Back to Journals » Journal of Asthma and Allergy » Volume 14
CoronaVac COVID-19 Vaccine-Induced Anaphylaxis: Clinical Characteristics and Revaccination Outcomes
Authors Laisuan W, Wongsa C , Chiewchalermsri C , Thongngarm T , Rerkpattanapipat T, Iamrahong P, Ruangwattanachok C, Nanthapisal S , Sompornrattanaphan M
Received 7 August 2021
Accepted for publication 16 September 2021
Published 7 October 2021 Volume 2021:14 Pages 1209—1215
DOI https://doi.org/10.2147/JAA.S333098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Wannada Laisuan,1 Chamard Wongsa,2 Chirawat Chiewchalermsri,3 Torpong Thongngarm,2 Ticha Rerkpattanapipat,1 Pansa Iamrahong,4 Chulapha Ruangwattanachok,4 Sira Nanthapisal,5 Mongkhon Sompornrattanaphan2
1Division of Allergy, Immunology and Rheumatology, Department of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Division of Allergy and Clinical Immunology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 3Department of Medicine, Panyananthaphikkhu Chonprathan Medical Center, Srinakharinwirot University, Nonthaburi, Thailand; 4Clinical Pharmacy Section, Pharmacy Division, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 5Department of Pediatrics, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand
Correspondence: Mongkhon Sompornrattanaphan
Division of Allergy and Clinical Immunology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Tel +66 2419 8263
Fax +66 2419 8263
Email [email protected]
Abstract: Anaphylaxis to CoronaVac, an inactivated vaccine against COVID-19, is extremely rare. We report 12 cases of anaphylaxis after CoronaVac administration, focusing on clinical characteristics and management outcomes. Skin test and graded vaccine challenge were successfully performed in our cases and might be considered if an inactivated vaccine is the only remaining option.
Keywords: anaphylaxis, COVID-19 vaccine, drug allergy, excipient, vaccine allergy, COVID-19, skin testing
Introduction
Anaphylaxis associated with vaccination against Coronavirus Disease-2019 (COVID-19) is a rare and potentially life-threatening serious adverse event following immunization (AEFI) with onset usually minutes to hours after vaccination.1 CoronaVac (Sinovac Life Sciences, Beijing, China) is an inactivated vaccine against COVID-19, produced by Vero cells inoculated with the CZ02 strain of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has a two-dose priming schedule given 2–4 weeks apart, and no booster schedule has been recommended to date.2 It was approved for use in adults 18–59 years of age by the Thai Federal Drug Agency for emergency use on February 22, 2021.3 Since then until June 27, 2021, immediate reactions clinically compatible with anaphylaxis associated with its use have occurred at a rate of 2.2 per million doses of CoronaVac.4 We report a case series of anaphylaxis after vaccination with CoronaVac to help healthcare workers diagnose and manage this serious AEFI.
Materials and Methods
We collected data from three tertiary hospitals in the greater Bangkok Metropolitan Region of Thailand including Siriraj Hospital, Ramathibodi Hospital, and the Panyananthaphikkhu Chonprathan Medical Center between March 20, 2021 and July 20, 2021. Twelve cases were identified by retrospective chart review that were classified as anaphylaxis according to the Brighton Collaboration case definition criteria.5 The protocol for this study was approved by the institutional review boards of the faculties of medicine for Siriraj Hospital (approval no. 597/2564, IRB2), and Ramathibodi Hospital (approval no. 2021/477), Mahidol University. The inclusion criteria were anaphylaxis occurring within four hours following CoronaVac administration. The exclusion criteria were patients with a history of non-immediate type hypersensitivity (eg, maculopapular exanthem, vasculitis, serum sickness-like reaction, and petechiae), a history incompatible with vaccine hypersensitivity, eg, numbness, fever, myalgia, headache, fatigue, and localized injection site reaction.
