Back to Journals » Clinical Ophthalmology » Volume 16
Correlations of Immediate Corneal Tomography Changes with Preoperative and the Elapsed Phaco Parameters
Authors Haddad JS , Borges C, Daher ND , Mine A , Salomão M , Ambrósio Jr R
Received 4 May 2022
Accepted for publication 19 July 2022
Published 4 August 2022 Volume 2022:16 Pages 2421—2428
DOI https://doi.org/10.2147/OPTH.S363185
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jorge Selem Haddad,1,2 Clainijane Borges,2 Nathalie Dalloul Daher,2 Alexandre Mine,2 Marcella Salomão,1,3 Renato Ambrósio Jr3
1Department of Ophthalmology, Federal University of São Paulo, São Paulo, SP, Brazil; 2Instituto Oftalmológico Paulista, São Paulo, SP, Brazil; 3Instituto de Olhos Renato Ambrósio, Rio de Janeiro, RJ, Brazil
Correspondence: Jorge Selem Haddad, Instituto Oftalmológico Paulista, 35 Alameda Casa Branca, São Paulo, SP, Brazil, Tel +55 11 98999-1212, Email [email protected]
Purpose: The ability to predict corneal edema and understand its relationship with imaging parameters enables optimization of decision-making in terms of cataract surgery. Therefore, we aimed to elucidate the immediate tomographic alterations after phacoemulsification.
Patients and Methods: In this prospective study, we evaluated clinical and corneal tomographic data of 30 patients with cataracts, obtained using a rotating Scheimpflug tomographic system before and after cataract surgery with a phacoemulsification system. Corneal thickness and volume were measured, and Pentacam Nucleus Staging, keratometry, and specular microscopy were performed preoperatively and immediately postoperatively. The Wilcoxon signed-rank test was used to compare pre-and postoperative values. We calculated the correlations between the changes in these values and multiple parameters related to phacodynamics, including “ultrasound (US) elapsed” (phaco time), “US average” (average power used), and “US absolute” (energy effectively dissipated, a product of the other two parameters).
Results: There were increases in corneal volume (p< 0.0001) and pachymetry (p< 0.0001), and a decrease in endothelial cell count (p< 0.0001) after surgery. The mean differences in pre- and postoperative specular microscopy, corneal volume, and pachymetry were − 335.13± 236.21 cells/mm3, 1.33± 0.56 mm3, and 61.33± 23.73 microns, respectively. The difference in pre-and postoperative corneal volume in patients with US elapsed ≥ 40 s was 0.75 mm3 greater than that in patients with US elapsed < 40 s (95% confidence interval [CI]: 0.24– 1.25; p=0.005); that of pachymetry in patients with US elapsed ≥ 40 s was 31.76 microns greater than that in patients with US elapsed < 40 s (95% CI: 9.55– 53.97; p=0.007). Spearman correlation revealed that, for every 1% increase in cataract density, the US average value increased by 0.31% (coef.: 0.3110; 95% CI: 0.0741– 0.5490; p=0.012).
Conclusion: Knowledge of Pentacam Nucleus Staging and the effect of US elapsed on differences in corneal volume and pachymetry before and after cataract surgery should be of particular value for surgeons who routinely encounter patients with hard cataracts.
Keywords: cataract surgery, corneal volume, pachymetry, phacoemulsification, ultrasound
Introduction
Cataract surgeons face a considerable challenge in achieving satisfactory results, considering the patients’ expectations of satisfactory refractive outcomes. Beyond good uncorrected visual acuity with good quality of vision, patients value the minimization of complications and a speedy recovery. Several pre- and intraoperative factors can affect the final result.1,2 Therefore, the understanding of the interrelationships between preoperative corneal properties and the parameters used in phacoemulsification is paramount.3 Such knowledge would minimize intraoperative damage to corneal tissue and facilitate predictability.
