Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Correlation analysis of metabolic syndrome and its components with thyroid nodules
Authors Liu J , Wang C, Tang X, Fu S, Jing G, Ma L, Sun W, Li Y, Wu D, Niu Y, Niu Q, Guo H, Song P
Received 11 June 2019
Accepted for publication 12 August 2019
Published 30 August 2019 Volume 2019:12 Pages 1617—1623
DOI https://doi.org/10.2147/DMSO.S219019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Jingfang Liu, Chenge Wang, Xulei Tang, Songbo Fu, Gaojing Jing, Lihua Ma, Weiming Sun, Yujuan Li, Dan Wu, Ying Niu, Qianglong Niu, Huiping Guo, Pei Song
Department of Endocrinology, The First Hospital of Lanzhou University, Lanzhou, Gansu 730000, People’s Republic of China
Correspondence: Jingfang Liu; Xulei Tang
Department of Endocrinology, The First Hospital of Lanzhou University, 1 Donggang West Road
, Lanzhou, Gansu 730000, People’s Republic of China
Tel +86 0 931 835 6470
Email [email protected]
[email protected]
Objective: This study aimed to analyze the relationship between the metabolic syndrome (MetS) and its components with the occurrence of thyroid nodules.
Methods: A total of 2719 volunteers from some areas of Gansu Province, China, who participated in the national survey of thyroid diseases and iodine nutrition status (Tide) and diabetes prevalence, were selected. Their height, weight, waist circumference (WC), systolic blood pressure (SBP), and diastolic blood pressure were recorded. The fasting plasma glucose (FPG), 2-h plasma postprandial glucose (2hPG), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and glycosylated hemoglobin (HbA1C) levels were measured. The prevalence of MetS and thyroid nodules was evaluated, and the correlation between each component of MetS and thyroid nodules was studied.
Results: The prevalence of MetS and thyroid nodules was 15.4% and 17.2%, respectively. WC, SBP, body mass index, FPG, 2hPG, TG, TC, and thyroid-stimulating hormone levels were significantly higher in the thyroid nodule group. The prevalence of thyroid nodules was significantly higher in the MetS group. A positive correlation was found between the degree of metabolic disorder and the occurrence of thyroid nodules. WC was found to be a risk factor for the occurrence of thyroid nodules. For WC≥90 cm, an increase in the independent variables led to a significant rise in the incidence of thyroid nodules.
Conclusion: The prevalence of thyroid nodules was higher in the MetS group. The WC of the MetS components might be an independent risk factor for the occurrence of thyroid nodules.
Keywords: metabolic syndrome, risk factors, thyroid nodules, waist circumference
Introduction
A thyroid nodule is a common thyroid disease. In the last 30 years, the incidence of thyroid nodules has shown an increasing trend, which may be attributed to the popularity of thyroid ultrasound. An epidemiological survey showed that the detectable rate of thyroid nodules via palpation was 3–7%, while it was as high as 20–67% using high-resolution ultrasound.1 Metabolic syndrome (MetS) refers to the pathological states of metabolic disorders caused by proteins, fats, carbohydrates, and other substances in the human body. It is characterized by central obesity, hypertension, abnormal glucose metabolism, and high triglyceride and low- and high-density lipoprotein levels. The economic development and change in human diet and lifestyle have led to an increasing incidence of MetS globally.2,3 In China, 12.7% men and 14.2% women were diagnosed with MetS.4 The MetS was closely related to cardiovascular diseases, type 2 diabetes, and the occurrence of tumors.5,6 Similarly, previous studies showed that patients with diabetes had a higher incidence of thyroid nodules compared with healthy people.7 Moreover, the body mass index (BMI) was associated with thyroid volume and the occurrence of thyroid nodules.8–10 However, whether MetS was related to the occurrence of thyroid nodules was not clear. In the present study, the incidence of MetS and thyroid nodules in some areas of Gansu province, China, was analyzed to explore the correlation between MetS and its components with thyroid nodules.
Participants and methods
Participants
A total of 2719 volunteers from some areas of Gansu province, China, who participated in the national survey of thyroid diseases and iodine nutrition status (Tide) and diabetes prevalence, were included in this study.
All the participants were ethnic Han Chinese, aged more than 18 years and with more than 5 years of living history in the community (or village) of the survey areas. These participants were not accepted for iodine-containing contrast agents or ethamidone for nearly 3 months.Participants with a history of thyroid diseases or pregnant women were excluded from this survey. Written and oral information of the protocol was announced before the screening, and informed consent was obtained from each eligible participant.
