Back to Journals » International Journal of General Medicine » Volume 15
Correlation of Inflammation and Coagulation Markers with the Incidence of Deep Vein Thrombosis in Cancer Patients with High Risk of Thrombosis
Authors Setiawan B , Budianto W, Sukarnowati TW, Rizky D , Pangarsa EA , Santosa D , Setiabudy RD, Suharti C
Received 7 May 2022
Accepted for publication 7 July 2022
Published 19 July 2022 Volume 2022:15 Pages 6215—6226
DOI https://doi.org/10.2147/IJGM.S372038
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Budi Setiawan,1 Widi Budianto,1 Tri Wahyu Sukarnowati,1 Daniel Rizky,1 Eko Adhi Pangarsa,1 Damai Santosa,1 Rahajuningsih Dharma Setiabudy,2 Catharina Suharti1
1Hematology-Medical Oncology Division, Internal Medicine Department, Faculty of Medicine, Diponegoro University/Dr. Kariadi Hospital, Semarang, Indonesia; 2Clinical Pathology Department, Faculty of Medicine, University of Indonesia, Jakarta, Indonesia
Correspondence: Budi Setiawan, Hematology-Medical Oncology Division, Internal Medicine Department, Faculty of Medicine, Diponegoro University/Dr. Kariadi Hospital, Jl. Dr. Soetomo No. 16, Semarang, Indonesia, Email [email protected]
Background: Deep vein thrombosis (DVT) is a common complication and the second leading cause of death in cancer patients. Pro-inflammatory stimuli in the cancer microenvironment induce nuclear factor kappa B (NF-κB) signaling pathway that plays an integral role in immunothrombosis mechanism.
Objective: To investigate the role of inflammatory and coagulation biomarkers in the development of DVT in cancer patients with high risk of thrombosis (Khorana score ≥ 2).
Subjects and methods: This study was a cross-sectional study at Dr. Kariadi General Hospital. The serum levels of proinflammatory cytokines, ie, NF-κB, interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and coagulation biomarkers, ie, tissue factor (TF), prothrombin fragment F1+2 (F1+2), fibrinogen and D-dimer were measured in newlydiagnosed cancer patients with a highrisk of thrombosis. Color duplex sonography was used for DVT screening.
Results: From January to November 2021, there were 83 eligible patients. DVT was confirmed in 8 subjects (9.63%). Univariate analysis revealed a significant difference between the median age of patients with DVT compared to non-DVT patients, 49.5 years (range: 23– 60 years) and 42 years (range: 19– 60 years), with p=0.046. D-dimer level was higher in DVT patients [(6.020 μg/L, range 2.090– 20.000) vs (1.940 μg/L, range 270– 20.000), p=0.005]. Multivariate analysis revealed age and D-dimer were significantly correlated with DVT incidence. In all patients, there were significant positive correlations between several inflammatory and coagulation activation parameters, which were IL-6 with D-dimer and F1+2, CRP with F1+2 and D-dimer as well as TNF-α with F1+2. However, these findings were not shown in DVT patients.
Conclusion: In cancer patients with a high risk of thrombosis, age and D-dimer level are the significant variables towards the incidence of DVT. In patients with DVT, there was no significant correlation between inflammatory and coagulation activation parameters.
