Back to Journals » Clinical Ophthalmology » Volume 18
Correlational Analysis of the Effective Optical Zone with Myopia, Myopic Astigmatism, and Spherical Equivalent in LASIK, PRK, and SMILE
Authors Moshirfar M , Herron MS , Cha DS, Santos JM , Miller LT, Hoopes PC Sr
Received 16 September 2023
Accepted for publication 23 January 2024
Published 5 February 2024 Volume 2024:18 Pages 377—392
DOI https://doi.org/10.2147/OPTH.S440608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Majid Moshirfar,1– 3 Michael S Herron,4 David S Cha,5 Jordan M Santos,6 Levi T Miller,7 Phillip C Hoopes Sr1
1Hoopes Vision Research Center, Hoopes Vision, Draper, UT, 84020, USA; 2John A. Moran Eye Center, University of Utah School of Medicine, Salt Lake City, UT, USA; 3Utah Lions Eye Bank, Murray, UT, USA; 4University of Nevada, Reno School of Medicine, Reno, NV, USA; 5Saint Louis University School of Medicine, St. Louis, MO, USA; 6University of Arizona, Phoenix School of Medicine, Phoenix, AZ, USA; 7Utah State University, Department of Electrical and Computer Engineering, Logan, UT, USA
Correspondence: Majid Moshirfar, Hoopes Vision Research Center, 11820 S. State St. #200, Draper, UT, 84020, USA, Tel\Fax +1 801-568-0200, Email [email protected]
Purpose: We assess the relationship between preoperative myopic sphere, astigmatism, and spherical equivalent and effective optical zone (EOZ) size, shape, and decentration within individual populations of post-LASIK, PRK, and SMILE patients.
Patients and Methods: A retrospective chart review was conducted with 118 LASIK, 144 PRK, and 41 SMILE eyes from 179 total patients that underwent compound myopic ablation. One-year postoperative Pentacam tangential difference maps were used for EOZ data measurements. Correlational analysis between compound myopic measures [sphere, cylinder, manifest refractive spherical equivalent (MRSE)] and EOZ parameters was performed, and differences between groups of myopic sphere and cylinder within each surgery type were assessed.
Results: An increase in absolute myopic sphere (and subsequent MRSE) is associated with a smaller EOZ area in SMILE (r=0.454, p=0.003) and a more circular EOZ shape in LASIK (r=0.396, p< 0.001) and PRK (r=0.563, p< 0.001). An increase in absolute myopic cylinder is associated with an increased EOZ area in all three surgery types [LASIK (r=− 0.459, p< 0.001), PRK (r=− 0.716, p< 0.001), SMILE (r=− 0.429, p=0.005)] and a more elliptical EOZ in LASIK (r=− 0.491, p< 0.001) and PRK (r=− 0.538, p< 0.001).
Conclusion: While astigmatism may be correlated to EOZ size within all three refractive surgery types, myopic sphere alone is insufficient to estimate EOZ size differences for procedures with a large blend zone of ablation like LASIK or PRK. Shape is just as important a factor as size to consider when examining corneal EOZ differences; reported correlative findings likely result from inherent differences in surgical technique and abruptness of planned surgical ablation borders.
Keywords: EOZ, OZ, Pentacam, tangential difference map
Introduction
The concept of effective optical zone (EOZ) within corneal refractive literature is one that has received much attention in recent years. Corneal EOZ refers to the central region of the cornea that has been treated during refractive ablation surgery such as LASIK, PRK, or SMILE that provides the intended optimal postoperative visual acuity1 and is currently measured using corneal topography maps by many. It is critical for surgeons to understand the impact that adequate sizing and centration of this zone has, as it affects patient outcomes such as visual quality and ultimately overall patient satisfaction. Decreased EOZ size2,3 and increased EOZ decentration4 have been linked to increased postoperative higher-order aberrations, which cause subjective glare and halos as frequently reported side effects.5 Over the past few decades, marked improvements have been made to reduce these effects in corneal refractive treatments using topography guided ablations, wave-front optimization, pupil tracking systems, increased planned optical zone (OZ) sizes,6,7 and transition zones of ablation.8 Currently, surgeons use these tools and techniques to perform LASIK, PRK, and SMILE with the goal of creating an EOZ of adequate size and visual axis-centration that will provide excellent visual acuity and minimize side effects.