Methods
The basic information and history of diseases (including thyroid diseases, diabetes, hypertension, and so on) of each participant were collected. The height, weight, waist circumference (WC), systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate of each participant were measured. After fasting for 8 h at night, the venous blood samples were collected in the morning. The fasting plasma glucose (FPG), 2 hr postprandial plasma glucose (2hPG), total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using the Bs-220 Automatic Biochemical Analyzer (Mairui Biotechnology Co. Ltd, China). The glycosylated hemoglobin (HbA1C) levels were detected using the Bio-Rad Glycosylated Hemoglobin Analyzer (Bio-Rad, USA).
Thyroid ultrasonography was performed by professional ultrasound physicians. The diameter of each part of the thyroid was measured routinely using LOGIQ100 PRO (GE, Germany) and 7.5-MHz probes.
The diagnostic criteria of MetS were proposed by the Chinese Diabetes Society in 2004.11 The eligible participants experienced at least three of the following conditions: (1) High blood pressure (SBP ≥140 and/or DBP ≥90 mm Hg) and/or hypertension diagnosed and treated; (2) TG >150 mg/dL (1.7 mmol/L); (3) HDL-C <35 mg/dL (0.90 mmol/L) in male participants and <39 mg/dL (1.0 mmol/L) in female participants; (4) overweight and/or obese (BMI ≥25.0 kg/m2 or WC >85 cm in male participants and >80 cm in female participants); (5) FPG ≥6.1 mmol/L (110 mg/dL) and/or 2hPG ≥7.8 mmol/L (140 mg/dL) and/or diabetes diagnosed and treated for elevated glucose levels.
Statistical analysis
All data were analyzed using SPSS 21.0 software and expressed as 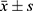 The two groups were compared using the independent-sample Student's t test. The chi-square test was used for comparing the enumeration data between the two groups. The relationship between each component of MetS and the occurrence of thyroid nodules was evaluated using the single-factor binary logistics regression analysis, and the independent risk factors of the occurrence of thyroid nodules were evaluated using multiple-factor binary logistics regression analysis. A P-value <0.05 was considered as statistically significant.
The two groups were compared using the independent-sample Student's t test. The chi-square test was used for comparing the enumeration data between the two groups. The relationship between each component of MetS and the occurrence of thyroid nodules was evaluated using the single-factor binary logistics regression analysis, and the independent risk factors of the occurrence of thyroid nodules were evaluated using multiple-factor binary logistics regression analysis. A P-value <0.05 was considered as statistically significant.
Results
Prevalence of MetS and thyroid nodules
A total of 2719 participants (1423 male and 1296 female) were included in the present study. Of these, 418 participants (15.4%) were diagnosed with MetS, including 280 male and 138 female. The prevalence of MetS was higher in male participants than in female participants (10.3% vs.5.1%, χ2=42.50; P<0.0001). All the parameters of the MetS and non-MetS groups are shown in Table 1.
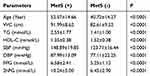 |
Table 1 Comparison between the MetS (+) group and MetS (-) group |
The thyroid nodules were detected in 469 participants (17.2%), and the prevalence of thyroid nodules was significantly higher in female participants than in male participants (9.5% vs 7.7%, χ2=12.261; P<0.0001).
Comparison of metabolic parameters between thyroid nodule and non-thyroid nodule groups
The levels of WC, SBP, BMI, FPG, 2hPG, TG, TC, and thyroid-stimulating hormone (TSH) were significantly higher in the thyroid nodule group than in the non-thyroid nodule group (all P<0.05). However, no significant difference was found in DBP, heart rate, HDL-C, LDL-C, and HbA1C levels between the two groups (Table 2).
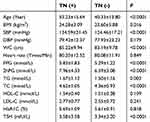 |
Table 2 Comparation of related parameters between TN (+) group and TN (-) group |
Relationship between thyroid nodules and MetS
A total of 116 participants with thyroid nodules in the MetS group and 353 participants with thyroid nodules in the non-MetS group were found. The prevalence of thyroid nodules in the MetS group was significantly higher in the non-MetS group than in the non-MetS group (27.8% vs 15.3, χ2=38.17; P<0.0001).
A single-factor logistic regression analysis showed thatthe occurrence of thyroid nodules was positively correlated with MetS [OR =2.120; P<0.0001; 95% CI (1.664–2.701)].