Keywords: inflammation, activation of coagulation, DVT, high-risk thrombosis, cancer patients
Introduction
Venous thromboembolism (VTE) is a common condition, which can be life-threatening in cancer patients. VTE risk increases nine-times in cancer patients compared to non-cancer patients.1 Studies reported that the incidence of thrombosis in patients with cancer has been increasing overtime, partly due to its increasing incidence in recent years.2
The annual incidence of VTE in cancer patients is estimated to be 0.5%, compared to 0.1% in general population. Cancer patients who experience VTE at the time of diagnosis tend to have poorer prognosis compared to cancer patients without VTE.3 Thrombotic events are the second leading cause of mortality after the cancer progression itself.4
Cancer is an acquired thrombophilia, which is prone to increase the risk of VTE. Tumor cells can activate coagulation pathway through various mechanism, including procoagulant production, pro-aggregation process, release of proinflammatory and proangiogenic cytokines, as well as direct interaction between blood vessels and blood cells through adhesion molecules.5
In cancer, there was an exposure of proinflammatory stimuli in the cancer microenvironment,6 causing endothelial cell damage which induce inflammatory responses.7,8 Inflammatory stimuli such as tumor necrosis factor (TNF)-α, interleukin (IL)-1a, IL-6, IL-17, IL-18 and epidermal growth factor (EGF) mediate inflammation through interaction with Toll-like receptors (TLRs), IL-1 receptors (IL-1R), IL-6 receptors (IL-6R), and TNF receptors (TNFR).8,9 The activation of receptors induces intracellular signaling pathway of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) to produce proinflammatory cytokines, namely IL-6, TNF α, IL-1, IL-8 and CRP.8
These proinflammatory cytokines play a key role in promoting procoagulant status, particularly by inducing the expression of tissue factor (TF),10 which causes the activation of coagulation cascade to form thrombus, characterized by an increase in markers for thrombin formation, including F1+2, TAT, FpA, fibrinogen, and D-Dimer.11 In addition, NF-κB signaling pathway induces the production of adhesion molecules, eg, ICAM-1, ECAM-1, PCAM-1, selectin and chemokines (MCP-1, IL-18, MP-2, CXCL), which leads to the event of thrombosis.12–14
Khorana risk score can be utilized to stratify risk of VTE in cancer patients into low, moderate, and high risks.15 Ambulatory cancer patients with a high risk of thrombosis (Khorana score ≥2) might be offered to receive pharmacologic thromboprophylaxis during receiving systemic chemotherapeutic agents. However, hospitalized cancer patients with active disease and immobility should also be offered pharmacologic thromboprophylaxis.16,17
Based on the aforementioned reasons, this study aims to investigate the role of proinflammatory cytokines (NF-κB, TNF-α, IL-6, and CRP) in promoting procoagulant status with activation of coagulation biomarkers (TF, prothrombin F1+2, fibrinogen, and D-dimer), which are the risk factors for DVT in cancer patients with high risk of thrombosis. This study is expected to provide a better understanding of the pathophysiology of cancer-associated thrombosis, thus contributing to an early primary evaluation for particular cancer patients based on DVT risk stratification.
Methods
Study Setting
This is a cross-sectional study performed in Dr. Kariadi Hospital, the main teaching hospital for Medical Faculty of Diponegoro University, Semarang, Indonesia. This study aims to investigate the role of proinflammatory biomarkers (NF-κB, TNF-α, IL-6, and CRP) and biomarkers for coagulation activation (TF, prothrombin F1+2, fibrinogen, and D-dimer) in the development of DVT in cancer patients with high risk of thrombosis who never received chemotherapy. All patients were evaluated for their Khorana score. Cancer patients with high risk of thrombosis (Khorana score ≥2) were referred for vascular duplex ultrasound evaluation. This study was approved by Dr. Kariadi Hospital institutional review board and complies with the Declaration of Helsinki.
Patient Selection and Data Collection
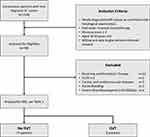
From January 2021 to November 2021, we included a total of 310 consecutive and unselected newly-diagnosed cancer patients for screening. Eighty-three cancer patients with high risk of thrombosis were enrolled in the study. Figure 1 presents the study cohort and patient distribution. The inclusion criteria were as follows: 1) newly diagnosed with cancer as confirmed with histological examination, 2) had never received chemotherapy, 3) high risk of thrombosis (Khorana score ≧2), 4) aged 18–60 years, and 5) is willing and able to give informed consent for participation in the study. Exclusion criteria include patients who 1) underwent surgery in the last 14 days, 2) were pregnant, 3) were receiving antithrombotic therapy, 4) Eastern Cooperative Oncology Group (ECOG) ≥3, 45) congenital disorders affecting coagulation system, (6) creatinine clearance <30 mL/minute, (7) ALT level more than 3 times of upper reference value, (8) total bilirubin level >5 mg/dl, (9) cardiac and cerebrovascular diseases, (10) signs of infection, (11) active bleeding, and (12) severe thrombocytopenia (<20.,00/µL). All patients were screened for DVT using probability test for Wells score and Doppler duplex ultrasound.