Factors inherent to patient anatomy such as preoperative corneal shape and power influence subsequent surgical technique, ablation profiles, and resultant EOZ size, which make them an area of particular interest. Many studies have been conducted that compare EOZ between surgery types such as LASIK and PRK,9–11 but relatively few thus far have examined the relationship between EOZ size and compound myopic factors within a single surgery type. For example, only two studies to our knowledge have directly compared EOZ size across levels of astigmatism. Ding et al and Wang et al recently described the relationship between level of preoperative myopic cylinder and postoperative EOZ in patients who underwent SMILE to be positive; higher astigmatism resulted in larger resultant EOZ size.12,13 No studies have directly examined the relationship between level of myopic sphere and EOZ within a surgical type thus far. The purpose of this study is to examine the relationships between EOZ size and decentration across levels of myopic sphere, myopic cylinder, and spherical equivalent within individual populations of all types of corneal refractive surgery including LASIK, PRK, and SMILE.
Materials and Methods
Patients and Comparison Groups
A retrospective analysis was conducted on patients who underwent laser refractive treatments (specifically LASIK, PRK, and SMILE) between 2017 and 2022. Adult patients (18+ years) with compound myopia and/or myopic astigmatism who had a postoperative BCVA of 20/25 or better were included. Exclusion criteria included patients with macular degeneration, keratoconus, glaucoma, cataracts, retinal detachment, herpes simplex virus, systemic lupus erythematosus, hyperopic compound spherical equivalent, and patients who underwent enhancement. All participants provided written consent prior to study in accordance with the declaration of Helsinki, and full approval was granted by the Biomedical Research Alliance of New York Institutional Review Board (#A20-12-547-823). This study analyzes the differences in EOZ across myopic sphere, cylinder, and spherical equivalent; as such, all surgery types were reviewed and analyzed separately from each other. The study includes 117 eyes from 70 LASIK patients, 144 eyes from 86 PRK patients, and 41 eyes from 23 SMILE patients. The classification of groups was based on widely accepted thresholds for myopia. The high, moderate, and low myope groups consisted of eyes with a myopic sphere of −6 diopters (D) or higher, between −3 and −6 D, and less than −3 D, respectively. Similarly, the high, moderate, low, and no cylinder groups were determined using the following criteria: high cylinder as −2.5 D or greater, moderate cylinder as −1.5 to −2.5 D, low cylinder as −0.5 to −1.5 D, and no cylinder as 0 to −0.5 D.
Surgical Technique
A single surgeon (MM) conducted all surgical procedures at the same location. When performing LASIK and PRK, a predetermined optical zone measuring 6.5 mm was utilized, with a smooth transition to 9 mm. The equipment employed for LASIK consisted of the FS200 femtosecond laser (Alcon Laboratories, Fort Worth, TX, USA) for creating or removing epithelial flaps in LASIK and PRK, respectively. Subsequent ablation was carried out using the WaveLight Allegretto Wave Excimer Laser System (Alcon Laboratories, Fort Worth, TX, USA). In the case of pure myopic SMILE patients (defined as having manifest refraction cylinder magnitude equal to or less than −0.25D), a planned optical zone with a spherical lenticule of 6.5 mm was employed; correction was performed in terms of spherical equivalent, without any transition zone. However, for SMILE myopic astigmatism needing toric treatment (defined as manifest cylinder magnitude of −0.5D or greater), a zone of 6.0 mm with a gradual transition to 6.5 mm was utilized using the Visumax toric lenticule. Manifest refraction cylinder of −0.5D received a correction of 0.75D due to USA FDA-approved Visumax SMILE software restrictions, while all greater cylinder values received an equivalent power correction. For lenticule extraction in SMILE surgeries, the Visumax 500 kHz femtosecond laser (Carl Zeiss Meditec, Jena, Germany) was used. No software versions changed for the excimer laser or flap performing device throughout the duration of the study.
