Back to Journals » Medical Devices: Evidence and Research » Volume 13
Coronary Sinus Reducing Stent for the Treatment of Refractory Angina Pectoris: A Health Technology Assessment
Authors Stanak M, Rothschedl E, Szymanski P
Received 14 May 2020
Accepted for publication 23 July 2020
Published 16 September 2020 Volume 2020:13 Pages 259—276
DOI https://doi.org/10.2147/MDER.S255440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Michal Stanak,1 Eleen Rothschedl,1 Piotr Szymanski2
1Austrian Institute for Health Technology Assessment (AIHTA), Vienna, Austria; 2MSWiA Central Clinical Hospital, Centre of Postgraduate Medical Education, Warsaw, Poland
Correspondence: Michal Stanak
Austrian Institute for Health Technology Assessment, Garnisongasse 7/20, Vienna 1090, Austria
Email [email protected]
Aim: To summarize the evidence on the clinical effectiveness and safety of coronary sinus reducing stent (CSRS) therapy in refractory angina pectoris (AP) patients.
Methods: We performed a systematic literature search in common databases (n=4). The evidence obtained was summarized according to GRADE methodology. A health technology assessment (HTA) was conducted using the HTA Core Model® for Rapid Relative Effectiveness Assessment. Primary outcomes for the clinical effectiveness domain were the proportion of patients with improvement in two or more Canadian Cardiovascular Society (CCS) angina score classes, overall mean reduction of CCS class, and Seattle Angina Questionnaire (SAQ) quality of life (QoL) score improvement. Outcomes for the safety domain were adverse device effects (ADEs) and serious adverse device effects (SADEs).
Results: One randomized controlled trial (RCT) was identified. Outcomes that showed statistically significant differences between CSRS and sham treatment (in favor of CSRS) were CCS angina score improvement of one or two classes, overall mean reduction of CCS class, and SAQ QoL score improvement. Concerning safety, the sham-controlled trial data indicate that there were fewer SADEs in the intervention group (19%) than in the control group (46%). SADEs reported in observation studies ranged from none to 30%. The most frequently reported SADEs were death and stable angina. In the RCT, the only case of death occurred in the control group. Concerning clinical effectiveness, the risk of bias (RoB) was rated to be low, and concerning safety, the RoB was rated to range from low to moderate. As assessed by GRADE, the overall strength of evidence for effectiveness and safety was moderate. Internal and external validity of the evidence base were low.
Conclusion: Even though the current evidence indicates that the assessed technology, CSRS, is potentially more effective than sham intervention for refractory AP patients, the lack of internal validity of the studies undermines the partially positive results.
Keywords: refractory angina pectoris, coronary artery disease, coronary sinus reducing stent, coronary sinus
Introduction
Cardiovascular disease at large is a major cause of health loss across all regions of the world.1 The Global Burden of Disease project 2015 estimated that 442.7 million prevalent cases of cardiovascular disease were present worldwide, which caused an estimated 17.92 million deaths.1 Ischemic heart disease was one of the leading causes of all health loss globally.1 Exact estimates of the incidence and prevalence of refractory angina pectoris (AP) are not available, but a guessed estimate by the 2002 European Society of Cardiology (ESC) Joint Study Group suggests that refractory AP occurs in 5–10% of all AP patients.2 Refractory AP is conventionally defined as a chronic condition (≥3 months in duration) characterized by angina in the setting of ischemic heart disease, which cannot be controlled by a combination of optimal medical therapy, angioplasty, or bypass surgery, and where reversible myocardial ischemia has been clinically established to be the cause of the symptoms.2 The estimated incidence of newly diagnosed patients with refractory angina in the USA ranges between 50,000 and 100 000 per year, while in Europe, the incidence is estimated to range between 30,000 and 50,000 new cases per year.3
The coronary sinus reducing stent (CSRS) aims to treat refractory AP patients and is suggested to be put in place once all the other therapeutic options are exhausted. Except for palliative management, the only alternative option for refractory AP that is based on controlled evidence is external counterpulsation.4,5
According to the ESC 2019 guideline, CSRS received the recommendation 2b, which means that the usefulness of CSRS is less well established by evidence/opinion, but that it may be considered for use in clinical practice.5 There is also limited information on the effectiveness and safety of the CSRS, which would be published in the form of health technology assessment (HTA) reports.6,7 For our assessment, we used the European Network for Health Technology Assessment (EUnetHTA) Core Model® for rapid Relative Effectiveness Assessment (REA), which is used for assessing the clinical effectiveness and safety of pharmaceuticals, diagnostic technologies, medical and surgical interventions, and screening technologies. We used this model to evaluate the clinical effectiveness and safety of CSRS therapy in refractory AP patients.