Factors such as the corneal incisions, keratometric measurements, and posterior corneal astigmatism have already been discussed at length.4,5 For instance, the location and size of incisions play a role in the outcome of cataract surgery.6–8 There is scientific evidence that a small corneal incision (2.8 mm) induces little refractive change, at least in eyes with low preoperative corneal cylinders, regardless of its site.4
Preoperative measurements with modern, sophisticated equipment, accompanied by state-of-the-art formulas and artificial intelligence, were reported as positive predictive factors in terms of the outcomes of cataract surgery.9,10 The Pentacam system, for example, is a non-contact computed tomographic scanner that provides tomographic maps of the anterior and posterior corneal surfaces, corneal thickness, lens densitometry, and anterior chamber depth. It uses a Scheimpflug swivel camera to image the anterior segment of the eye with proven effectiveness and precision.11–13 Corneal volume (CV) is one of the many morphological parameters that can be measured with this system.3,14 The CV is a single value that describes topographic and pachymetric changes. The Pentacam system also comes with optional software, called Pentacam Nucleus Staging (PNS), that can be used to quantify cataract density from the Scheimpflug images. This software measures the volume and optical density of the cataract to classify it into one of five stages.
The concurrent use of ultrasound (US) and PNS may provide the surgeon with supplemental information during evaluation of the impact of cataract surgery on keratometric measurements. Using US in this study, we aimed to evaluate the immediate tomographic changes in the postoperative cataract period.
Materials and Methods
Patients
Our study involved 30 consecutive individuals diagnosed with cataracts (17 women and 13 men; 15 right eyes and 15 left eyes; mean ± standard deviation age: 69±8.4 years) who underwent cataract operations at Instituto de Olhos Renato Ambósio between July 25, 2021 and August 30, 2020. Their demographic and clinical data were collected. The study protocol was approved by the Ethics Committee of the Federal University of São Paulo. Informed consent was waived as the data were collected as a part of the standard practice of care and adhered to the tenets of the Declaration of Helsinki. Only patients with operable cataracts and no history of corneal disease were included in the study. Patients who developed any postoperative complications were excluded. A patient was deemed to have against-the-rule astigmatism if they had a corneal astigmatism axis of 90 ± 20 degrees (negative cylinder) or 180 ± 20 degrees (plus cylinder), based on preoperative keratometry readings.
Preoperative and Postoperative Measures
Keratometry was performed before surgery and in the immediate postoperative period, by using the Pentacam system (Oculus Optikgeräte GmbH, Wetzlar, Germany). Its rotating Scheimpflug camera was used to determine the CVs in zones around the center of the cornea (diameters of 3, 5, and 7 mm), as well as the central corneal thickness at 0, 2, 4, and 6 mm from the center of the cornea.
The patients were seated and their heads stabilized by using a chin rest and forehead strap. They were instructed to look at a fixed target for approximately 1.5–2 s while the Scheimpflug camera was rotated. All measurements were performed by the same observer. Patient biometrics were obtained by using the IOLMaster 500 optical biometer (Carl Zeiss AG, Oberkochen, Germany), for partial coherence interferometry. The power of the intraocular lens (IOL) was calculated by using the optimized Haigis formula.
The PNS software automatically generates a cylindrical template for densitometry. The template volume used in the study was defined as follows: 2.0-mm diameter, 0.6-mm height, 8.0-mm front curvature, and 4.0-mm back curvature. This three-dimensional template was placed in the center of the nucleus, excluding the anterior and posterior cortices. The average and maximum density parameters were observed for each densitometric mode.
Surgical Procedure
On the day of surgery, the patient’s pupil was dilated with drops of 0.8% tropicamide and 5% phenylephrine. The surgery was performed with the patient under retrobulbar anesthesia. All surgeries were performed by one of the authors, who is an experienced surgeon (A.M.). A sterile, disposable 2.75-mm blade was used to create a self-sealing, triplanar corneal tunnel incision near the corneal limbus, extending into the clear corneal zone by 1 mm. Continuous curvilinear capsulorhexis was performed with a 26G cystotome through the main tunnel under a hydroxypropyl-methylcellulose viscoelastic covering. Blue trypan 0.4% was used in white cataracts, preceded by an air bubble, to avoid contact with the corneal endothelium. Hydrodissection were subsequently performed. Cataracts were removed via phacoemulsification with the Millennium Microsurgical System (Bausch & Lomb Surgical, Laval, Canada). The technique used (phaco chop in all instances) was at the surgeon’s discretion. The lens was inserted into the main incision by using an injector. A single polymethylmethacrylate IOL was implanted into the capsular bag. The self-sealing incision was left unsutured after verification that there was no leakage. The patients had ketorolac 0.5%, moxifloxacin 0.5%, and prednisolone 1% as a postoperative drop regimen.