Effective Optical Zone Measurement
Pentacam tangential difference (▲TG) maps were used to measure the effective optical zones with a baseline “pre” scan at the preoperative visit, and the “post” scan at the one-year postoperative visit. These maps are particularly sensitive to local curvature changes11 and utilize the equation “Dpt=(1.3375–1)*(1000)/R[mm]” to express power values corresponding to Placido topographers. In the Pentacam system, corneal vertex is approximated as equivalent to the vertex normal of Purkinje-Sanson Reflex 1 and serves as the central point of the ▲TG maps. To maintain consistency with this system, this point is consistently referred to as the corneal vertex in the present study. Twelve points where a 0 D difference was detected between the pre- and post-scans were measured at every consecutive 30 degrees moving counterclockwise from 0° in order to approximate the EOZ.11,12,14 Python 3.11 (Python Software Foundation) was used to run the publicly available NumPy best fitting ellipse program, which provides data such as the ellipse’s center point, area, axis lengths, and eccentricity. In this study, the major and minor axes are called major and minor diameters, and they denote the transected distance in mm between the two elliptical points that are farthest from, and closest to, each other, respectively. All reported decentration values represent the distance in mm from the corneal vertex and the center point of the measured ellipse. All areas are a measure of the zone contained within the borders of the ellipse in mm2.
Statistical Analysis
Individual surgery populations were assessed and controlled for preoperative characteristics of interest; categorical variables are reported using frequency while numerical variables are reported using mean ± standard deviation with associated ranges. All reported manifest refractive spherical equivalent (MRSE) values have been corrected to account for the corneal refractive index. Preoperative characteristics and measured EOZ parameters were assessed for normality using the Shapiro–Wilk test, with most showing nonparametric distributions. Thus, Spearman correlations were used to assess relationships between EOZ parameters and myopic sphere, MRSE, and myopic cylinder. Simple linear regressions were plotted for relationships of interest. The Kruskal–Wallis test was used to compare groups of myopic sphere and myopic cylinder, and all relationships that showed significance underwent pairwise comparisons with Bonferroni adjustments. The standard visual outcomes15 were plotted. Using G*power3.1 (Franz Faul, Universitat Kiel, Germany), retrospective power analysis was performed for all correlation analyses. A power of β=0.90 was achieved to reject the null hypothesis of zero correlation by taking the smallest sample and lowest significant correlation into account, with a significance level of α=0.05. All analyses were performed using SPSS software (version 29.0, IBM Corp.).
Results
Preoperative Characteristics
Individual populations of patients who underwent LASIK, PRK, or SMILE were assessed separately.
Within the LASIK population, data from 117 eyes of 44 male and 26 female patients with an average age of 33.5 ± 6.9 years were analyzed. Preoperative mean keratometry (Km) of these eyes was measured at 43.6 ± 1.2 D, anterior corneal Q was measured at −0.33 ± 0.13, minimal corneal thickness (MCT) at 548.6 ± 23.3 µm, and planned OZ at 6.5 ± 0.0 mm. Mean preoperative MRSE was measured at −3.23 ± 1.59 D, with the spherical component being −3.02 ± 1.75 D and the cylinder component being −0.75 ± 0.67 D.
For the PRK population, 144 eyes of 40 male and 46 female patients were assessed. These patients had an average age of 33.9 ± 5.7 years, Km of 44.1 ± 1.3 D, anterior corneal Q of −0.35 ± 0.12, MCT of 522.9 ± 27.3 µm, and planned OZ of 6.5 ± 0.0 mm. Mean preoperative MRSE was measured at −3.63 ± 1.51 D with a spherical component of −3.36 ± 1.72 D and a cylinder component of −0.92 ± 0.81 D.
The SMILE population included 41 eyes of 10 male and 13 female patients aged 32.9 ± 5.5 years. Preoperative Km of these eyes was 43.9 ± 1.0 D, anterior corneal Q was −0.33 ± 0.12, MCT was 549.1 ± 28.7 µm, and planned OZ was 6.3 ± 0.2 mm. Mean preoperative MRSE was measured at −5.00 ± 1.46 D, with the spherical component being −5.11 ± 1.68 D and the cylinder component being −0.47 ± 0.38 D.