Relationship between thyroid nodules and degree of metabolic disorder
All participants were grouped according to the degree of metabolic disorder: participants with one component of MetS as group 1, participants with two components of MetS as group 2, participants with three components of MetS as group 3, participants with four components of MetS as group 4, and participants without any component of MetS as group 5 (control group). The logistic regression analysis showed that the higher the degree of metabolic disorder, the more likely the occurrence of thyroid nodules (Table 3).
 |
Table 3 Correlation between the degrees of metabolic disorder and thyroid nodules |
After adjusting for gender and age, no difference in the risks of thyroid nodules was found between group 1 and group 5. However, for patients with two or more components of MetS, the more the number of ingredients of MetS, the greater the risks of thyroid nodules, although the significance decreased (Table 3).
Relationship between each component of MetS and thyroid nodules
The correlation between each component of MetS and thyroid nodules was analyzed by single-factor logistic regression. The results indicated that WC, SBP, FPG, 2hPG, and TG levels were risk factors for the occurrence of thyroid nodules (Table 4). Moreover, the TSH levels also significantly correlated with the occurrence of thyroid nodules (OR =1.048; P=0.001).
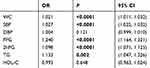 |
Table 4 Single factor regression analysis of each component of MetS and thyroid nodules |
The multivariate logistic regression analysis showed that the TSH, SBP, DBP, and 2hPG levels were the risk factors for the occurrence of thyroid nodules. After adjusting for TC, HDL-C, HbA1C, TSH, SBP, DBP, and 2hPG levels, the TSH, SBP, DBP, and 2hPG levels still correlated with the occurrence of thyroid nodules. Moreover, the WC, HDL-C, and LDL-C levels were also risk factors for the occurrence of thyroid nodules (Table 5). After further adjusting for gender and age, WC was found to be an independent risk factor for thyroid nodules. No correlation was observed between the TSH, SBP, DBP, FPG, 2hPG, TG, TC, HDL-C, LDL-C, and HbA1C levels and the occurrence of thyroid nodules (Table 5).
 |
Table 5 Multiple factors regression analysis of the components of MetS and thyroid nodules |
Thyroid nodules and WC levels
All the participants were grouped according to the WC levels, and those with WC <80 cm were taken as the control group. The single-factor logistic regression analysis showed no difference in the incidence of thyroid nodules between the group with WC <80 cm and that with WC =80–89 cm. However, for groups with WC ≥90 cm or higher, an increase in the independent variables led to a significant rise in the incidence of thyroid nodules. Although the significance reduced after adjusting for gender and age, the correlation persisted (Table 6).
 |
Table 6 Correlation between the group of the WC and thyroid nodules |
WC and TSH levels
The participants were divided into two groups based on their WC levels: WC ≥90 cm and WC <90 cm. The TSH levels between the two groups were compared. The results showed that the TSH levels were significantly higher in the WC ≥90 cm group than in the WC <90 cm group (3.23±2.88vs. 3.45±3.41, P<0.05).
Discussion
MetS is a complex disease involving multiple systems and various metabolic disorders. The core of MetS is insulin resistance, while obesity, especially central obesity, is closely related to the occurrence of insulin resistance. This study showed that SBP, plasma glucose, blood lipid, and WC levels all increased in patients with thyroid nodules, and the prevalence rates of thyroid nodules increased in patients with MetS. Shin et al reported that the incidence of thyroid nodules was significantly higher in patients with MetS than in those without MetS, which was consistent with the results of the present study.12 In patients with MetS, the hyperinsulinemia, hyperglycemia, and hyperlipidemia might stimulate thyroid cell hyperplasia.12 The risk of thyroid nodules increased with the increase in MetS components, and the correlation persisted after adjusting for gender and age. Moreover, patients with two or more MetS components were more likely to develop thyroid nodules than those without or with one MetS component. Patients with poor MetS control, especially those with poor glucose metabolism control, were more likely to develop thyroid nodules.
This study showed that the WC in MetS components was an independent risk factor for the occurrence of thyroid nodules. WC was a reliable indicator to reflect central obesity and also one of the components of MetS. Compared with BMI, WC better reflected the distribution of fat, predicted the amount of visceral fat, and had a better correlation with insulin sensitivity. Considering participants with WC <80 cm as the control group, the present study showed that the incidence of thyroid nodules increased significantly with the increase in WC for participants with WC ≥90 cm. Panagiotou et al reported that not only central obesity was related to the occurrence and number of thyroid nodules but also the hip circumference positively correlated with the maximum diameter of nodules.13
The relationship between WC and the occurrence of thyroid nodules might be attributed to the following aspects.