Methods
Systematic Literature Search
We conducted a systematic literature search on 10–13 December 2019 in four databases (Medline via Ovid, Embase, The Cochrane Library, and CRD [DARE, NHS-EED, HTA]) without a limit to years of publication, but limited to German and English. We searched for published clinical studies on CSRS in refractory AP patients. The search strategies can be provided upon request. In order to identify ongoing and unpublished studies, we conducted a search in three clinical trials registries (ClinicalTrials.gov, WHO-ICTRP, and EU Clinical Trials) on 29–30 January 2020, resulting in 13 potentially relevant hits.
Study Selection and Internal Validity Assessment
The inclusion criteria for the literature selection were defined using the Population–Intervention–Comparison–Outcome–(Study design) model (PICOs) shown in Table 1. No limit was set on the minimum number of study participants, but individual case reports were excluded. Two researchers selected references for inclusion and systematically extracted relevant studies into data-extraction tables. Internal validity was assessed using the risk of bias (RoB) tool for RCTs of the Cochrane Collaboration,8 as well as by the checklist for single-arm studies of the Institute of Health Economics (IHE).9 No cases of disagreement occurred.
 |
Table 1 PICOs Inclusion Criteria |
Outcome Measures
Outcomes were selected in accordance with EUnetHTA guidelines for rapid REAs, which state that clinical endpoints relevant for patients should be selected whenever possible (mortality, morbidity, health-related quality of life, and treatment satisfaction). Primary outcomes for clinical effectiveness were: Canadian Cardiovascular Society (CCS) angina score improvement of one or two classes, overall mean reduction of CCS class, and Seattle Angina Questionnaire (SAQ) quality of life (QoL) score improvement. For the assessment of safety, adverse device effects (ADEs) and serious adverse device effects (SADEs) were included.
Synthesis of Evidence
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology was used for summarizing and evaluating the strength of the evidence.11 Categories of high, moderate, low, and very low were applied and only critical outcomes were assessed. No meta-analysis was performed as only one prospective controlled trial was identified.
Methodological Framework and Reporting
An adaptation of the EUnetHTA Core Model for REAs was used for this HTA. The generic questions from Core Model (version 4.2) were translated into actual research questions. This analysis was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.12
Results
Search Results
Through a systematic search, we found 349 relevant citations. An additional 14 were found by a hand search, which resulted in overall 363 hits. The specific search strategy employed can be provided by the authors upon request. Concerning clinical effectiveness, one randomized controlled trial (RCT)13 met the inclusion criteria. Concerning safety, seven studies met the inclusion criteria: one RCT used also in the clinical effectiveness assessment13 and six prospective observational non-comparative studies.14–19 No retrospective study was included in the assessment. All the extracted data can be found in Tables 2, 3, and 4.
 |  |  |
Table 2 CSRS: Results from RCTs |
 |  |  |
Table 3 CSRS: Results from Observational Studies (Part 1) |
 | 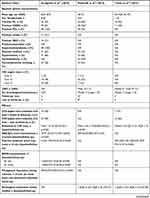 |  |
Table 4 CSRS: Results from Observational Studies (Part 2) |
 |
Table 5 Risk of Bias – Study Level (RCT)5 |
Study and Patient Characteristics
Study Characteristics
Concerning clinical effectiveness, one controlled trial was found (an RCT) that compared CSRS with a sham procedure (study name COSIRA, NCT01205893) and was sponsored by the manufacturer Neovasc Inc.13 It was conducted between April 2010 and April 2013 in 11 centers (in Belgium, Canada, Denmark, the Netherlands, Sweden, and the UK). The RCT included 104 patients (52 were in the intervention group [IG] and 52 in the control group [CG]) and the patient population was followed for 6 months. No patient was lost to follow-up.
