIP and VIP, frontal cortex in and near the expected locations of the FEF, areas of the MT complex (MTc, FST, and MST), DL, and parts of temporal cortex. Other regions have been variously reported (see Palmer and Rosa21,55 for review).
Here, we reported quantitative data on the strengths of connections, which were somewhat variable across the three cases, and different across tracers. As expected, most of the ipsilateral cortical connections of MT were intrinsic, with 23%–64% of neurons labeled by MT injections confined to MT. Other major sources of inputs to MT included those from V1, V2, V3, and MST. Projections from parietal cortex, largely from a region that could include LIP and VIP, were slight, generally 1%–2%, but over 5% for one injection. Relatively few neurons were also labeled in the region of the FEF (under 2%). Many of these connections reflect visual inputs to MT (V1–V3), or feedback to MT related to visual motion signals sent by MT to posterior parietal and frontal cortex. Some of the variability in the strengths of connections across cases and injection sites in our material likely reflects our approach of flattening cortex and cutting sections parallel to the cortical surface. Such sections are ideal for locating labeled neurons relative to a surface view and architectonic borders, but there are few sections, 20 or so, across the cortical thickness, and half of these sections are used for architecture. Thus, sampling of cortical sublayers may vary, and results across cases and injection sites would more accurately reflect connection strengths. In spite of this variability, we can compare the present quantitative results with those obtained after injections in MT of marmosets, another New World monkey. Palmer and Rosa21 compared the proportions of neurons projecting to MT, and did not include neurons labeled within MT. However, they subdivided cortex somewhat differently than in our present study. As in our present study, V1 and V2 provided proportionately large inputs to MT. The greatest input to marmoset MT was from MTc, which, in our study in owl monkeys, projected densely to MT only in our case where the injection was close to the MT side of the MT/MTc border. Thus, some contamination of MTc by our injection was possible. In addition, MTc was defined as a proportionately smaller area in our present study. As in our study, Palmer and Rosa21 found that connection strengths were variable across injections in different cases.
The functional significance of MT connections
The major activating inputs to MT are from visual areas V1, V2, and V3. Our injections in MT, and those indicated in other studies, indicate that these inputs are retinotopically organized, and suitable for creating, through a moderate level of convergence, the larger excitatory receptive fields of the smaller MT, while creating the retinotopy of MT. As for V2 and V3, MT neurons appear to be dependent on V1 for above-threshold activation, as lesions or chemical blocking of V1 in adult owl monkeys render MT neurons unresponsive to visual stimuli.47,51 As the projections from V1 are from orientation-sensitive neurons selectively activated by the magnocellular inputs to V1 (eg, Tigges et al in 198156 and Maunsell et al in 199057), they likely create the direction of movement- and orientation-selective modules in MT.58 The magnocellular geniculate pathway to V1 also activates layer three neurons projecting to orientation-selective band-like modules in V1 and V3, which project to MT.20,37,58,59 These indirect feedforward inputs are thought to contribute to the sensitivities of MT neurons to binocular disparity, a signal for perceptual depth, and processing motion at high speeds.60
Our injections also labeled widespread connections within MT. These intrinsic connections likely contribute a low-level excitation, possibly below spiking thresholds, between functionally matched orientation selective specific modules in MT revealed a patchy pattern of lateral connections to clusters of neurons within MT that were functionally similar to the injected module.54,61 The larger injection cores in our experiments would have involved several orientation modules, and the orientation-specific networks were not revealed.
