Back to Journals » International Journal of General Medicine » Volume 14
Correlation Between Promoter Hypomethylation and Increased Expression of Syncytin-1 in Non-Small Cell Lung Cancer
Authors Fu Y, Zhuang X , Xia X, Li X, Xiao K, Liu X
Received 27 November 2020
Accepted for publication 19 February 2021
Published 19 March 2021 Volume 2021:14 Pages 957—965
DOI https://doi.org/10.2147/IJGM.S294392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yang Fu,1 Xuewei Zhuang,2,3 Xiyan Xia,4 Xiaohui Li,3 Ke Xiao,3 Xiaojing Liu3
1Department of Reproductive Medicine Center, Jinan Maternity and Child Care Hospital, Jinan, 250001, People’s Republic of China; 2Department of Clinical Laboratory Medicine, Shandong Provincial Third Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250031, People’s Republic of China; 3Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250012, People’s Republic of China; 4Department of Microbial Immune, Jinan Vocational College of Nursing, Jinan, 250012, People’s Republic of China
Correspondence: Xuewei Zhuang Tel +86-531-81656669
Fax +86-531-86927544
Email [email protected]
Introduction: Syncytin-1 is a human endogenous retroviral (HERVW) envelope protein, which has been implicated in trophoblast and cancer cell fusions as well as in immunomodulatory functions. We investigated syncytin-1 expression and promoter methylation in non-small cell lung cancer (NSCLC) and the adjacent, para-carcinoma tissues. In addition, the correlation to patient survival differentiation of between 5-year survival and death group was analyzed.
Methods: Survival ratio was calculated by Kaplan-Meier survival curve. Death risk assessment was executed by Cox risk regression model. The 5ʹ-LTR methylation level of HERVW promoter was detected by EpiTYPER method.
Results: Syncytin-1 expression in NSCLC tissue was found to be significantly higher than in para-carcinoma tissues. Moreover, the 5-year survival group has a lower syncytin-1 expression than the death group. Clinical stage and the percentage of syncytin-1 positive cells were top risk factors according to Cox ratio risk regression model analysis. While the methylation level of the 5ʹ-LTR in HERVW gene promoter was relatively lower in NSCLC than para-carcinoma tissues, the methylation status of a CpG-2 site overlapping the Oct-1 binding site was found to be an important element potentially involved in the epigenetic regulation of HERVW gene expression.
Conclusion: These findings suggest that syncytin-1 could be a biomarker for the diagnosis/prognosis of NSCLC, and further studies are required to elucidate the exact role of syncytin-1 in the development of NSCLC as well as the underlying molecular mechanism for syncytin-1 function and regulation.
Keywords: non-small cell lung cancer, syncytin-1, epigenetic regulation, prognosis, DNA methylation
Introduction
More than 25% tumor mortality is caused by lung cancer worldwide,1 and 85% of lung cancers belong to non-small cell lung cancer.2 According to an updated report from the American Cancer Society (ACS), it was estimated that in 2020, 1,806,590 new cancer cases and 606,520 cancer deaths were projected to occur in the United States alone.3 Approximately 75% of lung cancers are diagnosed in late stages that are associated with a low 5-year survival rate of 15.6%.4,5 In these late stage cases, treatments including surgery, radiotherapy or chemotherapy are of very limited efficacy, highlighting an urgent need for early diagnosis and treatment.
Syncytin-1 is a functional glycoprotein encoded by the env gene located at chromosome 7q21-22. As a member of the human endogenous reverse transcription virus W gene family,6,7 syncytin-1 is highly expressed in human placenta and several carcinoma tissues. Maliniemi et al. reported syncytin-1 expression in 15 of 30 epidermic malignant lymphoma.8 Sun et al. reported significantly increased expressions of syncytin-1 in archaeocytes, lymphocytes, granulocytes and monocytes during acute myelocytic leukemia.9 Larsson et al. reported that 38% of 165 premenopausal node-negative breast cancers are positive for syncytin-1 expression, and this positivity correlates with the poor outcomes of patients.10 Strissel et al. disclosed that syncytin-1 is over-expressed in late-stage and poorly differentiated endometrial carcinoma rather than the early-stage and highly differentiated one.11 The expression of syncytin-1 in non-small cell lung cancer tissues remained poorly understood.