Concerning safety, seven studies met the inclusion criteria. One RCT, described above,13 four prospective case series,14–16,19 and two prospective registries.17,18 The total number of patients analyzed in safety analysis who received the CSRS therapy was 348 (plus 52 patients in CG). The observational studies were conducted between October 2004 and April 2017 in Germany, India, Israel, Italy, and Belgium. The follow-up ranged from 414 to 24 months.15 No observational study stated the source of funding.
Patient Characteristics
In the RCT included in the assessment of clinical effectiveness analysis, patients were evenly distributed between the IG and CG. All patients belonged to CCS angina class III or IV, despite attempted optimal pharmacological therapy for 30 days prior to screening, and all had evidence of reversible ischemia with limited options for revascularization. Mean left ventricular ejection fraction (LVEF) ranged between 53.5% and 54.8%. Most of the patients had experienced the following conditions or received the following interventions: previous coronary artery bypass grafting (CABG), previous percutaneous coronary intervention (PCI), previous myocardial infarction (MI), current/previous smoking, diabetes mellitus, hypertension, hypercholesterolemia, and intake of one or more antianginal medications.13 Exclusion criteria were highly specific and are presented in detail in Tables 2, 3, and 4.
In the trials included in the assessment of safety, all patients belonged to CCS angina classes II–IV and had an LVEF of more than 25/30%. Inclusion criteria in all prospective observational studies were homogeneous with respect to the definition of severe refractory AP despite pharmacological therapy, ineligibility for CABG and/or PCI, and objective myocardial ischemia. Exclusion criteria were more heterogeneous, as three observational studies excluded patients with MI and CABG/PCI in less than 3 (to 7) months and patients with the presence of life-threatening arrhythmias, decompensated heart failure, and severe valvular heart disease.16,17,19 While tricuspid valve replacement/repair was an exclusion criterion in two studies,16,19 presence of a pacemaker lead was an exclusion criterion in four studies,15,16,18,19 acute coronary syndrome in less than 3 months was an exclusion criterion in three studies,14,15,18 and right atrial pressure of more than or equal to 15 mmHg was a criterion in all studies.14–19
Clinical Effectiveness
Data from the only controlled trial found (an RCT) served as the only source for reporting on clinical effectiveness outcomes and were reported at the 6-month follow-up.13 No longer-term data were found and so no results on progression and/or recurrence are present. Concerning the outcome of mortality, one case of SADE of death was reported in the RCT. It occurred in the CG, while no cases of death occurred in the IG. Concerning morbidity, the outcome of CCS angina score improvement of at least two classes occurred in 35% of IG and 15% of CG patients (p=0.02). CCS angina score improvement by one class occurred in 71% of IG and 42% of CG patients (p=0.003), and the overall mean reduction of CCS class was 1.1 classes in the IG and 0.5 classes in the CG (p=0.001).
In terms of the effect of CSRS on the patient’s body functions, two outcomes were considered relevant: Wall Motion Score Index (WMSI) improvement and total exercise duration improvement. While the WMSI improved by 14% in the IG and 8% in the CG (p=0.20), the total exercise duration improved by 59 seconds (13%) in the IG and by 4 seconds (1%) in the CG (p=0.07).
Disease-specific QoL was reported with respect to improvement in SAQ QoL score, and while IG patients improved by 17.6 points, CG patients improved by 7.6 points (p=0.048). Patient satisfaction was reported with respect to SAQ treatment satisfaction score, which improved by a mean of 2.9 points in both the IG and the CG. See Table 2 for further details.