Our injections also revealed projections to MT from other areas in the MT complex, namely FSTd, MTc, and MST. MST has long been recognized as a major target of MT that provides a higher level of processing visual motion.62 Dorsal MST appears to get wide-field motion information from wide-field domains in MT.61 The feedback projection neurons labeled in MST by our MT injections may be more broadly distributed. FSTd, FSTv, and MTc are more recently defined subdivisions of visual cortex.25 FST was first identified by Ungerleider and Desimone19 on the basis of inputs from MT and directionally selective neurons. The region was further subdivided by Kaas and Morel into FSTd with dense connection with MT and FSTv with dense connection with MTc, a narrow area surrounding much of MT.25 MTc includes the smaller, incomplete representation V4t.19 MTc can be identified by its modular appearance as a string of CO-dense patches,25 and it forms a long retinotopically organized area that is congruent with MT retinotopy along their common border.63 MTc has neurons with smaller receptive fields than the very large receptive fields in FST and MST, and most neurons are directionally selective. Our separate injections in MT labeled adjacent parts of MTc, as expected for connections between retinotopically organized areas, but they labeled largely overlapping zones in MST and FSTd, consistent with previous evidence that receptive fields are large in these areas and that they show little retinotopy. MT appears to project local motion information to FSTd.61
Other areas with connections with MT included dorsal stream DM, part of the PPC, and visual motor cortex in the frontal lobe, including the region of the FEF. There is general agreement that DM is retinotopically organized, but it has become clear that the early identifying maps of DM52 likely included parts of V3,28,49 and parts of V3 of other congruently adjoining areas may have been included in DM of other microelectrode mapping studies. However, the connection pattern of MT with the DM region that was revealed in the present study is consistent with other evidence that the lower visual quadrant is represented caudal and medial to that of the upper visual quadrant in DM.49,52,64 DM is a motion-sensitive area with a high proportion of neurons that are selective for direction of motion and stimulus orientation.7 Our MT injections also labeled distributions of neurons in the caudal part of PPC that showed no obvious retinotopic pattern. An injection in this PPC region in an owl monkey labeled neurons in MT, MST, MTc, DM, and the FEF.65 The connected region has not yet been identified, but the lateral intraparietal and ventral intraparietal regions (LIP and VIP) in macaques have MT connections.17,66 LIP is considered to be a visuomotor area with connections to the FEF,24 while VIP may be a visuomotor area for arm and body movements that protect the head.67 In the present cases, feedback connections from the FEF to MT were retinotopic in that the more caudal injection in MT labeled more lateral FEF neurons than rostral injections, corresponding to the representation of smaller eye movements in lateral FEF.53
Our injections in MT also labeled neurons in DL (V4) and IT, regions generally considered to be parts of the ventral stream of visual processing involved in object identification.12,13 The connections of MT with DL reflected the retinotopic organization of DL or V4 with the upper visual quadrant represented lateral to the lower visual quadrant, although the early map of DL included the MTc region.52 However, DL may have dorsal stream as well as ventral stream functions. Although DL is the major source of inputs for ventral stream processing in IT,40 projections from V2, which have been used to define the DL/V4 territory, appear to be connected in the caudal half of DL, leaving the rostral half relatively free of V2 inputs in squirrel monkeys.40 In addition, the rostral half of DL has dense connections with MT and DL. A similar arrangement of rostral and caudal DL/V4 areas may exist in macaque monkeys.68 Alternatively, DL/V4 may be subdivided into an alternation of dorsal stream modules with neurons sensitive to stimulus motion and orientation, and ventral stream modules sensitive to color.69 Thus, parts of DL/V4 could provide MT with information about object motion.
Finally, the labeling of neurons in various locations in IT (Figure 5) was unexpected. The finding that the proportions of such labeled neurons was variable across injectios in each case, and across cases, suggests that the evidence for large proportions of labeled neurons in IT for the CTB injection (15% and 23%) should be treated with caution, as MT connections with IT have not been reported in most other studies. However, MT injections in owl monkeys, squirrel monkeys, marmosets, and galagos labeled patterns of neurons across much of IT in some but not all cases.20 In a similar manner, Palmer and Rosa21 found that MT injections variably labeled clusters of neurons in marmoset IT.
Conclusion
Injections of multiple, distinguishable tracers across the retinotopic map in MT revealed projections from other areas of ipsilateral cortex. Early visual areas, including V1, V2, V3, DL, and DM, all provided inputs to MT from retinotopically matched regions. MT also had dense intrinsic connections, and inputs from the other areas of the MT complex (MTc, MST, and FST). Other regions within the superior temporal sulcus, near the inferior occipital sulcus, and throughout IT projected to MT. More input to MT arose from the cortex in and around the lateral sulcus, particularly in its caudal tip, and the PPC. Finally, our injections also revealed projections from frontal regions, including the regions of FEF and FV.