No differences were found between groups of myopic sphere or cylinder in any of these parameters except for levels of MRSE across myopic sphere (p<0.001) for all three surgeries, and for planned OZ in SMILE (p<0.001) (Table1 and Table 2).
 |
Table 1 Preoperative Characteristics of LASIK, PRK, and SMILE Across Levels of Myopic Sphere (Myopia) |
 |
Table 2 Preoperative Characteristics of LASIK, PRK, and SMILE Across Levels of Myopic Cylinder (Astigmatism) |
Correlations Between Myopia and Effective Optical Zone Measurements
No significant correlation is noted between the level of myopic sphere and area of EOZ in LASIK (r=0.085, p=0.362) or PRK (r=0.119, p=0.156). In SMILE, EOZ area is positively correlated with the level of myopic sphere (r=0.454, p=0.003). LASIK and PRK do, however, have a statistically significant positive correlation between myopic sphere and EOZ eccentricity (r=0.396, p<0.001 and r=0.563, p<0.001, respectively), while SMILE does not (r=0.246, p=0.121). For LASIK and PRK, sphere is positively correlated with major diameter (r=0.228, p=0.013 and r=0.280, p=0.001, respectively) and negatively correlated with minor diameter (r=−0.338, p<0.001 and r=−0.422, p<0.001, respectively), while SMILE is positively correlated with both major and minor diameter (r=0.508, p=0.001 and r=0.331, p=0.034, respectively). Patterns of correlation between MRSE and EOZ are comparable to those found between myopic sphere and EOZ, with a notable exception of significance between MRSE and major diameter length in both LASIK (r=0.113, p=0.227) and PRK (r=0.140, p=0.095). As absolute myopic sphere or MRSE increases, EOZ areas become smaller in SMILE and EOZ shape becomes more circular in LASIK and PRK (Table 3, Figures 1 and 2).
 |
Table 3 Spearman Correlation Between Effective Optical Zone, Myopia, Myopic Astigmatism, and Spherical Equivalent in LASIK, PRK, and SMILE, Presented as r Value (p value) |
 |
Figure 1 Linear regression of effective optical zone area and eccentricity with myopic sphere in separate populations of LASIK, PRK, and SMILE. |
 |
Figure 2 Linear regression of effective optical zone area and eccentricity with manifest refractive spherical equivalent in separate populations of LASIK, PRK, and SMILE. |
The level of myopic cylinder is negatively correlated with EOZ area, major diameter, and minor diameter in all surgeries, with a single exception between myopic cylinder and minor diameter in LASIK (r=−0.088, p=0.343). All correlations between myopic cylinder and EOZ size are moderate to strong [LASIK: (r=−0.459, p<0.001), PRK: (r=−0.716, p<0.001), SMILE: (r=−0.429, p=0.005)]. Myopic cylinder has a negative correlation with EOZ eccentricity in both LASIK (r=−0.491, p<0.001) and PRK (r=−0.538, p<0.001), but not in SMILE (r=−0.171, p=0.286). A weak negative correlation is found between myopic cylinder and absolute decentration from vertex in PRK (r=−0.327, p<0.001). As absolute myopic cylinder increases, EOZ areas increase in all three surgery types and EOZ shape becomes more elliptical in LASIK and PRK (Table 3 and Figure 3).
 |
Figure 3 Linear regression of effective optical zone area and eccentricity with myopic cylinder in separate populations of LASIK, PRK, and SMILE. |
Across Groups of Myopic Sphere and Cylinder
In terms of myopic sphere, EOZ shape is more circular among high and moderate myopes when compared to low myopes in both LASIK (p=0.001 and p=0.009, respectively) and PRK (p<0.001 for both). High, moderate, and low myope groups had measured eccentricities at 0.26 ± 0.07, 0.35 ± 0.12, and 0.44 ± 0.17 for LASIK and 0.28 ± 0.03, 0.35 ± 0.11, and 0.52 ± 0.17 for PRK. This stems from smaller measured minor diameters in lower myope groups (LASIK: 4.75 ± 0.28 mm, PRK: 4.73 ± 0.26 mm) and greater measured major diameters in lower myopes (LASIK: 5.49 ± 0.83 mm, PRK: 5.83 ± 1.03 mm). These diameter differences from moderate and high myope groups were all significant except for major diameters in LASIK (p=0.283). No difference was noted in EOZ eccentricity across groups of myopic sphere in SMILE (p=0.518), and no difference was noted in EOZ area across groups of myopic sphere in LASIK, PRK, or SMILE (p=0.994, p=0.273, and p=0.132, respectively) (Table 4).