Before this study was conducted, all patients had been informed about the study details in an individual interview. The informed consent must be signed by the patient before participating in the study. Then, the patients’ cancer history, tumor site, tumor histology and tumor stage were documented. Patients who met the inclusion criteria were selected as the subjects of this study. Samples were examined to see the plasma levels of NFκB, TNF-α, IL-6, CRP, TF, Prothrombin F1+2, fibrinogen and D-dimer.
Prediction Score
Two prediction scores were used in this study: the Khorana and Wells. For each patient, we calculated the Khorana risk score to stratify the risk of VTE in cancer patients undergoing chemotherapy. Patients were assigned to two risk categories: low risk (score of <2) and high risk (score of ≥2).16 Wells score considers 1 point each for active cancer, paralysis, paresis, recent plaster immobilization of lower limb, recently bed-ridden for >3 days, major surgery in the past 4 weeks, localized tenderness along distribution of deep venous system, entire leg swollen, calf swelling >3 cm compared to asymptomatic leg, pitting edema, and collateral superficial veins. DVT is considered likely in patients with a Wells score of 2 or above and unlikely in patients with a score of less than 2.18
Outcome and Measures: Deep Vein Thrombosis
The endpoint of the study was the occurrence of DVT, either asymptomatic, symptomatic or fatal VTE, confirmed by color duplex ultrasound. Color duplex ultrasound was performed once patients meet inclusion and exclusion criteria and was performed at the Radiology Department of Dr. Kariadi Hospital, Semarang, Indonesia. All patients were assessed for DVT using Logiq 7 pro US imaging system (Logiq 7 pro; GE Healthcare, USA) with the 7–10 Hz linear probe. The diagnosis of DVT was based either on the presence of a non-compressible segment (compression ultrasound test – CUS) or flow impairment on color Doppler imaging. Patients were examined for both proximal (popliteal, femoral, and common femoral vein) and distal (peroneal and tibial veins) DVT. To avoid investigator-related variations of the results, color duplex ultrasound was performed in each patient by the same investigator.
Laboratory Measurements
Complete blood count was measured by an automatic flow cytometry technique using Sysmex XN 1000 automated hematology analyzer (Sysmex Corporation, Japan). Plasma prothrombin time, activated partial thromboplastin time, fibrinogen, and D-dimer levels were measured by the turbidimetry chromogenic immunoassay method using Sysmex CS-2100i automated blood coagulation analyzer (Sysmex Corporation, Japan).
The plasma levels of NF-κB, TNF-α, IL-6, CRP, TF, F1+2 were measured using venous blood specimens collected through sterile and atraumatic antecubital venipuncture. The specimens were collected in 5 mL citrate vacutainer tubes SST, containing 0.5 mL of liquid anticoagulant. Plasma NFκB levels, plasma TNF-α levels, and plasma IL-6 levels were measured using ELISA method (kit catalog numbers E-EL-H1386 96T, E-EL-H0109 96T, and E-EL-H0102 96T, respectively, Elabscience Biotechnology USA). Plasma CRP levels were measured using anti-human CRP mouse monoclonal antibody-coated latex (Sekisui Medical Co., LTD, Japan) following the manufacturer’s instruction. Plasma TF levels and Prothrombin F1+2 levels were measured using ELISA method (kit catalog numbers E-EL-H0040 96T and E-EL-H1793, respectively, Elabscience Biotechnology, USA). Plasma fibrinogen, was measured with coagulation method, and D-dimer levels was measured using the turbidimetry chromogenic immunoassay method with Sysmex CS-2100i automated blood coagulation analyzer (Sysmex Corporation, Japan).