Acknowledgments
The authors thank Mary Feurtado for help in surgery and animal care, Laura Trice and Mary Varghese for tissue processing, and Dr Iwona Stepniewska and Emily Rockoff for help during surgery and comments on this manuscript. JHK was supported by an NIH Grant EY002686. Christine E Collins’ present affiliation is Eli Lilly and Company, Indianapolis, IN, USA.
Author contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JHK, CEC, CMC. Acquisition of data: CMC, CEC. Analysis and interpretation of data: CMC, JHK. Drafting of the manuscript: CMC, CEC, JHK.
Disclosure
The authors report no conflicts of interest in this work.
References
Allman JM, Kaas JH. A representation of the visual field in the caudal third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus). Brain Res. 1971;31(1):85–105. | |
Allman JM, Kaas JH. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus). Brain Res. 1971;35(1):89–106. | |
Allman JM, Kaas JH, Lane RH. The middle temporal visual area (MT) in the bushbaby, Galago senegalensis. Brain Res. 1973;57(1):197–202. | |
Malonek D, Tootell RB, Grinvald A. Optical imaging reveals the functional architecture of neurons processing shape and motion in owl monkey area MT. Proc Biol Sci. 1994;258(1352):109–119. | |
Xu X, Collins CE, Kaskan PM, Khaytin I, Kaas JH, Casagrande VA. Optical imaging of visually evoked responses in prosimian primates reveals conserved features of the middle temporal visual area. Proc Natl Acad Sci U S A. 2004;101(8):2566–2571. | |
Zeki S. The response properties of cells in the middle temporal area (area MT) of owl monkey visual cortex. Proc R Soc Lond B Biol Sci. 1980;207(1167):239–248. | |
Baker JF, Petersen SE, Newsome WT, Allman JM. Visual response properties of neurons in four extrastriate visual areas of the owl monkey (Aotus trivirgatus):a quantitative comparison of medial, dorsomedial, dorsolateral, and middle temporal areas. J Neurophysiol. 1981;45(3):397–416. | |
Felleman DJ, Kaas JH. Receptive-field properties of neurons in middle temporal visual area (MT) of owl monkeys. J Neurophysiol. 1984;52(3):488–513. | |
Salzman CD, Murasugi CM, Britten KH, Newsome WT. Microstimulation in visual area MT: effects on direction discrimination performance. J Neurosci. 1992;12(6):2331–2355. | |
Komatsu H, Wurtz RH. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J Neurophysiol. 1989;62(1):31–47. | |
Groh JM, Born RT, Newsome WT. How is a sensory map read out? Effects of microstimulation in visual area MT on saccades and smooth pursuit eye movements. J Neurosci. 1997;17(11):4312–4330. | |
Ungerleider L, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982:549–586. | |
Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15(1):20–25. | |
Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev. 2007;55:285–296. | |
Spatz WB, Tigges J. Experimental-anatomical studies on the “middle temporal visual area (MT)” in primates. I. Efferent cortico-cortical connections in the marmoset Callithrix jacchus. J Comp Neurol. 1972;146(4):451–464. | |
Wall JT, Symonds LL, Kaas JH. Cortical and subcortical projections of the middle temporal area (MT) and adjacent cortex in galagos. J Comp Neurol. 1982;211(2):193–214. | |
Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3(12):2563–2586. | |
Weller RE, Wall JT, Kaas JH. Cortical connections of the middle temporal visual area (MT) and the superior temporal cortex in owl monkeys. J Comp Neurol. 1984;228(1):81–104. | |
Ungerleider L, Desimone R. Cortical connections of visual area MT in the macaque. J Comp Neurol. 1986;248(2):190–222. | |
Krubitzer LA, Kaas JH. Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Vis Neurosci. 1990;5(2):165–204. | |
Palmer SM, Rosa MG. Quantitative analysis of the corticocortical projections to the middle temporal area in the marmoset monkey: evolutionary and functional implications. Cereb Cortex. 2006;16(9):1361–1375. | |
Rosa MG, Soares JG, Fiorani M Jr, Gattass R. Cortical afferents of visual area MT in the Cebus monkey: possible homologies between New and Old World monkeys. Vis Neurosci. 1993;10(05):827–855. | |