It is well recognized that genetic changes such as site mutation, deletion, translocation, and/or amplification as well as epigenetic changes including alterations in histone modifications, patterns of DNA methylation and/or microRNA can all contribute to the carcinogenesis of lung cancers.1,12 Multiple genetic mutations including translocation of ALK gene, mutation of EGFR gene and rearrangement of ROS-1 gene have been identified and the oncogenic mechanisms well characterized.13 Epigenetic changes, through their impact on gene transcription, may lead to activation of oncogene and silence of tumor suppressor genes.14 HERVW gene is not only overexpressed in various types of tumors, recent studies indicated that the gene products may be involved in modulation of cell cycle and cell apoptosis in placental trophoblasts.15 Its expression and epigenetic regulation in lung cancers, however, remains uninvestigated.
In this study, syncytin-1 expression in carcinoma and para-carcinoma tissues of NSCLC is detected by immunohistochemistry. The 5ʹ-LTR methylation of HERVW promoter is determined by EpiTYPER method. Correlation of syncytin-1 expression and carcinogenesis of NSCLC is analyzed. These studies may provide novel information regarding its involvement in the carcinogenic mechanism of NSCLC. Such knowledge could be useful for the improvement of diagnosis, prognosis and management of NSCLC cases.
Materials and Methods
Patients and Tissue Samples
Thirty patients clinically diagnosed as NSCLC from Jan 1999 to Oct 2001 in Qilu Hospital, Shandong University, were included in this study, of which 23 were male and 7 female, ages ranging from 37–75 (57.87±1.72 in average), 14 glandular and 16 squamous, 12 TNM stage I, 6 stage II and 12 stage III, 15 with lymphatic metastasis and 15 without. None of the patients received radiotherapy or chemotherapy before surgery. The inclusive criterion was: Survived for more than 1 month after surgery and did not die of any cause other than lung cancer during the follow-up time of 1–78 month (34 on average). Tissue samples were collected from pulmonary primary lesions with pathological characteristics identified under microscope. This study was authorized and administrated by the Ethics Committee of Qilu Hospital, Shandong University. Para-carcinoma refers to the adjacent regions 1–2 cm from the edge of carcinoma.
Immunohistochemistry
Carcinoma and para-carcinoma tissues removed in surgery were fixed with 10% neutral formalin for 12–24 h and embedded in paraffin. Tissues were sectioned into slides in thickness of 6 μm, dewaxed in xylene for 5 min twice, and dehydrated with 100%, 95%, 85%, 75% alcohol for 60 seach. Tissue sections were boiled in EDTA repairing fluid for 2.5 min and rinsed with PBS three times for antigen retrieval after cooling. Endogenous peroxidase was blocked with 100 μL 3% hydrogen peroxide by incubation at 37°C for 10 min before being rinsed with PBS three times. Antibody solution is obtained by 1:100 dilution from the rabbit anti-human syncytin-1 monoclonal antibody (GeneTex, USA). The tissue slides were exposed to 50 μL of antibody solution at room temperature for 2 h. After extensive rinsing with PBS, the tissue slides were stained with the secondary antibody (Zhongshan Jinqiao, Beijing) by incubation at 37°C for 30 min. Color development was performed with DAB solution (Zhongshan Jinqiao, Beijing) for 3–5 min. After counter staining with hematoxylin, the slides were dehydrated, cleared, and mounted for microscopic observation.
Evaluation of Staining
Syncytin-1 protein in cytoplasm and cell membrane was positively stained in brownish color and the nuclei was in blue color. The percentage of positive cells was calculated, and semi-quantitative analysis was performed by categorization of the staining density into four classes: negative (0 positivity), weakly positive (lower than 25% positivity), moderately positive (25–50% positivity) and strongly positive (more than 50% positivity).
EpiTYPER Methylation Detection
The paraffin-fixed tissues were sliced into 10 μm thickness, and 5–10 tissue slides were used for each DNA extraction. PCR primer sequences were designed by the EpiDesigner software: 5ʹ-TTTTTTTTGGGATGAGGGTAAA and 3ʹ-TTAAAACAAACCCAAACACTTAACC. PCR amplification was executed at 94°C for 4 min as initial denaturalization, followed by 45 cycles of denaturalization at 94°C for 20 s, annealing at 56°C for 30 s, extension at 72°C for 1 min, with hydrosulfite DNA as templates. The PCR products were visualized by 1.5% agarose gel electrophoresis under 160 V for 20 min as follows (Figure 1). PCR products were digested with SAP at 37°C for 20 min, 85°C for 5 min, and dotted into MassArray sampler for mass spectroscopy detection. The results were analyzed with the EpiTYPER software.