Samples for ELISA were immediately centrifuged at 2500 g for 15 minutes, and plasma samples were aliquoted, coded, and stored at −80°C until the assays were performed. Samples were prepared according to manufacturer’s instructions, and plates were read on an Elx808 plate reader (Biotek, Vermont, USA) at 450 nm wavelength. Results were normalized to total protein using the bicinchoninic acid (BCA) assay (Pierce Rockford, Illinois, USA) and reported as ng/mg total protein. Measurements were done in a blinded fashion. All samples were assayed in duplicate and those showing values above the standard curve were retested with appropriate dilutions.
Statistical Analysis
Statistical analysis was performed to analyze the difference between patients with DVT and without DVT. Multivariate analysis was performed on variables with p<0.1 and parameters which theoretically have close associations with the incidence of DVT. Analysis was performed to investigate the correlation between inflammatory parameters and coagulation activation parameters in all patients who fulfilled the inclusion criteria. Data were then analyzed to investigate the correlation between inflammatory parameters and coagulation parameters in patients with DVT.
Results
Patients’ Characteristics
From January 2021 to November 2021, we included a total of 310 consecutive and unselected newly-diagnosed cancer patients for screening. Eighty-three consecutive cancer patients with high risk of thrombosis were enrolled in the study. From the 83 patients, 8 were diagnosed with DVT. The characteristics of study subjects are shown in Table 1.
 |
Table 1 Clinical Characteristics of Study Participants |
The median age of the subjects was 42 years (range: 19–60 years) and the data distribution was not normal. There was a significant difference between the median age of patients with DVT, (49.5 years, range: 23–60 years), and patients without DVT (42 years, range: 19–60 years), with p=0.046.
There were more male patients compared to female patients (50.6% vs 49.4%). There was no significant difference in terms of gender between patients with DVT and patients without DVT. Most patients were overweight (50.6%). However, there was no significant difference in terms of BMI between patients with DVT and patients without DVT.
In this study, the most common cancer type was colorectal cancer (33.7%). Statistical analysis revealed that there was no significant difference in terms of cancer type between patients with DVT and patients without DVT (p=0.367). This result might be caused by the abnormal distribution of cancer type among two groups. The majority of patients had stage IV cancer (48.1%) and there was no significant difference in terms of cancer stage at the time of diagnosis between patients with DVT and patients without DVT (p=0.270).
Most patients had ECOG score of 0 (63.9%) and there was no significant difference in terms of ECOG between patients with DVT and patients without DVT (p=0.088). The majority of patients had Wells score of 1, identified in 77 subjects (92.8%). Most patients had Khorana score of 2, identified in 54 subjects (65%), and there was no significant difference in terms of Wells score and Khorana score between patients with DVT and patients without DVT (p=0.492 and p=0.582, respectively).
Based on laboratory examination, the median Hb level was 11.3 g/dL (7.3–15.9 g/dL), median WBC count was 12,200/µL (5100–37,000/µL), and the median platelet count was 440,000/µL (219,000–951,000/µL), the data distributions were not normal. Statistical analysis revealed that there was no significant difference in Hb, WBC, and platelet counts between patients with DVT and patients without DVT (p=0.654; p=0.459; and p=0.445, respectively).
In terms of inflammatory parameters, the differences of the median NF-KB, IL-6, CRP and TNF-α levels in patients with DVT were not significant compared to those in patients without DVT (p=0.793; p=0.379; p=0.109; and p=0.781, respectively) and data distributions were not normal. As for the coagulation activation parameters, the median TF and D-dimer in patients with DVT were higher compared to those in patients without DVT (p=0.080 and p=0.005, respectively). However, the F1+2 and fibrinogen levels in patients with DVT were not significantly different than those in patients without DVT (p=0.241 and p=0.167, respectively).