Safety
Comparative Studies
In the only RCT,13 10 (19%) SADEs occurred in the IG as opposed to 24 (46%) in the CG, and no SADEs occurred with more frequency in the IG than the CG. Almost all cases of SADEs occurred in no more than two patients in the IG or CG, with the exception of the following: atypical chest pain (IG=1, CG=6), unstable angina (IG=1, CG=4), and stable angina (IG=1, CG=5). With respect to ADEs, they were reported in 32 patients (64%) in the IG and 37 (69%) in the CG.
Prospective Observational Non-Comparative Studies
All of the observational evidence reported SADEs and ADEs at 4–6 months of follow-up, except for one study with the longest follow-up (which reported results at 24 months).15 This was also the study with the highest number of SADEs (30%).
While two observational studies reported no SADEs,14,19 the remaining four studies reported 14 (10%), five (22%), six (13%), and 15 patients (30%) suffering from SADEs, respectively.15–18 The SADE of death occurred in 14 patients (10%),18 in one patient (4%),16 in three patients (6%),17 and in five patients (10%).15 MI occurred in one study15 in three patients (6%), and stable angina in two studies16,17 in four (17%) and two patients (4%), respectively. Coronary artery disease (CAD) progression further occurred in seven patients (14%)15 and unstable angina in one patient (2%).17 With respect to ADEs, those reported were hospitalization, coronary angiogram, revascularization, and device migration. They were not reported in two studies16,19 and were reported to be none in another study.14 Furthermore, they were reported to occur in 64 patients (45%),18 four patients (8%),17 and 13 patients (26%).15 See Tables 2, 3, and 4 for further details.
RoB and Quality of Evidence
The RoB of the RCT was rated to be low, the RoB of observational studies was rated to range from low14,15,17,19 to moderate16,18 (Tables 5 and 6), and the strength of evidence assessed by GRADE was rated moderate (Table 7).
 |
Table 6 Risk of Bias – Study Level (Case Series)21 |
 |
Table 7 Evidence Profile: Efficacy and Safety of CSRS in Patients with Angina Pectoris |
Discussion
For the analysis of clinical effectiveness, one RCT with 104 patients was included,13 indicating a statistically significant improvement in two crucial outcomes (CCS angina score improvement by one/two classes and SAQ QoL score). The third crucial outcome (SAQ treatment satisfaction) did not improve in a statistically significant way (p=0.981), neither did the more objective outcomes (total exercise duration or WMSI). For the analysis of safety, additional six prospective observational studies with 296 patients were included as well. In total, 348 patients received the CSRS therapy. Based on the RCT data, there were fewer SADEs associated with CSRS compared to the CG and the only case of death occurred in the CG. With respect to observational evidence, there remains a point of concern as the SADEs range from none14,19 to 30%15 (with the highest number of SADEs occurring in the study with the longest follow-up). Furthermore, 8% of patients from observational studies died, while 5% of deaths were explicitly claimed not to be related to the CSRS.15–18
In our systematic search, we found only one other HTA on the CSRS therapy, which, however, included both prospective and retrospective evidence and arrived at a positive conclusion toward the CSRS therapy.7 Our HTA is solely based on prospective evidence and our conclusion is more reserved. Also, even though CSRS therapy seems to be a promising treatment for refractory AP patients (with respect to two crucial outcomes and a relatively positive safety profile), the internal and external validity of the studies in the present evidence base is uncertain.
Internal Validity
Regardless of the relatively positive assessment of the evidence quality (low to moderate RoB and moderate strength of evidence), the following issues need to be considered when interpreting the findings on both clinical effectiveness and safety.
Clinical Effectiveness
When interpreting the clinical effectiveness findings, issues with inappropriate inclusion criteria, mechanism of action, placebo effect, sample size, and the randomization procedure should be considered.