Results
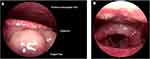
Table 1 summarizes the clinical characteristics of 12 cases, and Table 2 summarizes the result of allergy skin test and outcomes of vaccine re-administration. No cases had had previous allergic reactions or anaphylaxis to any other vaccines. One case met the criteria for Brighton Collaboration level 1 of diagnostic certainty (highest confidence) while 10 and one met levels 2 and 3, respectively. Ten cases were treated at the vaccination units while 2 cases were hospitalized for treatment. No cases were admitted to intensive care units. Six cases received epinephrine administration. Treatment outcomes were discharged to home (n=12), and there were no cases of anaphylaxis-related mortality. Of the 12 cases of anaphylaxis, nine events occurred after the first dose of CoronaVac, two events occurred after the second dose, and one after both the first and second dose. The median interval from vaccination to symptom onset was 30 min (range, 6–180 min). One-third of the patients had onset within 15 minutes and two-thirds within 30 minutes. The most common signs and symptoms were urticaria (50%, 6/12) and angioedema (50%, 6/12). Patient 11 had lip angioedema, voice change, difficulty swallowing, resulting in an otolaryngologist consultation (Figure 1). Forty-two percent of the patients had a documented history of drug hypersensitivity or intolerance. Two-thirds of the patients had at least one atopic disease including allergic rhinitis (58%, 7/12), shellfish allergy (16%, 2/12), and asthma (8%, 1/12). One case (8%, 1/12) had had a previous anaphylactic episode. No cases had had previous allergic reactions or anaphylaxis to any other vaccines. Anaphylaxis severity gradings were 8% grade 1, 75% grade 2, and 17% grade 3. Fifty percent (6/12) of the patients had available paired serum samples for tryptase measurement. By using a consensus tryptase threshold, a peak serum tryptase value exceeding 1.2 multiplied by the baseline serum tryptase plus 2,6 all available paired samples were interpreted as negative.
 |
Table 1 Clinical Characteristics of 12 Cases with Anaphylaxis After CoronaVac Vaccination |
 |
Table 2 Results of Allergy Skin Tests and Outcomes of Vaccine Re-Administration |
Cases were invited to undergo allergy tests within 4–8 weeks. Ten patients agreed to undergo and were performed skin testing. A total of two patients had positive skin test results, of which one had a positive skin prick test (undiluted vaccine), and the other had positive results for both skin prick test and intradermal test (diluted 1:100 with normal saline), yielding a total proportion of positive skin tests of 20% (2/10). Regarding vaccine re-administration, four cases decided to switch to the ChAdOx1 nCoV-19 vaccine (AstraZeneca) while five agreed to perform graded challenges. A two-step graded challenge was performed in skin test-negative patients while a five-step graded challenge was performed in skin test-positive patients. Three (3/5) had an adverse vaccine reaction on revaccination with CoronaVac with signs and symptoms including nausea, urticaria at the face, palpitations, and generalized pruritus. Patient 1 had symptoms compatible with an immediate hypersensitivity reaction during the graded challenge. Eliciting doses ranged from 0.35 to 0.5 mL. Most reactions were successfully managed with antihistamine and antiemetic. All five patients completed the graded challenge testing.
Discussion
This is the first report of clinical characteristics and management outcomes of 12 cases with CoronaVac vaccine-induced anaphylaxis. Although no serious adverse event related to CoronaVac vaccination was identified up to February 3, 2021, from a Phase I/II study,7 allergic reaction was reported (0.08% in the vaccine group) in a CoronaVac Phase III study in Turkey. A systemic allergic reaction was reported in only one patient, which occurred more than 24 hours after the first dose and resolved uneventfully in the following 24 hours.8 From a real-world study in Chile, 2.3 events per 100,000 doses were reported as serious AEFI with 69 patients reported as anaphylaxis.9 However, no data on allergologic workup and the outcome of revaccination have been reported to date.