In this study, we performed multivariate analysis on variables with p<0.1 and parameters that theoretically have close associations with the incidence of DVT. From logistic regression multivariate analysis, we found that significant variables associated with the incidence of DVT include age (p=0.020; hazard ratio [HR]=1.593; 95% confidence interval [CI]=1.076–2.359), D-dimer (p=0.028; HR=1.001; 95% CI=1.000–1.001), and F1+2 (p=0.024; HR=0.999; 95% CI=0.998–1.000) (Table 2).
 |
Table 2 Multivariate Analysis of Age, Coagulation Activation Parameters, and Inflammatory Parameters in Correlation with the Incidence of Deep Vein Thrombosis |
This study found that age, D-dimer, and F1+2 had significant association with the incidence of DVT. It can be concluded that the patients’ risk increase as they age, at 1.593 times increment every year. Similarly, every increase of one point of D-dimer level increases the risk of DVT by 1.001 times. Although F1+2 level had significant association with the incidence of DVT, the HR was less than 1.
In cancer patients with high risk of thrombosis, the correlation between inflammatory parameters and coagulation parameters can be seen in Figure 2. There was significant positive correlation between inflammatory parameters and coagulation activation parameters, which were IL-6 and D-dimer (r=0.3), IL-6 and F1+2 (r=0.46), CRP and F1+2 (r=0.24), CRP and D-dimer (r=0.29) as well as TNF-α and F1+2 (r=0.39). On the other hand, there was significant negative correlation between TF and NF-KB (r=−0.41), TF and TNF-α (r=−0.22), as well as TF and CRP (r=−0.25) (Figure 3). However, in DVT patients, there were no significant correlations between inflammatory parameters and coagulation parameters, as shown in Figure 4 All data distributions were abnormal and statistical analysis was performed with Spearman analysis.
 |
Figure 3 Scatterplot of significant correlation between inflammatory parameters and coagulation activation parameters. |
 |
Figure 4 Correlation between inflammatory parameters and coagulation activation parameters in cancer patients with high risk of thrombosis (Khorana score ≥2) who also had DVT. |
From the 83 patients, Doppler ultrasound showed that 8 patients had DVT. Data analysis of the eight patients revealed no significant correlation between inflammatory parameters and coagulation activation parameters in patients with DVT.
Discussion
Univariate and multivariate analyses revealed significant differences between patients with DVT and patients without DVT in terms of age and D-dimer, as seen in Table 1. The incidence of vein thrombosis increases as the age increases. Vein thrombosis is very rare in young individual (<1 event per 10,000 people-years). However, it increases to 1% per year in elderly, which showed that age is one of the most common risk factors for vein thrombosis.19 A study by Khorana et al, showed that increasing age is a risk factor for VTE in cancer patient population. In a retrospective cohort study, cancer patients aged 65 years old are more prone to develop VTE compared to younger patients.20
D-Dimer, a product of fibrin degradation that usually increases in acute thrombosis, is a global indicator for coagulation activation and fibrinolysis. There is a close correlation between the development of cancer and the activation of coagulation system. Several studies have shown that D-dimer was associated with VTE risk in cancer patients. Additionally, in CATS, D-dimer is proven to be an important biomarker for predicting VTE in cancer patients. D-dimer as a predictive parameter has been validated in an independent cohort study of CATS patient.21,22 A study by Setiawan et al reported that a combination of D-dimer (≥5030 μg/L), hs- CRP (≥51.05 mg/L) and Wells score ≥3 can predict DVT incidence in cancer patients.23
The results of this study support the notion that age and D-dimer are important factors in the development of DVT; thus, cancer patients who are of older age with high D-dimer level can be considered at high risk of thrombosis and could be candidates for thromboprophylaxis.