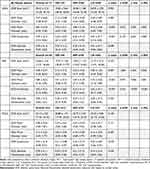 |
Table 4 Effective Optical Zone Parameters in LASIK, PRK, and SMILE Across Levels of Myopic Sphere (Myopia) |
Across groups of myopic cylinder, differences were found among measured EOZ areas in LASIK, PRK, and SMILE (p<0.001, p<0.001, and p=0.28, respectively) and among EOZ eccentricity in LASIK and PRK (p<0.001 for both). In LASIK, EOZ area ranged from 28.57 ± 7.12 mm2 in the high cylinder group to 19.22 ± 1.58 mm2 in the low cylinder group, with a progressive decrease in each group and significance in 3 of the 6 pairwise comparisons. In PRK, EOZ area also showed a progressive decrease that ranged from 27.92 ± 5.50 mm2 in the high cylinder group to 18.84 ± 1.38 mm2 in the low cylinder group, with significance in 5 of the 6 pairwise comparisons. SMILE showed a significant difference in EOZ area among the two measured groups, namely “low” and “no” cylinder (p=0.028). In LASIK, EOZ eccentricity ranged from 0.65 ± 0.09 in the high cylinder group to 0.32 ± 0.11 in the low cylinder group, with a progressively more circular shape in each group and significance in 4 of the 6 pairwise comparisons. In PRK, EOZ eccentricity again showed a progressive increase in circular shape that ranged in eccentricity values of 0.67 ± 0.15 in the high cylinder group to 0.35 ± 0.11 in the low cylinder group, with significance in 4 of the 6 pairwise comparisons. In SMILE, no difference was noted in eccentricity across groups of myopic cylinder (p=0.181) (Table 5).
 |
Table 5 Effective Optical Zone Parameters in LASIK, PRK, and SMILE Across Levels of Myopic Cylinder (Astigmatism) |
Refractive Outcomes
The standard visual and refractive outcomes of each individual surgical group are included for reference15 (Figure 4).
 |
Figure 4 Continued. |
Discussion
The Effect of Myopia and Spherical Equivalent on Effective Optical Zone
The relationships discovered between EOZ parameters and corneal power exhibited remarkable similarity between the sphere and MRSE. This similarity is seen because MRSE is determined by summing the myopic sphere power with half the myopic cylinder power. Additionally, most participants had a much greater myopic sphere than cylinder, further reinforcing these consistent relationships observed in the present study. Because the sphere significantly surpassed the cylinder power, this remarkable similarity between the sphere and MRSE had only a few exceptions. Relationships found in the SMILE population, however, were different from those seen in both LASIK and PRK.
In SMILE, as both myopic sphere and MRSE increase, the EOZ size decreases, while in LASIK and PRK, the EOZ shape becomes more circular. These novel associations reported here likely stem from inherent differences in surgical technique between the three procedures.16 In SMILE, the singular complete extraction of an intact lenticule is performed, as opposed to the many micro-ablations employed in LASIK and PRK, which likely contribute at least in part to the observed relationship with size. Additionally, the greater blend zone in the latter surgeries also appears to effectively avoid a sharp boundary on the measured EOZ. Without such a significant blend zone in SMILE, larger tissue removal results in smaller zones. In LASIK and PRK, a relationship is found only with shape rather than size, perhaps due to smoother transitions across varied levels of sphere and MRSE. While it might have been expected based on surgical intuition that EOZ size would decrease as myopic sphere increases in all types of surgery, this pattern is only observed in SMILE. Apart from size and shape in these specific scenarios, no other changes related to myopia are noted across different surgical procedures, including decentration.