Statistical Analysis
Data were analyzed using STATA 14.0 software (StataCorp LP, College Station, TX, USA). Frequency tables were used for descriptive analyses. Continuous variables were evaluated for normality using the Shapiro–Wilk test. Pre-and postoperative continuous variables were compared using the Wilcoxon signed-rank test. Uni- and multivariable linear regression analyses were used to assess the effect of covariates on pre- vs postoperative differences; stepwise forward selection was used for modeling, with increments of 0.2. Spearman correlation test was used to assess the correlation between continuous variables. A two-sided p-value less than or equal to 0.05 was considered statistically significant.
Results
Table 1 summarizes the characteristics of the 30 included patients. Table 2 contains the US values for the surgical procedure. There were statistically significant increases in astigmatism (p=0.0350), CV (p<0.0001), and pachymetry (p<0.0001) in the postoperative period compared with the preoperative period (Table 3). There was also a statistically significant decrease in cell count upon specular microscopy (p<0.0001) in the postoperative period compared with the preoperative period. Figure 1 illustrates the mean differences in postoperative astigmatism, specular microscopy, CV, and pachymetry compared to the preoperative period were 0.76±1.83 D, −335.13±236.21 cells/mm3, 1.33±0.56 mm3, and 61.33±23.73 microns, respectively.
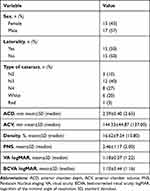 |
Table 1 Patient Characteristics |
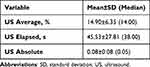 |
Table 2 Ultrasound Values Referring to the Surgical Procedure |
 |
Table 3 Comparison of Astigmatism, Specular Microscopy, Corneal Volume, and Pachymetry Before and Immediately After Surgery |
 |
Figure 1 Box plots for astigmatism, specular microscopy, corneal volume, and pre- and postoperative pachymetry. |
Uni- and multivariable linear regression analyses were applied to evaluate the effects of average (%), elapsed (s) and absolute values of US on observed differences in astigmatism, specular microscopy, CV, and pachymetry after adjustment for preoperative values and interaction between US average and elapsed. No statistically significant effects of US average, US elapsed, US absolute, or preoperative parameters on the differences between pre- and postoperative astigmatism or specular microscopy cell counts were observed (p>0.05). Table 4 summarizes the uni- and multivariable regression analyses for CV and pachymetry. Although US average, US absolute, preoperative CV, and the interaction between US average and US elapsed did not have an effect on the observed differences between preoperative and postoperative CV (p>0.05), US elapsed had a statistically significant effect (p=0.005). The difference in pre- and postoperative CV in patients with US elapsed ≥40 s was 0.75 mm3 greater than that in patients with US elapsed <40 s (coef.: 0.75; 95% confidence interval [CI]: 0.24 to 1.25; p=0.005). Likewise, although US average, US absolute, preoperative corneal pachymetry, and the interaction between US average and US elapsed had no effect on the observed differences between preoperative and postoperative pachymetry values (p>0.05), US elapsed did have a statistically significant effect (p=0.007). The difference in pre- and postoperative pachymetry in patients with US elapsed ≥40 s was 31.76 microns greater than that in patients with US elapsed <40 s (coef.: 31.76; 95% CI: 9.55 to 53.97; p=0.007).
 |
Table 4 Multiple Linear Regression Analyses for Differences Between Pre- and Postoperative Outcomes |
Figure 2 illustrates the relationship between US average and cataract density. For every 1% increase in cataract density, according to the Scheimpflug grading score, the US average value increased by 0.31% (coef.: 0.3110; 95% CI: 0.0741–0.5490; p=0.012).