 |
Figure 1 Detection for the PCR products of tissue DNA by 1.5% agarose gel electrophoresis. The marker: DL2000 bands are 2000 bp, 1000 bp, 750 bp, 500 bp, 250 bp, and 100 bp from top to bottom. |
Statistical Analysis
The syncytin-1 staining positivity of NSCLC carcinoma and para-carcinoma were compared with Student’s t-test using the SPSS 17.0 statistical software, and P<0.05 was considered statistically significant. Survival curve was constructed with the Kaplan-Meier method and intro-curve comparison was performed with the Log rank test. Prognosis was evaluated by Cox ratio risk recurrence model. The 5ʹ-LTR methylation of HERVW promoter was analyzed by mass spectroscopy and EpiTYPER software. The average methylation ratio and standard deviation was calculated with the SPSS 17.0 statistical software, and compared with non-parametric rank sum test and Mann–Whitney U-test. P<0.05 was considered statistically significant.
Results
Comparison of Syncytin-1 Staining Positivity Between NSCLC Carcinoma and Para-Carcinoma Tissues
Syncytin-1 protein staining positivity was 96.7% (29/30), with 65.5% (19/29) strongly positive (Figure 2A) and 34.5% (10/29) moderately positive (Figure 2B) in carcinoma tissues, and 33.3% lowly expressed in para-carcinoma tissues (Figure 2C). Syncytin-1 positive cell rate in carcinoma tissues (35.41%±0.22%) was significantly higher than in para-carcinoma tissues (2.17%±0.03%) (P<0.01) (Figure 3). High expression is defined as higher than 25% positive cell rate.
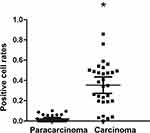 |
Figure 3 Syncytin-1 positive cell rate in NSCLC tissues and para-carcinoma tissues. The syncytin-1 positive cell rate in NSCLC tissues was higher than that in adjacent tissues. *Represents P<0.05. |
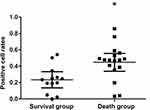
Comparison of Syncytin-1 Expression Between 5-Year Survival and 5-Year Death Group
Syncytin-1 expression positivity was 44.67% ± 0.21% in the 5-year death group and 23.30% ± 0.16% in the 5-year survival group. The former was significantly higher than the latter (P<0.01) (Figure 4).
Survival Analysis
Five-year mortality of syncytin-1 high-expression group was 73.7% and the low-expression group was 27.3%, with a significant difference between the two groups (P<0.05). The average survival period of the low-expression group was 64 months while the high-expression group was only 28 months, and the difference was statistically significant (P<0.01) (Figure 5) according to the Kaplan-Meier survival curve analysis. Clinical stage and positive cell percentage were found to be lethal factors of NSCLC based on the Cox ratio risk recurrence model (Table 1).
 |
Table 1 Multivariate Cox Regression Analysis Results of Non-Small Cell Lung Cancer Patients |
Comparison of Total Methylation Ratio and CpG2 Site Methylation Ratio
The total methylation ratios of NSCLC tissues and para-carcinoma tissues were 77.50%±10.19% and 78.87% ± 11.54%, respectively (Figure 6), with no significant difference (P>0.05). The CpG2 site methylation ratios of NSCLC tissues and para-carcinoma tissues were 81.91% ± 12.38% and 94.33% ± 4.93%, respectively, showing a significant difference between the two groups (P<0.05) (Figure 7, Table 2) according to the non-parametric rank sum test and Mann–Whitney U-test of two independent samples.
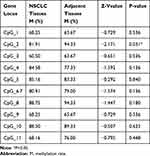 |
Table 2 Comparison of Methylation Rates of CpG Sites in Non-Small Cell Lung Cancer Tissues and Adjacent Tissues |
Discussion
Lung cancer was particularly prevalent during the twentieth century due to the popularity of tobacco consumption, especially in developed countries where lung cancer ranks at the top of the morbidity and mortality list. The carcinogenesis of lung cancer is a complicated process influenced by environmental, genetic, epigenetic, physical, chemical, immunological, and infectious factors, e.g., the activation of oncogenes and inactivation of suppressor genes. An earlier diagnosis and therapy would lead to a more optimistic prognosis. The 5-year survival ratio of local lung cancer or bronchogenic carcinoma is 54.8%, while this ratio drops sharply to 27.4% and even 4.2%, respectively, for regional and widespread lung cancers.13 Hence, a novel lung cancer biomarker could be essential for developing tools for early diagnosis. Our results suggest that the increased syncytin-1 expression and/or the alteration of DNA methylation in the 5ʹ-LTR of HERVW promoter could be potential biomarkers for the diagnosis of lung cancers.