In this study, significant positive correlation between inflammatory parameters and coagulation activation were observed, which were IL-6 and D-dimer, IL-6 and F1+2, CRP and F1+2, CRP and D-dimer, as well as TNF-α and F1+2. In addition, there were significant negative correlation between TF and NF-KB as well as TNF-α and CRP. This might be the result of damages to the blood vessel due to cancer cells invasion, which leads to inflammation in the blood vessels. The direct toxic effects of chemotherapeutic agents toward endothelial cells and insertion of central vein catheter which activates endothelial cells also cause an increase in coagulation activation in cancer patients.24,25 Several evidences showed that immune cells and inflammatory process induce the development of DVT. The effect of immune cells on endothelial cells activity and immune cascade leads to the expression of adhesion receptor on endothelial cells.25 The main event in the initiation of VTE is most likely the inflammation on the venous wall. This had been demonstrated in studies reporting associations between VTE and inflammatory markers, including CRP, IL-6, IL-8, and TNF-α.10
In cancer, there was an exposure towards proinflammatory stimuli in the cancer microenvironment.6 Additionally, administration of chemotherapeutic agents induces endothelial cell damage, which leads to inflammatory responses.7,8 Inflammatory stimuli, such as TNF-α, IL-1a, IL-6, IL-17, IL-18 and epidermal growth factor (EGF), mediate inflammation through interaction with Toll-like receptors (TLRs), IL-1 receptors (IL-1R), IL-6 receptors (IL-6R), and receptors TNF (TNFR).8,9 Activation of these receptors induces intracellular signaling pathway of NF-κB and MAPK to produce proinflammatory cytokines, including IL- 6, TNF α, IL-1, IL-8 and CRP.8
These proinflammatory cytokines play a role in promoting procoagulant status, particularly by inducing the expression of TF,10 which activates the coagulation cascade to form thrombus, characterized by an increase in marker for thrombin formation, namely F1+2, TAT, FpA and D Dimer.11 In addition, NF-κB signaling pathway produces adhesion molecules, such as ICAM-1, ECAM-1, PCAM-1, selectin, and chemokines (MCP-1, IL-18, MP-2, CXCL), leading to the event of thrombosis.12–14
Theoretically, cancer patients with high risk of thrombosis who were diagnosed with DVT would have higher inflammatory parameters, which lead to activation of coagulation and subsequently the development of DVT. However, in this study, further analysis investigating the role of inflammation and coagulation activation in DVT patients showed that there was no significant association between inflammatory parameters and coagulation activation parameters with DVT. This result may be caused by the big difference in the number of patients in both groups. Other inflammation markers that might be more correlated with the coagulation markers should be further evaluated.
VTE prophylaxis anticoagulants, eg, unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), direct oral anticoagulant (DOAC), such as rivaroxaban or apixaban have been recommended for cancer patients with in international guidelines such as from the American Society of Clinical Oncology (ASCO),16 International Initiative on Thrombosis and Cancer (ITAC),17 National Comprehensive Cancer Network (NCCN)26 as well as national guidelines from the Indonesian Society of Thrombosis and Hemostasis.27 Several clinical studies had shown the benefits and safety of VTE prophylaxis for medical patients. Those studies used enoxaparin, dalteparin, and fondaparinux compared to placebo with acute medical patients. Enoxaparin was used in Medical Patients with Enoxaparin (MEDENOX),28 dalteparin was used in Prevention of VTE in Immobilized Patients (PREVENT),29 fondaparinux was used in Arixtra for Thromboembolism Prevention in a Medical Indications Study (ARTeMIS),30 as well as rivaroxaban was compared placebo in cancer patients with high risk of thrombosis in CASSINI study.31 All those studies showed significant decrease of VTE incidence. These results support the evidence-based recommendation for thromboprophylaxis in clinical practice.
Although the guidelines and studies regarding the benefits and safety of VTE prophylaxis exist, the use of thromboprophylaxis by clinicians remains infrequent.32–35 The main reasons for infrequent administration of prophylaxis in patients with high risk of VTE include the costs,32,34,36 concerns for bleeding complication,33–35 lack of knowledge or confidence regarding the thromboprophylaxis guidelines,33 lack of vigilance,34,37 and reluctance to administer injection daily for anticoagulant injection as prophylaxis.33
The most recent understanding on the mechanism of VTE showed the importance of immune system and inflammatory system activities in the pathogenesis of VTE, stating that VTE as a process is related not only to the coagulation process but also to immunity and inflammation, which leads to thrombosis. The aforementioned paradigm presents a new idea for studies regarding new therapy for VTE prophylaxis by inhibiting immune and inflammatory processes, instead of directly inhibiting clotting factors in the coagulation cascade, in order to reduce the risk of bleeding which can occur when administering anticoagulant as VTE prophylaxis.25
The limitation of the study is the uneven distribution of cancer types. This is due to the fact that our main inclusion criteria were the Khorana score (≥2), which contributes to the uneven distribution of cancer types in the study sample. This study also has a big difference in the number of patients in both groups.