Previous studies showed that WC positively correlated with insulin resistance and was a predictive factor for insulin resistance.14 Insulin resistance was related to the distribution, structure, and density of thyroid blood vessels, which might promote the growth of nodules. Insulin resistance may directly activate the proliferation pathway through insulin or insulin-like growth factor 1 and regulate the expression of thyroid genes and the proliferation and differentiation of thyroid cells.15 Thyroid cells may synthesize insulin-like growth factor 1 and express insulin-like growth factor 1 receptor. The expression level of insulin-like growth factor 1 was higher in thyroid cells with nodules than in those without nodules. In patients with insulin resistance, the volumes of thyroid nodules decreased obviously after metformin treatment for some time,16 further proving the relationship between insulin resistance and thyroid nodules.
Bastemir et al found that the TSH levels were significantly higher in obese people than in normal people. They also pointed out that the TSH level positively correlated with weight, BMI, and WC levels.17 In the present study, the TSH level was higher in patients with WC ≥90 cm than in those with WC <90 cm. Moreover, a study on the correlation between thyroid function and metabolic parameters indicated that the TSH level was independently related to WC, and was independent of insulin sensitivity and hemodynamic parameters.18 In terms of TSH secretion, obese patients had a lower sensitivity to FT4 than normal-weight patients, and they improperly secreted more TSH.18 Therefore, the TSH level gradually increased with the increase in WC. TSH might promote the proliferation of thyroid follicular epithelium cells. The higher TSH levels not only promoted the hyperplasia of normal thyroid tissues but also increased the occurrence of the nodules.
Compared with the individuals with a thin body, fat tissues of obese people expressed high levels of pro-inflammatory proteins, including tumor necrosis factor-alpha, interleukin-6, and so on.19 A study in Mexico showed that the leptin level positively correlated with WC in women with WC >85 cm and men with WC >90 cm. Leptin, as a product of obesity gene, plasma leptin, and its mRNA levels, reflected the body fat in the adipose tissues. It might be important in regulating weight and controlling the size of the adipose tissues.20 The present study also found that WC was an independent risk factor for the occurrence of thyroid nodules. However, whether the mechanism was related to the increase in plasma leptin levels needs further investigation. Central obese people produced more leptin than those with a normal body. Leptin is secreted by fat cells and is one of the important neuroendocrine regulatory factors that may promote the secretion of TSH through the hypothalamus–pituitary–thyroid–fat tissue axis. Long-term higher TSH levels can cause significant hyperplasia of thyroid glands and increase the incidence of thyroid nodules.
Leptin may promote tumor transformation, proliferation of cancer cells, and tumor angiogenesis.21 The increased leptin levels correlated with the occurrence of prostate cancer, colon cancer, breast cancer, and endometrial cancer. Although more than 90% of the thyroid nodules found by ultrasound were benign nodules, some nodules were malignant. Kitahara et al reported a study including 125,347 men and 72,363 women, during more than 1 year of follow-up. Of these, 106 men and 105 women were diagnosed with primary thyroid cancer. The WC (>102 cm for men and >88 cm for women) and body mass (BMI >30 kg/m2) were found to be the risk factors for thyroid cancer.22 Hyperglycemia and BMI were reported to be correlated with thyroid cancer.21,22 Patients with central obesity had insulin resistance, and the body secreted more insulin as a compensatory basis. Chronic hyperinsulinemia reduced the production of insulin-like growth factor binding proteins I and II, which had an inhibitory effect on insulin-like growth factor 1. The inhibitory effect on insulin-like growth factor 1 weakened, leading to increased circulating insulin-like growth factor 1 levels and cancer cells overexpressing insulin-like growth factor 1 and its receptors.23 Higher circulating insulin-like growth factor 1 level was found to be associated with an increased risk of breast, prostate, lung, and colorectal cancers.24 Chronic hyperinsulinemia might induce mutations in cells. Moreover, insulin-like growth factor 1 might, in turn, stimulate cell proliferation and help in the development and metastasis of tumors. Finally, hyperglycemia might also lead to dysfunction of the protein kinase C cell signaling system and accelerate tumor growth and metastasis.25
Ethics query
This study was approved by the First Hospital of Lanzhou University ethics committee, in accordance with the Declaration of Helsinki, and the participants provided written informed consent.
Data availability statement
The data that support the findings of this study are available from the corresponding author.
Acknowledgment
This work was financially supported by the Public Welfare Research Projects of China under Grant (201,402,005), and the Special Fund for Clinical Medical Research of Chinese Medical Association under Grant (15,010,010,589).
Disclosure
No potential conflicts of interest were reported by the authors in this work.
References
1. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010;16:1–43. doi:10.4158/10024.GL
2. Iqbal AZ, Basharat S, Basharat A, Basharat S. Prevalence of the metabolic syndrome and its component abnormalities among school age Pakistani children.J. Ayub Med Coll Abbottabad. 2014;26:194–199.