Larsen et al.16 performed a retrospective analysis on 140 colon and rectum cancer cases. It was found that syncytin-1 was commonly expressed in both carcinoma of colon and rectum, and the rectum carcinomas showed high syncytin-1 expression levels as well as low 5-year survival ratios. On the other hand, Larsson et al.10 reported that syncytin-1 expression could be an independent positive indicator for breast cancer prognosis since patients with higher syncytin-1 expression in their tumors had a higher non-palindromic survival ratio. In this study, higher expression of syncytin-1 in NSCLC tissue than in para-carcinoma tissue as shown by immunohistochemistry is consistent with previous studies in other carcinoma tissues. However, the syncytin-1 expression level was higher in the 5-year death group than the survival group, and the K-M survival curve also demonstrated that the survival ratio in the low-expression group was 27.3% (3/11) with an average survival time of 64 months, while the ratio in the high-expression group was 73.7% (14/19) with an average survival time of only 27 months. This finding suggested that for NSCLC, a high syncytin-1 expression level is associated with a worse prognosis. Among the factors considered, including age, gender, syncytin-1 positive cell percentage, clinical stage and TNM stage, and clinical stage, syncytin-1 positive cell percentage was found to be the most important factor of NSCLC mortality according to the Cox ratio risk recurrent model. Hence, syncytin-1 could be a novel biomarker for NSCLC diagnosis and prognosis.
Syncytin-1 mediates the fusion of chorionic trophoblasts (CT) to form syntrophoblasts (ST), and the reduction in syncytin-1 expression plays an essential role in the pathogenesis of intrauterine growth restriction (IUGR), preeclampsia (PE) and HELLP syndrome.17 Besides the function in cell fusion, decreased syncytin-1 expression led to cell apoptosis as observed in preeclampsia.18,19 Syncytin-1 was also reported to promote cell proliferation through activation of TGF-β1 and TGF-β3, to mediate immunosuppression during adaptation to pregnancy.20,21 Over 75% of bladder urothelial carcinoma has up-regulated syncytin-1 expression, which contributes to the proliferation of urothelial cells.22 Moreover, in the neuroblastoma cell line, manipulation of syncytin-1 led to abnormal regulation of the cAMP signaling pathway, and toxic excitation of neurons and subsequent neural system diseases.23 Thus, non-fusogenic activities of syncytin-1 may be involved in the regulation of a variety of functions in tumor cells. More studies are required to verify if NSCLC could be one of such tumors.
The high expression of syncytin-1 appears to be associated with the low-level methylation of 5ʹ-LTR promoter. Matouskova et al. argue that syncytin-1 expression in trophocytes is triggered by demethylation of the promoter region resulting in proliferation and differentiation of syntrophoblast cells.24 Zhuang et al. reported syncytin-1 down-regulation and increased 5ʹ-LTR methylation in the preeclamptic placenta.25 Zhou et al. reported that syncytin-1 over-expression correlated with a decreased promoter methylation level in endometrial cancers.26 Benesova et al. compared spermocytoma and nonseminoma and found a low-level methylation of HERVW promoter in the former and high-level methylation in the latter.27 In endometrial cancers, hypomethylated 5ʹ-LTR was associated with up-regulated expression of syncytin-1.11
At least two components, that is, U3 region of 5ʹ-LTR promoter and its upstream TSE function to regulate the transcription of HERVW gene owing to the CAAT box and octamer protein binding site of U3 region and 5ʹ-LTR upstream GCMa binding site, which interacts with GCMa transcription factor to increase syncytin-1 expression level in BeWo and JEG3 choriocarcinoma cell lines.21,28,29 In this study, 10 CpG sites of 5ʹ-LTR promoter of HERVW gene in NSCLC and para-carcinoma tissues have been investigated (Figure 8). The result demonstrates that HERVW promoter in NSCLC tissues has a relatively lower level of methylation than in para-carcinoma tissues, especially in the CpG2 site, suggesting a site-specific methylation of HERVW promoter in NSCLC tissue. Our findings are generally consistent with the above observations, all point to an epigenetic regulation of syncytin-1 expression in tumor cells.