 |
Figure 2 Regression analysis showing a moderate linear correlation between US average and cataract density. |
Discussion
Despite the advances in surgical techniques and devices used in cataract surgery, there are still notable differences between the preoperative and immediate postoperative corneal anatomy.15,16 In this study, these differences are summarized in Table 3. The influence of, eg, laser-assisted cataract surgery and the use of US during the procedure on the final results are well documented.17,18
The literature has already demonstrated utilizing diagnostic imaging tools in cataract surgery prediction. For example, in a retrospective study, Fernando Faria-Correia et al19 showed a good correlation between lens densitometry and phacodynamics. Therefore, the Pentacam device, including the PNS, is a good tool for preoperative cataract surgery planning and predicting cataract hardness.20,21
The parameters used in phacoemulsification influence the CV and postoperative astigmatism.22–24 Especially with very hard cataracts, the absolute ultrasound time directly influences the degree of corneal edema in the immediate postoperative period, as described in this study. The surgeon determines the phacoemulsification parameters according to the same principle regardless of the device used. These settings are displayed as “US AVE”, “US Elapsed”, and “US Absolute”. US AVE is the average phaco power used during the surgery, US Elapsed is the elapsed phaco time, and US absolute is a product of the phaco power and time. The mean values for US average, US elapsed, and US absolute used by the surgeon in this study were 14.90% ± 6.35%, 45.53 ± 27.81 s, and 0.08 ± 0.08, respectively. In our study, neither these parameters nor the interaction between US average and US elapsed had a statistically significant effect on the differences in pre- and postoperative astigmatism, specular microscopy, or pachymetry results.
In our study, we discovered moderate linear correlation between PNS and US parameters. This supplemental information may help the surgeon to predict corneal edema when faced with hard nucleus cataracts or corneal endothelial fragility.
Notably, US elapsed had a statistically significant effect on the difference in CV and pachymetry between the pre- and postoperative periods. A larger US elapsed value was associated with a more clinically significant increase in those parameters, leading to a larger degree of edema immediately after surgery.
Conclusion
PNS may help the surgeon to predict cataract hardness and, thus, provide an estimate for the degree of US required for each cataract. US elapsed is another important predictive factor and phacoemulsification parameter; its indiscriminate use may increase corneal pachymetry and volume. In addition, the concurrent use of tomographic measurements, obtained immediately after cataract surgery, and US parameters, obtained during phacoemulsification, proved helpful. This data is of particular value for surgeons who routinely encounter patients with hard cataracts.
Abbreviations
CI, confidence interval; CV, corneal volume; IOL, intraocular lens; PNS, Pentacam Nucleus Staging; US, ultrasound.
Funding
No funding or sponsorship was received for this study or publication of this article.
Disclosure
Dr. Haddad, Dr. Daher, Dr. Mine, Dr. Borges, and Dr. Salomão have no financial or proprietary interest in any product mentioned herein. Dr. Ambrosio is a consultant for Oculus®, Wetzlar, Germany. Dr. Ambrosio is also a Consultant to Alcon, Mediphacos, and Zeiss.
References
1. Aristodemou P, Sparrow JM, Kaye S. Evaluating refractive outcomes after cataract surgery. Ophthalmology. 2019;126(1):13–18. doi:10.1016/j.ophtha.2018.07.009
2. Rementería-Capelo LA, García-Pérez JL, Gros-Otero J, Morán A, Sánchez-Pina JM, Contreras I. Visual and refractive outcomes of cataract surgeries performed in one year in a private practice setting: review of 2714 procedures. J Ophthalmol. 2020;2020:2421816. doi:10.1155/2020/2421816
3. Sedaghat MR, Sharepoor M, Hassanzadeh S, Abrishami M. The corneal volume and biomechanical corneal factors: is there any correlation? J Res Med Sci. 2012;17(1):32–39.