We diagnosed anaphylaxis based on clinical criteria and measured paired serum tryptase to confirm the diagnosis after a reaction while the demonstration of positive skin tests confirmed the trigger and is considered proof of IgE sensitization.10 Many mechanisms of immediate vaccine hypersensitivity have been proposed, including IgE-mediated mast cell activation, non-IgE-mediated mast cell activation, complement activation, and direct mast cell activation (eg, MRGPRX2).11–13
All patients in our study had negative tryptase results. The majority of patients (83%, 10/12) had negative skin tests while the minority of patients (17%, 2/12) had positive skin tests, suggesting IgE-mediated mechanism. This heterogeneity reflects the various mechanisms related to immediate hypersensitivity in our case series, which is similar to previous reports.11,12 Some reactions might relate to IgE/FcER1-dependent mast cell activation (ie, allergic reactions). In contrast, the majority of reactions might not relate to IgE/FcER1 (ie, non-allergic reactions).11,12 All patients with negative skin tests had no tryptase elevation while two patients with positive skin tests had no available sera for tryptase measurement. Interestingly, patient 1 had a positive skin test, suggesting an allergic mechanism. The patient reacted to the first vaccine exposure. It is possible that the previous sensitization occurred from an environment exposure or cross-reactivity to previously sensitized substances.
Excipients are generally considered as the major contributor to specific IgE-mediated and immediate reactions associated with vaccines.14 In contrast, the vaccine antigen and non-human protein residual are regarded as less probable causes of the allergic reaction.11 The excipients contained in CoronaVac include aluminum hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, and sodium hydroxide.3 Aluminum has been proposed as the possible allergen with the most potential to stimulate a type I hypersensitivity reaction. A previous study demonstrated immediate contact urticaria from patch testing using aluminum material.15 In addition, aluminum can stimulate Th2 responses, causing IgE production. There is evidence of an increase in total and specific-IgE to tetanus toxoid with the use of the aluminum hydroxide-absorbed tetanus toxoid vaccine.16 However, no previous studies have demonstrated aluminum-specific IgE from vaccine-allergic patients. Another proposed mechanism of aluminum hypersensitivity is the activation of the complement system, which is supported by an in vitro study.13 Complement fragments, such as C3a and C5a, can activate mast cells, leading to degranulation.
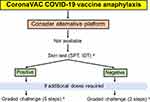
The administration of the second dose with a different COVID-19 vaccine platform and different vaccine components is considered safe in patients who have anaphylaxis to the first dose of a COVID-19 vaccine. However, many cases of successful revaccination of the second dose with the same platform after experiencing an immediate hypersensitivity reaction to the first dose have been reported.17–21 We provide the recommendation for individuals with anaphylactic reactions to the CoronaVac COVID19 vaccine, summarized in Figure 2.
Mass vaccination with a vaccine against COVID-19 is the intervention of choice to reduce morbidity and mortality and control the COVID-19 pandemic, and the benefits of all vaccines against COVID-19 outweigh the risks of the vaccines. Mass vaccination with CoronaVac (Sinovac Life Sciences, Beijing, China) has conditional or emergency use approval in 32 countries and territories as of May 24, 2021, and was approved for use by the World Health Organization in May 2021.2,22
Conclusion
Although anaphylaxis associated with CoronaVac is extremely rare, the preparedness for vaccination centers to recognize and treat anaphylaxis early is important. Collaboration with an allergist for diagnostic workup is essential to plan for future vaccination with patients. Skin testing with the vaccine and an appropriate control is recommended to determine whether the vaccine is responsible for the reaction.23,24 Graded vaccine challenges could be considered if an inactivated vaccine is the only remaining option or in a setting where the culprit vaccine is the only remaining choice and no alternative vaccine platforms are available. This consideration should be based on the risk-benefit analysis after discussion with the patients. Another factor to be considered is the presence of a variant of concern at that time because differential immune responses might be observed between vaccine platforms.
Ethical Approval
This study was approved by the institutional review boards and ethics committees of the faculties of medicine for Siriraj Hospital and Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (approval no. 2021/477, 597/2564). All patients provided informed consent to participate and for the data to be published. This study was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
All patients provided informed consent to participate and for the data to be published. The patients were informed that de-identified data would be used in scientific research and publications.
Acknowledgments
We would like to thank Dr. Anthony Tan for editing the English language. We also thank Dr. Jarungjit Kraiwattanapong for the endoscopic examination photographs.
Author Contributions
All authors have made substantial contributions to the conception and design of the research, the acquisition, analysis or interpretation of the data, and the drafting or revising of the manuscript. All authors have agreed to submit the manuscript to the current journal, have given their final approval of the version to be published, and have agreed to be accountable for all aspects of the work.