The Effect of Astigmatism on Effective Optical Zone
Correlation analysis conducted within the SMILE population demonstrates consistency with the findings reported by Ding et al and Wang et al regarding the relationship between the size of the effective optical zone (EOZ) and astigmatism.12,13 The present study reveals that in SMILE, as the myopic cylinder increases, the area of the EOZ also increases. Notably, this trend is observed in both LASIK and PRK procedures as well. Irrespective of the type of surgery, higher levels of astigmatism result in a larger postoperative EOZ area. Furthermore, these findings indicate that LASIK and PRK exhibit a more elliptical measured EOZ with increased myopic cylinder, whereas such an effect is not observed in SMILE. This discrepancy can again be attributed to differences in surgical techniques. In the case of SMILE, the posterior portion of the lenticule maintains an oval shape, even though blending on the anterior surface occurs to form a circle. This unique characteristic of SMILE may be contributing to the lack of measured difference in overall postoperative EOZ shape, which is distinct from the findings in both LASIK and SMILE. Each of the latter surgeries resulted in more elliptical shapes among higher levels of astigmatism.
Considerations for Interpretation
Populations here were controlled for preoperative characteristics across groups of myopic sphere and cylinder; as such, no comparisons can meaningfully be made between surgical type, but conclusions drawn from comparisons across level of sphere and astigmatism are more applicable to groups with similar preoperative values. Because myopic sphere is the main component of spherical equivalent, this is the one parameter that showed statistical difference between groups of myopic sphere for all three surgery types. Surgical groups also inherently differ from one another in choosing the most appropriate, safest, and effective procedure for the patient (ie SMILE is most suitable for patients with high myopic sphere and low levels of astigmatism, while LASIK and PRK provide adequate correction in low myopes requiring a greater degree of astigmatic correction). These clinical considerations caused variation in sample size for each surgical cohort, weighting them toward the safer option, and resulting in a complete lack of data for SMILE in “high” and “moderate” myope groups. Nonetheless, a significance level of α=0.05 was used and a power of 90% was achieved for all reported significant correlations.
One particular strength of this study is the methods by which EOZ is measured. While many methods for measurement can and have been used, the discriminatory 12-point system with best-fit ellipses used here provides a more comprehensive understanding of the resulting postoperative EOZ shape in comparison to those that estimate zone based on a circular shape while using one average diameter. In comparison to other methods that were trialed, this was the most reproducible across various levels of myopia and cylinder, along with having particular utility in determining decentration.17 By employing this comprehensive approach, we were able to provide a more nuanced impression of EOZ shape without the need for analyzing multiple Pentacam map types, while preserving the integrity of each parameter of interest.
One limitation of this study is that due to the need for toric treatment, OZ size varied among some groups. Specifically, some participants who underwent SMILE and PRK had an OZ of 6 mm. Sufficient patients with 6 mm OZ were present in the SMILE “low cylinder” group to result in a statistically significant difference in OZ between levels of myopic cylinder, but no other differences were noted across cylinder in preoperative characteristics. Due to this, the resultant significant differences seen in EOZ area and major diameter can be attributed, at least in part, to these differences in preoperative characteristics. Another potential limitation is that rather than assigning arbitrary cutoff points for groups to form equal distributions in sample size, groups were intentionally formed using widely accepted limits for sphere and cylinder in mind; this caused some groups to have a much smaller sample size than others. Sample size is most important when attempting to establish a lack of correlation or a lack of difference between groups due to the potential for type II statistical error, while significant relationships are presumed credible due to the low probability of finding such relationships. Despite reporting comparisons that demonstrate no statistically significant differences between groups with smaller samples, this does not negate the existence of such differences, and future studies may mitigate this limitation by employing a prospective design. This would also ensure adequate representation of each cohort and allow for exploration of causative relationships, while still maintaining tangibility of widely accepted cutoff values used in clinical practice.