Conclusion
In conclusion, in cancer patients with high-risk thrombosis, there were significant positive correlations between inflammatory parameters and coagulation activation, which were IL-6 and D-dimer, IL-6 and F1+2, CRP and F1+2, CRP and D-dimer, as well as TNF-α and F1+2. No significant correlation between inflammatory parameters and coagulation parameters in patients with DVT was observed in the same population. Significant variables for the development of DVT include age and D-dimer. Further studies are needed to deepen the understanding the role of immune and inflammatory systems in the pathogenesis of VTE and finding other inflammatory markers that define better relationship between inflammatory and coagulation in VTE patients.
Acknowledgments
We sincerely thank Mika Lumban Tobing MD, Suyono, MD, from Haematology-Medical Oncology Division, Internal Medicine Department, Faculty of Medicine, Diponegoro University/Dr. Kariadi Hospital, Semarang. We would also like to thank A. Gunawan Santosa, MD, from Radiology Department, Faculty of Medicine, Diponegoro University/Dr. Kariadi Hospital, Semarang, for data support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Streiff MB, Agnelli G, Connors JM, et al. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombolysis. 2016;41(1):32–67. doi:10.1007/s11239-015-1317-0
2. Global Cancers. Facts and figures. 4th ed. American Cancer Society; 2018. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-4th-edition.pdf.
3. Abdol Razak N, Jones G, Bhandari M, Berndt M, Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers. 2018;10(10):380. doi:10.3390/cancers10100380
4. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. doi:10.1111/j.1538-7836.2007.02374.x
5. Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102(S1):S2–S9. doi:10.1038/sj.bjc.6605599
6. Karin M. NF- k B as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141.
7. Boccaccio C, Comoglio PM. Oncogenesis, cancer and hemostasis. In: Cancer-Associated Thrombosis. New York: Informa Healthcare USA, Inc; 2008:1–15.
8. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi:10.18632/oncotarget.23208
9. Park M, Hong J. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5(2):15. doi:10.3390/cells5020015
10. Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6(2):15. doi:10.3389/fped.2018.00142
11. van Gorp ECM, Suharti C, ten Cate H, et al. Review: infectious diseases and coagulation disorders. J Infect Dis. 1999;180(1):176–186. doi:10.1086/314829
12. Saghazadeh A, Hafizi S, Rezaei N. Inflammation in venous thromboembolism: cause or consequence? Int Immunopharmacol. 2015;28(1):655–665. doi:10.1016/j.intimp.2015.07.044
13. Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):17023. doi:10.1038/sigtrans.2017.23
14. Granger DN, Senchenkova E. Inflammation and the Microcirculatin. San Rafael (CA): Morgan & Claypool Life Sciences; 2010.