Further analysis of the CpG2 site located 95 nt downstream from the transcriptional start site found it overlaps with an Oct-1 transcription factor binding site. Oct-1 is a DNA-binding transcriptional factor regulating a large number of target genes including housekeeping genes and specific genes involved in immune, endocrine, and neural functions.30 High levels of Oct-1 expression have been found in somatic stem cells and cancer stem cells, and gastric, prostatic, cervical, and esophageal cancers.31 HERVW promoter demethylation may result in an increased binding by transcriptional factors such as Oct-1, to induce the expression of syncytin-1 protein. Thus, the CpG2 site could be a key epigenetic component mediating epigenetic and/or non-epigenetic regulation mechanisms of syncytin-1 expression under different sorts of pathological conditions.
The study had several limitations. Firstly, only one methylation site was found to show significant differences, which led to insufficient evidence on the relationship between methylation and protein expression, which may be related to the small number of samples or stale samples. Therefore, in the subsequent study, we will collect fresh tissue samples to carry out a multi-center joint study.
In this study, an over-expression of syncytin-1 was found in NSCLC tissues compared with adjacent tissues. The overexpression of syncytin-1 was associated with a worse prognosis of patient survival. A low-methylation level in the 5ʹ-LTR promoter region of the HERVW gene, especially a specific CpG site overlapping with an oct-1 binding site was identified as a key element for the regulation of syncytin-1 expression. Although the precise molecular mechanism remains elusive, the observations suggest that syncytin-1 could be a novel biomarker for lung cancer diagnosis/prognosis, and further studies along this line may be useful for improving the management of lung cancers.
Abbreviations
NSCLC, non-small cell lung cancer; MF, muscarinic fungoides; CT, chorionic trophoblast; ST, syntrophoblast; IUGR, intrauterine growth restriction; PE, preeclampsia.
Ethical Approval
Ethical approval for this project was obtained from the Ethics Committee of Qilu Hospital, Shandong University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Acknowledgments
This study was supported by grants from Shandong Provincial Nature Science Foundation (No. ZR2015CM030), Shandong Key Research and Development Program (No. 2016GSF201169).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Balgkouranidou I, Liloglou T, Lianidou ES. Lung cancer epigenetics: emerging biomarkers. Biomark Med. 2013;7(1):49–58. doi:10.2217/bmm.12.111
2. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. doi:10.1056/NEJMra0802714
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
4. Molina R, Filella X, Auge JM, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 2003;24(4):209–218. doi:10.1159/000074432
5. Nanavaty P, Alvarez MS, Alberts WM. Lung cancer screening: advantages, controversies, and applications. Cancer Control. 2014;21(1):9–14. doi:10.1177/107327481402100102
6. Blond JL, Besème F, Duret L, et al. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J Virol. 1999;73(2):1175–1185. doi:10.1128/JVI.73.2.1175-1185.1999
7. Voisset C, Bouton O, Bedin F, et al. Chromosomal distribution and coding capacity of the human endogenous retrovirus HERV-W family. Aids Res Hum Retroviruses. 2000;16(8):731–740. doi:10.1089/088922200308738
8. Maliniemi P, Vincendeau M, Mayer J, et al. Expression of human endogenous retrovirus-w including syncytin-1 in cutaneous T-cell lymphoma. PLoS One. 2013;8(10):e76281. doi:10.1371/journal.pone.0076281
9. Sun Y, Zhu H, Song J, et al. Upregulation of leukocytic syncytin-1 in acute myeloid leukemia patients. Med Sci Monit. 2016;22:2392–2403. doi:10.12659/MSM.899303
10. Larsson LI, Holck S, Christensen IJ. Prognostic role of syncytin expression in breast cancer. Hum Pathol. 2007;38(5):726–731. doi:10.1016/j.humpath.2006.10.018
11. Strissel PL, Ruebner M, Thiel F, et al. Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: emergence of new molecular targets. Oncotarget. 2012;3(10):1204. doi:10.18632/oncotarget.679
12. Brzeziańska E, Dutkowska A, Antczak A. The significance of epigenetic alterations in lung carcinogenesis. Mol Biol Rep. 2013;40(1):309–325. doi:10.1007/s11033-012-2063-4
13. Ansari J, Shackelford RE, Elosta H. Epigenetics in non-small cell lung cancer: from basics to therapeutics. Transl Lung Cancer Res. 2016;5(2):155. doi:10.21037/tlcr.2016.02.02
14. Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi:10.1016/j.cell.2007.01.029
15. Bolze PA, Mommert M, Mallet F. Contribution of syncytins and other endogenous retroviral envelopes to human placenta pathologies. Prog Mol Biol Transl Sci. 2017;145:111–162.