Conclusion
While astigmatism can serve as a predictive factor for estimating the size of the effective optical zone (EOZ) across all three types of corneal refractive surgeries, relying solely on myopic sphere measurements is inadequate when assessing differences in EOZ size for procedures with a significant blend zone of ablation, such as LASIK or PRK. It is crucial to consider factors that affect both the shape and size when examining variances in corneal EOZ. The reported correlations are likely a result of inherent disparities in surgical techniques and the abruptness of planned surgical ablation borders. In conclusion, both myopia and cylindrical power influence EOZ size in different ways for each surgery type. However, neither of these factors seems to affect EOZ decentration.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tabernero J, Klyce SD, Sarver EJ, Artal P. Functional optical zone of the cornea. Invest Ophthalmol Visual Sci. 2007;48(3):1053–1060. doi:10.1167/iovs.06-0867
2. Boxer Wachler BS, Huynh VN, El-Shiaty AF, Goldberg D. Evaluation of corneal functional optical zone after laser in situ keratomileusis. J Cataract Refract Surg. 2002;28(6):948–953. doi:10.1016/S0886-3350(02)01322-6
3. Nepomuceno RL, Boxer Wachler BS, Scruggs R. Functional optical zone after myopic LASIK as a function of ablation diameter. J Cataract Refract Surg. 2005;31(2):379–384. doi:10.1016/j.jcrs.2004.04.073
4. Liu M, Sun Y, Wang D, et al. Decentration of optical zone center and its impact on visual outcomes following SMILE. Cornea. 2015;34:392–397. doi:10.1097/ICO.0000000000000383
5. Fan-Paul NI, Li J, Miller JS, Florakis GJ. Night vision disturbances after corneal refractive surgery. Surv Ophthalmol. 2002;47(6):533–546. doi:10.1016/S0039-6257(02)00350-8
6. Morris AT, Ring CP, Hadden OB. Comparison of photorefractive keratectomy for myopia using 5 mm and 6 mm diameter ablation Zones. J Refract Surg. 1996;12(2):S275–S277. doi:10.3928/1081-597X-19960201-16
7. Hersh PS, Stulting RD, Steinert RF, et al. Results of Phase III excimer laser photorefractive keratectomy for myopia. The Summit PRK Study Group. Ophthalmology. 1997;104(10):1535–1553. doi:10.1016/s0161-6420(97)30073-6
8. MacRae S. Excimer ablation design and elliptical transition zones. J Cataract Refract Surg. 1999;25(9):1191–1197. doi:10.1016/S0886-3350(99)00144-3
9. He S, Luo Y, Chen P, et al. Prospective, randomized, contralateral eye comparison of functional optical zone, and visual quality after SMILE and FS-LASIK for high myopia. Trans Vision Sci Technol. 2022;11(2):13. doi:10.1167/tvst.11.2.13
10. Damgaard IB, Ang M, Mahmoud AM, Farook M, Roberts CJ, Mehta JS. Functional Optical Zone and Centration Following SMILE and LASIK: a Prospective, Randomized, Contralateral Eye Study. J Refract Surg. 2019;35(4):230–237. doi:10.3928/1081597X-20190313-01
11. Hou J, Wang Y, Lei Y, Zheng X. Comparison of effective optical zone after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2018;44(10):1179–1185. doi:10.1016/j.jcrs.2018.06.046
12. Ding X, Fu D, Wang L, Zhou X, Yu Z. Functional optical zone and visual quality after small-incision lenticule extraction for high myopic astigmatism. Ophthalmol Ther. 2021;10(2):273–288. doi:10.1007/s40123-021-00330-9
13. Wang X, Xia L. Evaluation of the effects of myopic astigmatism correction and anterior corneal curvature on functional optical zone after SMILE. J Refract Surg. 2023;39(2):135–141. doi:10.3928/1081597X-20221215-01
14. Liu S, Zhang X, Niu L, Yu Z, Zhou X, Zhao J. Comparison of the functional optical zone in eyes with high myopia with high astigmatism after SMILE and FS-LASIK. J Refract Surg. 2022;38(9):595–601. doi:10.3928/1081597X-20220725-01
15. Waring G, Reinstein D, Dupps W, et al. Standardized graphs and terms for refractive surgery results. J Refract Surg. 2011;27:7–9. doi:10.3928/1081597X-20101116-01
16. Shah R. History and results; indications and contraindications of SMILE compared with LASIK. Asia Pac J Ophthalmol. 2019;8(5):371–376. doi:10.1097/01.APO.0000580132.98159.fa
17. Liang C, Yan H. Methods of corneal vertex centration and evaluation of effective optical zone in small incision lenticule extraction. Ophthalmic Res. 2023;717–726. doi:10.1159/000529922
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