Disclosure
All authors declare no personal or professional conflicts of interest in this work.
References
1. McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141(2):463–472. doi:10.1016/j.jaci.2017.12.971
2. World Health Organization. Interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac; 24 May 2021. Available from: https://apps.who.int/iris/bitstream/handle/10665/341454/WHO-2019-nCoV-vaccines-SAGE-recommendation-Sinovac-CoronaVac-2021.1-eng.pdf.
3. Thai Food and Drug Administration (Thai FDA). Available from: https://www.fda.moph.go.th/sites/drug/Shared%20Documents/Vaccine/U1DR1C1072640000311C-SPC-EN.pdf.
4. Data from the Ministry of Public Health. Thailand. Available from: https://ddc.moph.go.th/uploads/ckeditor2//files/Daily%20report%202021-05-30.pdf.
5. Ruggeberg JU, Gold MS, Bayas JM, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–5684. doi:10.1016/j.vaccine.2007.02.064
6. Valent P, Bonadonna P, Hartmann K, et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe systemic mast cell activation and mast cell activation syndrome. Int Arch Allergy Immunol. 2019;180(1):44–51. doi:10.1159/000501079
7. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, Phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi:10.1016/S1473-3099(20)30831-8
8. Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, Phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi:10.1016/S0140-6736(21)01429-X
9. Jara A, Undurraga EA, Gonzalez C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi:10.1056/NEJMoa2107715
10. Muraro A, Worm M, Alviani C, et al. EAACI guideline: anaphylaxis (2021 update). Allergy. 2021. doi:10.1111/all.15032
11. Kounis NG, Koniari I, de Gregorio C, et al. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9(3):221.
12. Risma KA, Edwards KM, Hummell DS, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147(6):2075–82 e2. doi:10.1016/j.jaci.2021.04.002
13. Guven E, Duus K, Laursen I, Hojrup P, Houen G. Aluminum hydroxide adjuvant differentially activates the three complement pathways with major involvement of the alternative pathway. PLoS One. 2013;8(9):e74445. doi:10.1371/journal.pone.0074445
14. Stone CA
15. Kutlu A, Ucar R, Aydin E, Arslan S, Caliskaner AZ. Could aluminum be a new hidden allergen in type 1 hypersensitivity reactions when used as a drug additive? Postepy Dermatol Alergol. 2016;33(3):243–245. doi:10.5114/ada.2016.60620
16. Cogne M, Ballet JJ, Schmitt C, Bizzini B. Total and IgE antibody levels following booster immunization with aluminum absorbed and nonabsorbed tetanus toxoid in humans. Ann Allergy. 1985;54(2):148–151.
17. Krantz MS, Kwah JH, Stone CA
18. Krantz MS, Bruusgaard-Mouritsen MA, Koo G, Phillips EJ, Stone CA
19. Mustafa SS, Ramsey A, Staicu ML. Administration of a second dose of the moderna COVID-19 vaccine after an immediate hypersensitivity reaction with the first dose: two case reports. Ann Intern Med. 2021;174(8):1177–1178. doi:10.7326/L21-0104
20. Wolfson AR, Robinson LB, Li L, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9):3308–3320.e3. doi:10.1016/j.jaip.2021.06.010
21. Kessel A, Bamberger E, Nachshon L, Rosman Y, Confino-Cohen R, Elizur A. Safe administration of the Pfizer-BioNtTech COVID-19 vaccine following an immediate reaction to the first dose. Allergy. 2021. doi:10.1111/all.15038
22. World Health Organization. Background document on the inactivated vaccine Sinovac-CoronaVac against COVID-19. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-background-2021.1.
23. Dreskin SC, Halsey NA, Kelso JM, et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9(1):32. doi:10.1186/s40413-016-0120-5
24. Wood RA, Setse R, Halsey N; Clinical Immunization Safety Assessment Network Hypersensitivity Working G. Irritant skin test reactions to common vaccines. J Allergy Clin Immunol. 2007;120(2):478–481. doi:10.1016/j.jaci.2007.04.035
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.