16. Larsen JM, Christensen IJ, Nielsen HJ, et al. Syncytin immunoreactivity in colorectal cancer: potential prognostic impact. Cancer Lett. 2009;280(1):44–49. doi:10.1016/j.canlet.2009.02.008
17. Ruebner M, Strissel PL, Ekici AB, et al. Reduced syncytin-1 expression levels in placental syndromes correlates with epigenetic hypermethylation of the ERVW-1 promoter region. PLoS One. 2013;8(2):e56145. doi:10.1371/journal.pone.0056145
18. Huang Q, Chen H, Wang F, et al. Reduced syncytin-1 expression in choriocarcinoma BeWo cells activates the calpain1-AIF-mediated apoptosis, implication for preeclampsia. Cell Mol Life Sci. 2014;71(16):3151–3164. doi:10.1007/s00018-013-1533-8
19. Langbein M, Strick R, Strissel PL, et al. Impaired cytotrophoblast cell-cell fusion is associated with reduced syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev. 2008;75(1):175–183. doi:10.1002/mrd.20729
20. Lu Q, Li J, Senkowski C, et al. Promoter hypermethylation and decreased expression of syncytin-1 in pancreatic adenocarcinomas. PLoS One. 2015;10(7):e0134412. doi:10.1371/journal.pone.0134412
21. Yu C, Shen K, Lin M, et al. GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem. 2002;277(51):50062–50068. doi:10.1074/jbc.M209316200
22. Yu H, Liu T, Zhao Z, et al. Mutations in 3|[prime]|-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene. 2013;33(30):3947–3958. doi:10.1038/onc.2013.366
23. Li S, Liu ZC, Yin SJ, et al. Human endogenous retrovirus W family envelope gene activates the small conductance Ca2+-activated K+ channel in human neuroblastoma cells through CREB. Neuroscience. 2013;247(13):164–174. doi:10.1016/j.neuroscience.2013.05.033
24. Matousková M, Blazková J, Pajer P, Pavlícek A, Hejnar J. CpG methylation suppresses transcriptional activity of human syncytin-1 in non-placental tissues. Exp Cell Res. 2006;312(7):1011–1020.
25. Zhuang XW, Li J, Brost BC, et al. Decreased expression and altered methylation of syncytin-1 gene in human placentas associated with preeclampsia. Curr Pharm Des. 2014;20(11):1796–1802. doi:10.2174/13816128113199990541
26. Zhou H, Li J, Podratz KC, et al. Hypomethylation and activation of syncytin-1 gene in endometriotic tissue. Curr Pharm Des. 2014;20(11):1786. doi:10.2174/13816128113199990540
27. Benešová M, Trejbalová K, Kovářová D, et al. DNA hypomethylation and aberrant expression of the human endogenous retrovirus ERVWE1/syncytin-1 in seminomas. Retrovirology. 2017;14(1):20. doi:10.1186/s12977-017-0342-9
28. Cheng YH, Richardson BD, Hubert MA, Handwerger S. Isolation and characterization of the human syncytin gene promoter. Biol Reprod. 2004;70(3):694–701. doi:10.1095/biolreprod.103.023473
29. Gimenez J, Montgiraud C, Oriol G, et al. Comparative methylation of ERVWE1/syncytin-1 and other human endogenous retrovirus LTRs in placenta tissues. DNA Res. 2009;16(4):195–211. doi:10.1093/dnares/dsp011
30. Pankratova EV, Stepchenko AG, Krylova ID, Portseva TN, Georgieva SG. The regulatory interplay between Oct- 1isoforms contributes to hematopoiesis and the isoforms imbalance correlates with a malignant transformation of B cells. Oncotarget. 2018;9(52):29892–29905. doi:10.18632/oncotarget.25648
31. Pankratova EV, Stepchenko AG, Portseva T, Mogila VA, Georgieva SG. Different N-terminal isoforms of Oct- 1control expression of distinct sets of genes and their high levels in namalwa burkitt’s lymphoma cells affect a wide range of cellular processes. Nucleic Acids Res. 2016;44(19):9218–9230.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.