15. Blom JW. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715. doi:10.1001/jama.293.6.715
16. Key NS, Chb MB, Khorana AA, Kuderer NM, Bohlke K, Ayy L. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Oncol Pract. 2019;15:661–664. doi:10.1200/JOP.19.00368
17. Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566–e581. doi:10.1016/S1470-2045(19)30336-5
18. Geersing GJ, Zuithoff NPA, Kearon C, et al. Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: individual patient data meta-analysis. BMJ. 2014;348(mar10 3):g1340–g1340. doi:10.1136/bmj.g1340
19. Engbers MJ, Van Hylckama Vlieg A, Rosendaal FR. Venous thrombosis in the elderly: incidence, risk factors and risk groups. J Thromb Haemost. 2010;8(10):2105–2112. doi:10.1111/j.1538-7836.2010.03986.x
20. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339–2346. doi:10.1002/cncr.23062
21. Pabinger I, Thaler J, Ay C, Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122(12):2011–2018. doi:10.1182/blood-2013-04-460147
22. Schaefer JK, Jacobs B, Wakefield TW, Sood SL. New biomarkers and imaging approaches for the diagnosis of deep venous thrombosis. Curr Opin Hematol. 2017;24(3):274–281. doi:10.1097/MOH.0000000000000339
23. Setiawan B, Rosalina R, Pangarsa EA, Santosa D, Suharti C. Clinical evaluation for the role of high-sensitivity c-reactive protein in combination with D-Dimer and wells score probability test to predict the incidence of deep vein thrombosis among cancer patients. Int J Gen Med. 2020;13:587–594. doi:10.2147/IJGM.S261718
24. Kirwan CC, McCollum CN, McDowell G, Byrne GJ. Investigation of proposed mechanisms of chemotherapy-induced venous thromboembolism. Clin Appl Thromb. 2015;21(5):420–427. doi:10.1177/1076029615575071
25. Budnik I, Brill A. Immune factors in deep vein thrombosis initiation. Trends Immunol. 2018;39(8):610–623. doi:10.1016/j.it.2018.04.010
26. Khorana AA. The NCCN clinical practice guidelines on venous thromboembolic disease: strategies for improving VTE prophylaxis in hospitalized cancer patients. Oncologist. 2007;12(11):1361–1370. doi:10.1634/theoncologist.12-11-1361
27. Fadjari TH, Suharti C. Penatalaksanaan tromboemboli vena pada kanker. In: Tambunan KL, Suharti C, Sukrisman L, Fadjari T, Setiawan B, editors. Pedoman Nasional Tromboemboli Vena. 2018:53–65.
28. Alikhan R, Cohen AT, Combe S, et al. Prevention of venous thromboembolism in medical patients with enoxaparin. Blood Coagul Fibrinolysis. 2003;14(4):341–346. doi:10.1097/00001721-200306000-00004
29. Leizorovicz A, Cohen AT, Turpie AGG, Olsson C-G, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879. doi:10.1161/01.CIR.0000138928.83266.24
30. Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329. doi:10.1136/bmj.38733.466748.7C
31. Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med. 2019;380(8):720–728. doi:10.1056/NEJMoa1814630
32. Atmakusuma TD, Tambunan KL, Sukrisman L, et al. Underutilization of anticoagulant for venous thromboembolism prophylaxis in three hospitals in Jakarta. Acta Med Indones. 2015;47(2):136–145.
33. Mahé I, Chidiac J, Helfer H, Noble S. Factors influencing adherence to clinical guidelines in the management of cancer-associated thrombosis. J Thromb Haemost. 2016;14(11):2107–2113. doi:10.1111/jth.13483
34. Bradley CT, Brasel KJ, Miller JJ, Pappas SG. Cost-effectiveness of prolonged thromboprophylaxis after cancer surgery. Ann Surg Oncol. 2010;17(1):31–39. doi:10.1245/s10434-009-0671-6
35. Figueroa R, Alfonso A, López-Picazo J, et al. Insights into venous thromboembolism prevention in hospitalized cancer patients: lessons from a prospective study. PLoS One. 2018;13(8):e0200220. doi:10.1371/journal.pone.0200220
36. Hibbert PD, Hannaford NA, Hooper TD, et al. Assessing the appropriateness of prevention and management of venous thromboembolism in Australia: a cross-sectional study: Table 1. BMJ Open. 2016;6(3):e008618. doi:10.1136/bmjopen-2015-008618
37. Bump GM, Dandu M, Kaufman SR, Shojania KG, Flanders SA. How complete is the evidence for thromboembolism prophylaxis in general medicine patients? A meta-analysis of randomized controlled trials. J Hosp Med. 2009;4(5):289–297. doi:10.1002/jhm.450
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.