First, the main point of concern is the discrepancy between the inclusion criteria in all the studies included in the analysis and the definition of refractory AP (as defined by the ESC5). The ESC defines refractory AP as long-lasting symptoms (for ≥3 months) due to established reversible ischemia in the presence of obstructive CAD, which cannot be controlled by escalating medical therapy with the use of second- and third-line pharmacological agents, bypass grafting, or stenting, including PCI of chronic total coronary occlusion.5 Contrary to the definition, none of the studies included patients with symptoms lasting for more or equal to 3 months and, furthermore, while three studies did not report on previous pharmacological therapy,16,17,19 patients with one to five courses of pharmacological treatment were included in two studies,14,15 1.34–3.3 courses in one study,18 and in the RCT, 25% of patients had zero or one course of medication and 31% had at least three courses.13
Second, there is a lack of clarity behind the mechanism of action of the CSRS. The main hypothesis is that the CSRS alleviates symptoms by improving perfusion in ischemic myocardial territories, but no study has evaluated the effect of the CSRS on myocardial perfusion to demonstrate its mechanism of action.20 One of the potential issues is related to the claimed beneficial hemodynamic changes, which are at odds with one of the principles of use of intermittent and pressure-controlled increase in coronary sinus pressure – a release of obstruction22 resulting in rapid reduction of coronary sinus pressure after the prolongedplateau phase, which may induce a sort of aspirating effect on fluids and toxic metabolites that have accumulated in the ischemic segment.23 It is further known that coronary sinus flow at rest and hyperemic states are in agreement with myocardial blood flow values, and reduced coronary flow reserve measured at the coronary sinus level may have an association with adverse outcome.24
Moreover, it is unclear why there remains a 15–30% rate of non-responders.25 One assumption is that the lack of endothelialization may be at stake, ie that the surface of the device may not be completely covered by endothelium (the vein’s inner lining) and thus may not create the pressure gradient.26 Another assumption is that anatomic variants of the cardiac venous system of individual patients may lead to insufficient pressure gradient across the device.25
Third, there is an echoing concern in the academic literature over the potential large placebo effect associated with novel therapies in this specific patient group.5,20,27 It is further highlighted that such a placebo effect may not result in steady long-term benefit20 and the short-term follow-up (6 months) of the only RCT13 does not prove otherwise.
Fourth, the clinical benefit caused by the CSRS may be overstated as the sample size in the RCT is not big enough to reject a true null hypothesis.28
Fifth, there is a concern in the literature over the randomization process in the RCT.13 It was highlighted that intravenous heparin was used only in IG patients and hence post-procedural laboratory testing may have revealed to the patients who belonged to the IG and who to the CG.28
Safety
With regard to interpreting safety findings, underreporting of complications, obstruction of future therapy, and further potential SADEs need to be considered.
First, dual antiplatelet therapy (DAPT) with clopidogrel and aspirin is recommended for 6 months after the implantation of a CSRS.27 The complications related to DAPT are, however, not reported in the studies, even though they should be considered along with CSRS complications. While only two studies reported the use of DAPT,13,18 no studies reported on the SADE of bleeding events associated with DAPT.
Second, because heart failure may eventually develop in a large proportion of refractory AP patients, there is a concern that the CSRS may preclude the future use of the coronary sinus for implantation of the left ventricular pacing lead necessary for cardiac resynchronization therapy (CRT) (the established heart failure therapy).28
Third, potential SADEs related to individual anatomic considerations during implantation should be taken into account. The potential SADEs are related to the close proximity of the circumflex coronary artery, which may provoke an acute MI (if damaged), and the presence of a Thebesian valve or a valve of Vieussens, which could hamper device implantation in up to 85% of patients.29
External Validity
In terms of external validity, the data are considered not to be generalizable to other contexts as the CSRS patient population did not actually include refractory AP patients. Application of the highly specific inclusion and exclusion criteria in the real-world context also remains in question. Hence, in the light of the small population size and the selective sample of included patients, the conclusions about effectiveness and safety are considered inflated.