4. Tejedor J, Pérez-Rodríguez JA. Astigmatic change induced by 2.8-mm corneal incisions for cataract surgery. Invest Ophthalmol Vis Sci. 2009;50(3):989–994. doi:10.1167/iovs.08-2778
5. Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38(12):2080–2087. doi:10.1016/j.jcrs.2012.08.036
6. Nikose AS, Saha D, Laddha PM, Patil M. Surgically induced astigmatism after phacoemulsification by temporal clear corneal and superior clear corneal approach: a comparison. Clin Ophthalmol. 2018;12:65–70. doi:10.2147/OPTH.S149709
7. Yang J, Wang X, Zhang H, Pang Y, Wei RH. Clinical evaluation of surgery-induced astigmatism in cataract surgery using 2.2 mm or 1.8 mm clear corneal micro-incisions. Int J Ophthalmol. 2017;10(1):68–71. doi:10.18240/ijo.2017.01.11
8. Yoon YC, Ha M, Whang WJ. Comparison of surgically induced astigmatism between anterior and total cornea in 2.2-mm steep Meridian incision cataract surgery. BMC Ophthalmol. 2021;21(1):373. doi:10.1186/s12886-021-02131-x
9. Gutierrez L, Lim JS, Foo LL, et al. Application of artificial intelligence in cataract management: current and future directions. Eye Vis. 2022;9(1):3. doi:10.1186/s40662-021-00273-z
10. Tognetto D, Giglio R, Vinciguerra AL, et al. Artificial intelligence applications and cataract management: a systematic review. Surv Ophthalmol. 2021;S0039–6257(21):00187–00189. doi:10.1016/j.survophthal.2021.09.004
11. Haddad JS, Barnwell E, Rocha KM, Ambrosio R Jr, Waring GO. Comparison of biometry measurements using standard partial coherence interferometry versus new Scheimpflug tomography with integrated axial length capability. Clin Ophthalmol. 2020;14:353–358. doi:10.2147/OPTH.S238112
12. Barkana Y, Gerber Y, Elbaz U, et al. Central corneal thickness measurement with the Pentacam Scheimpflug system, optical low-coherence reflectometry pachymeter, and ultrasound pachymetry. J Cataract Refract Surg. 2005;31(9):1729–1735. doi:10.1016/j.jcrs.2005.03.058
13. Lackner B, Schmidinger G, Skorpik C. Validity and repeatability of anterior chamber depth measurements with Pentacam and Orbscan. Optom Vis Sci. 2005;82(9):858–861. doi:10.1097/01.opx.0000177804.53192.15
14. Abib F, Martins Dos Santos R. Corneal volume of the normal human corneas calculated by Pentacam. Invest Ophthalmol Vis Sci. 2019;60(11):B0137.
15. Dikci S, Demirel S, Fırat P, et al. Pre-, intra- and postoperative management in phacoemulsification surgery for completely monocular cases. Ann Res. 2019;26(5):859–863.
16. Day AC, Dhariwal M, Keith MS, et al. Distribution of preoperative and postoperative astigmatism in a large population of patients undergoing cataract surgery in the UK. Br J Ophthalmol. 2019;103(7):993–1000. doi:10.1136/bjophthalmol-2018-312025
17. Day AC, Gore DM, Bunce C, Evans JR. Laser-assisted cataract surgery versus standard ultrasound phacoemulsification cataract surgery. Cochrane Database Syst Rev. 2016;7(7):CD010735. doi:10.1002/14651858.CD010735.pub2
18. Horta GA, Horta RC, Steinfeld K, Koch CR, Mello GR, Kara-Junior N. Ultrasound power and irrigation volume in different lens opacity grades: comparison of femtosecond laser-assisted cataract surgery and conventional phacoemulsification. Clinics. 2019;74:e1294. doi:10.6061/clinics/2019/e1294
19. Faria-Correia F, Lopes BT, Ramos IC, Monteiro T, Franqueira N, Ambrósio R
20. Magalhães FP, Costa EF, Cariello AJ, Rodrigues EB, Hofling-Lima AL. Comparative analysis of the nuclear lens opalescence by the lens opacities classification system III with nuclear density values provided by Oculus Pentacam: a cross-section study using Pentacam Nucleus Staging software. Arq Bras Oftalmol. 2011;74(2):110–113. doi:10.1590/s0004-27492011000200008
21. Faria-Correia F, Ramos I, Lopes B, Monteiro T, Franqueira N, Ambrósio R
22. Kanellopoulos AJ, Asimellis G. Standard manual capsulorhexis/ultrasound phacoemulsification compared to femtosecond laser-assisted capsulorhexis and lens fragmentation in clear cornea small incision cataract surgery. Eye Vis. 2016;3:20. doi:10.1186/s40662-016-0050-x
23. Abell RG, Darian-Smith E, Kan JB, Allen PL, Ewe SYP, Vote BJ. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: outcomes and safety in more than 4000 cases at a single center. J Cataract Refract Surg. 2015;41(1):47–52. doi:10.1016/j.jcrs.2014.06.025
24. Menapace RM, Dick HB. [Femtosecond laser in cataract surgery. A critical appraisal]. Ophthalmologe. 2014;111(7):624–637. German. doi:10.1007/s00347-014-3032-1
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