Kaskan PM, Kaas JH. Cortical connections of the middle temporal and the middle temporal crescent visual areas in prosimian galagos (Otolemur garnetti). Anat Rec (Hoboken). 2007;290(3):349–366. | |
Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265(3):332–361. | |
Kaas JH, Morel A. Connections of visual areas of the upper temporal lobe of owl monkeys: the MT crescent and dorsal and ventral subdivisions of FST. J Neurosci. 1993;13(2):534–546. | |
Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299(4):421–445. | |
Jeffs J, Ichida JM, Federer F, Angelucci A. Anatomical evidence for classical and extra-classical receptive field completion across the discontinuous horizontal meridian representation of primate area V2. Cereb Cortex. 2009;19(4):963–981. | |
Lyon DC, Connolly JD. The case for primate V3. Proc Biol Sci. 2012;279(1729):625–633. | |
Rosa MG, Palmer SM, Gamberini M, et al. Connections of the dorsomedial visual area: pathways for early integration of dorsal and ventral streams in extrastriate cortex. J Neurosci. 2009;29(14):4548–4563. | |
Jeffs J, Federer F, Ichida JM, Angelucci A. High-resolution mapping of anatomical connections in marmoset extrastriate cortex reveals a complete representation of the visual field bordering dorsal V2. Cereb Cortex. 2013;23(5):1126–1147. | |
Stepniewska I, Qi HX, Kaas JH. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur J Neurosci. 1999;11(2):469–480. | |
Krubitzer LA, Kaas JH. The dorsomedial visual area of owl monkeys: connections, myeloarchitecture, and homologies in other primates. J Comp Neurol. 1993;334(4):497–528. | |
Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1:203–209. | |
Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats deomonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171(1):11–28. | |
Angelucci A, Clascá F, Sur M. Anterograde axonal tracing with the subunit B of cholera toxin: a highly sensitive immunohistochemical protocol for revealing fine axonal morphology in adult and neonatal brains. J Neurosci Methods. 1996;65(1):101–112. | |
Bruce K, Grofova I. Notes on a light and electron microscopic double-labeling method combining anterograde tracing with Phaseolus vulgaris leucoagglutinin and retrograde tracing with cholera toxin subunit B. J Neurosci Methods. 1992;45(1–2):23–33. | |
Lyon DC, Kaas JH. Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. J Neurosci. 2001;21(1):249–261. | |
Lyon DC, Kaas JH. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain Behav Evol. 2002;59(3):114–129. | |
Allman JM, Kaas JH. A crescent-shaped cortical visual area surrounding the middle temporal area (MT) in the owl monkey (Aotus trivirgatus). Brain Res. 1974;81(2):199–213. | |
Cusick CG, Kaas JH. Cortical connections of area 18 and dorsolateral visual cortex in squirrel monkeys. Vis Neurosci. 1988;1(2):211–237. | |
Weller RE, Kaas JH. Retinotopic patterns of connections of area 17 with visual areas V-II and MT in macaque monkeys. J Comp Neurol. 1983;220(3):253–279. | |
Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J Comp Neurol. 1996;371(4):649–676. | |
Weller RE, Kaas JH. Subdivisions and connections of inferior temporal cortex in owl monkeys. J Comp Neurol. 1987;256(1):137–172. | |
Burman KJ, Palmer SM, Gamberini M, Rosa MG. Cytoarchitectonic subdivisions of the dorsolateral frontal cortex of the marmoset monkey (Callithrix jacchus), and their projections to dorsal visual areas. J Comp Neurol. 2006;495(2):149–172. | |
Burman KJ, Rosa MG. Architectural subdivisions of medial and orbital frontal cortices in the marmoset monkey (Callithrix jacchus). J Comp Neurol. 2009;514(1):11–29. | |
Paxinos G, Watson C, Petrides M, Rosa M, Tokuno H. The Marmoset Brain in Stereotaxic Coordinates. New York: Academic Press; 2011. | |
Collins CE, Lyon DC, Kaas JH. Responses of neurons in the middle temporal visual area after long-standing lesions of the primary visual cortex in adult new world monkeys. J Neurosci. 2003;23(6):2251–2264. | |