3. Rubio-Ruiz ME, El Hafidi M, Pérez-Torres I, Baños G, Guarner V. Medicinal agents and metabolic syndrome. Curr Med Chem. 2013;20:2626–2640.
4. Wu GX, Wu ZS, Liu J, et al. A study on the incidence of cardiovascular disease on the metabolic syndrome in 11 provinces in China. Chin J Epidemiol. 2003;24:551–553.
5. Gorter PM, Olijhoek JK, van der Graaf Y, Algra A, Rabelink TJ, Visseren FL. Prevalence of the metabolic syndrome in patients with coronary heart disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm. Atherosclerosis. 2004;173:363–369. doi:10.1016/j.atherosclerosis.2003.12.033
6. Agnoli C, Grioni S, Sieri S, et al. Metabolic syndrome and breast cancer risk: a case-cohort study nested in a multicentreitalian cohort. PLoS One. 2015;10:e0128891. doi:10.1371/journal.pone.0128891
7. Guo H, Sun M, He W, et al. The prevalence of thyroid nodules and its relationship with metabolic parameters in a Chinese community-based population aged over 40 years. Endocrine. 2014;45:230–235. doi:10.1007/s12020-013-9968-0
8. Turcios S, Lence-Anta JJ, Santana JL, et al. Thyroid volume and its relation to anthropometric measures in a healthy cuban population. Eur Thyroid J. 2015;4:55–61. doi:10.1159/000371346
9. Xu W, Chen Z, Li N, et al. Relationship of anthropometric measurements to thyroid nodules in a Chinese population. BMJ Open. 2015;5:e008452. doi:10.1136/bmjopen-2015-008452
10. Chen H, Zhang H, Tang W, et al. Thyroid function and morphology in overweight and obese children and adolescents in a Chinese population.J. Pediatr Endocrinol Metab. 2013;26:489–496.
11. Research group on metabolic syndrome of diabetic society of China. Recommendations on metabolic syndrome from diabetic society of China. Chin J Diabetes. 2004;12:156–161.
12. Shin J, Kim MH, Yoon KH, Kang MI, Cha BY, Lim DJ. Relationship between metabolic syndrome and thyroid nodules in healthy Koreans. KoreanJ Intern Med. 2016;31:98–105. doi:10.3904/kjim.2016.31.1.98
13. Panagiotou G, Komninou D, Anagnostis P, et al. Association between lifestyle and anthropometric parameters and thyroid nodule features. Endocrine. 2017;56:560–567. doi:10.1007/s12020-017-1285-6
14. Stepien M, Rosniak-Bak K, Paradowski M, et al. Waist circumference, ghrelin and selected adipose tissue-derived adipokines as predictors of insulin resistance in obese patients: preliminary results. Med Sci Monit. 2011;17:PR13–R18. doi:10.12659/msm.882030
15. Song B, Zuo Z, Tan J, et al. Association of thyroid nodules with adiposity: a community-based cross-sectional study in China. BMC Endocr Disord. 2018;18:3. doi:10.1186/s12902-018-0232-8
16. Rezzónico J, Rezzónico M, Pusiol E, Pitoia F, Niepomniszcze H. Metformin treatment for small benign thyroid nodules in patients with insulin resistance. Metab Syndr Relat Disord. 2011;9:69–75. doi:10.1089/met.2010.0026
17. Bastemir M, Akin F, Alkis E et al. Obesity is associated with increased serum TSH level, independent of thyroid function. Swiss Med Wkly. 2007;137:431–434.
18. De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin Endocrinol (Oxf). 2007;67:265–269. doi:10.1111/j.1365-2265.2007.02874.x
19. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi:10.1079/bjn20041213
20. García-Jiménez S, Bernal Fernández G, Martínez Salazar MF, et al. Serum leptin is associated with metabolic syndrome in obese Mexican subjects. J Clin Lab Anal. 2015;29:5–9. doi:10.1002/jcla.21718
21. Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi:10.1002/jcp.20472
22. Kitahara CM, Platz EA, Park Y, Hollenbeck AR, Schatzkin A, Berrington de González A. Body fat distribution, weight change during adulthood, and thyroid cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2012;130:1411–1419. doi:10.1002/ijc.26161
23. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi:10.1038/nrc1408
24. Micucci C, Valli D, Matacchione G, Catalano A. Current perspectives between metabolic syndrome and cancer. Oncotarget. 2016;7:38959–38972. doi:10.18632/oncotarget.8341
25. Zhan YS, Feng L, Tang SH, et al. Glucose metabolism disorders in cancer patients in a Chinese population. Med Oncol. 2010;27:177–184. doi:10.1007/s12032-009-9189-9
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