Collins CE, Lyon DC, Kaas JH. Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. Anat Rec A Discov Mol Cell Evol Biol. 2005;285(1):619–627. | |
Lyon DC, Kaas JH. Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron. 2002;33(3):453–461. | |
Lyon DC, Xu X, Casagrande VA, Stefansic JD, Shima D, Kaas JH. Optical imaging reveals retinotopic organization of dorsal V3 in New World owl monkeys. Proc Natl Acad Sci U S A. 2002;99(24):15735–15742. | |
Collins CE, Xu X, Khaytin I, Kaskan PM, Casagrande VA, Kaas JH. Optical imaging of visually evoked responses in the middle temporal area after deactivation of primary visual cortex in adult primates. Proc Natl Acad Sci U S A. 2005;102(15):5594–5599. | |
Allman JM, Kass JH. The dorsomedial cortical visual area: a third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus). Brain Res. 1975;100(3):473–487. | |
Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15(6):4464–4487. | |
Malach R, Schirman TD, Harel M, Tootell RB, Malonek D. Organization of intrinsic connections in owl monkey area MT. Cereb Cortex. 1997;7(4):386–393. | |
Palmer SM, Rosa MG. A distinct anatomical network of cortical areas for analysis of motion in far peripheral vision. Eur J Neurosci. 2006;24(8):2389–2405. | |
Tigges J, Tigges M, Anschel S, Cross NA, Letbetter WD, McBride RL. Areal and laminar distribution of neurons interconnecting the central visual cortical areas 17, 18, 19, and MT in squirrel monkey (Saimiri). J Comp Neurol. 1981;202(4):539–560. | |
Maunsell JH, Nealey TA, DePriest DD. Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J Neurosci. 1990;10(10):3323–3334. | |
Kaskan PM, Dillenburger BC, Lu HD, Roe AW, Kaas JH. Orientation and direction-of-motion response in the middle temporal visual area (MT) of New World owl monkeys as revealed by intrinsic-signal optical imaging. Front Neuroanat. 2010;4:23. | |
Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland KS, editors. Primary Visual Cortex in Primates. New York: Plenum Press; 1994:201–259. | |
Ponce CR, Hunter JN, Pack CC, Lomber SG, Born RT. Contributions of indirect pathways to visual response properties in macaque middle temporal area MT. J Neurosci. 2011;31(10):3894–3903. | |
Berezovskii VK, Born RT. Specificity of projections from wide-field and local motion-processing regions within the middle temporal visual area of the owl monkey. J Neurosci. 2000;20(3):1157–1169. | |
Tanaka K, Saito H. Analysis of motion of the visual field by direction, expansion/contraction, and rotation cells clustered in the dorsal part of the medial superior temporal area of the macaque monkey. J Neurophysiol. 1989;62(3):626–641. | |
Rosa MG, Elston GN. Visuotopic organisation and neuronal response selectivity for direction of motion in visual areas of the caudal temporal lobe of the marmoset monkey (Callithrix jacchus):middle temporal area, middle temporal crescent, and surrounding cortex. J Comp Neurol. 1998;393(4):505–527. | |
Rosa MG, Palmer SM, Gamberini M, Tweedale R, Pinon MC, Bourne JA. Resolving the organization of the New World monkey third visual complex: the dorsal extrastriate cortex of the marmoset (Callithrix jacchus). J Comp Neurol. 2005;483(2):164–191. | |
Beck PD, Kaas JH. Cortical connections of the dorsomedial visual area in new world owl monkeys (Aotus trivirgatus) and squirrel monkeys (Saimiri sciureus). J Comp Neurol. 1998;400(1):18–34. | |
Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296(3):462–495. | |
Cooke DF, Taylor CS, Moore T, Graziano MS. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci U S A. 2003;100(10):6163–6168. | |
Stepniewska I, Collins CE, Kaas JH. Reappraisal of DL/VA boundaries based on connectivity patterns of dorsolateral visual cortex in macaques. Cereb Cortex. 2005;15(6):809–822. | |
Tanigawa H, Lu HD, Roe AW. Functional organization for color and orientation in macaque V4. Nat Neurosci. 2010;13(12):1542–1548. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.