Limitations of Evidence
Owing to the limitations concerning the internal as well as external validity of the evidence base, it remains a question to what extent the RCT identified by our systematic literature search is relevant for excluding placebo effects. Moreover, this result is further undermined by the large placebo effect associated with novel therapies in this specific patient population.5 Better powered RCTs with longer follow-up are needed for the sake of defining the role of treatment modalities for specific subgroups, for decreasing non-responder rates, and for ascertaining benefits beyond placebo effects.5
Socio-Economic and Ethical Considerations
When taking into consideration socio-economic and ethical aspects of the CSRS, the effects have to be reflected against the backdrop of principles of distributive justice, beneficence, non-maleficence, autonomy, and uncertainty. On the one hand, the CSRS is claimed to reduce healthcare spending as it decreased healthcare resource use and related costs in a 1-year timeframe under a spectrum of cost-effectiveness thresholds.30 Moreover, the CSRS targets a patient population where there is a therapeutic gap5 and so, if proven to be effective, the CSRS may secure the principles of medical beneficence and patient autonomy.
On the other hand, though, there is a lack of clarity behind the mechanism of action and there are no long-term data.20 There are further concerns of additional SADEs highlighted above that can, for instance, impede the use of CRT for future heart failure patients.28 For that reason, as stated above, to prevent breaching the principle of non-maleficence, better powered controlled trials are required. At this point in time, there is no larger RCT in the pipeline. The only RCT that is currently recruiting includes 40 patients and aims to measure the impact of the CSRS on exertional capacity measured by maximal oxygen consumption (VO2) during cardiopulmonary exercise testing; it aims to be completed by December 2021 (NCT04121845). Important to note is that there is an ongoing ISCHEMIA trial (NCT01471522) that may potentially determine the best management strategy for higher-risk patients with stable ischemic heart disease, and may change the guideline for refractory AP patients considerably.
Conclusion
It is not clear whether the CSRS can improve CCS angina score and QoL without causing more SADEs than the sham intervention (based on moderate quality of evidence). This is because of inconsistent results, incomplete safety data with regard to DAPT, inappropriate inclusion criteria in the studies, insufficient sample size, and incomplete blinding in the RCT. The potential of the CSRS to fulfill the therapeutic gap ought to be considered against the backdrop of its unclear mechanism of action, the lack of a long-term safety profile, and additional potential SADEs. Furthermore, the cost-effectiveness of the CSRS can only be established once the effectiveness of CSRS is established. In that respect, owing to the inconsistencies with internal and external validity of the evidence base, even the conclusions about placebo effects cannot be taken for granted.
Disclosure
Piotr Szymanski reports personal fees from Abbott Laboratories as a speaker, not related to coronary stenting. The authors report no other conflicts of interest in this work.
References
1. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:25. doi:10.1016/j.jacc.2017.04.052
2. Mannheimer C. The problem of chronic refractory angina. Report from the ESC Joint Study Group on the Treatment of Refractory Angina. 2002.
3. Gowdak LHW. Prevalence of refractory angina in clinical practice. Heart Metab. 2017;72:9–12.
4. Simons M, Laham RJ. New therapies for angina pectoris. UpToDate; 2019. Available from: https://www.uptodate.com/contents/new-therapies-for-angina-pectoris.
5. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2019;41(3):407–477.
6. Benedetto D, Abawi M, Stella PR, et al. Percutaneous device to narrow the coronary sinus: shifting paradigm in the treatment of refractory angina? A review of the literature. Front. 2016;3:42.
7. Bazoukis G, Brilakis ES, Tse G, et al. The efficacy of coronary sinus reducer in patients with refractory angina-A systematic review of the literature. J Interv Cardiol. 2018;31(6):775–779.
8. Higgins J, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928
9. Institute of Health Economics (IHE). Quality appraisal of case series studies checklist. Edmonton (AB): Institute of Health Economics; 2014. Available from: http://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about.
10. Stanak M, Rothschedl E. Percutaneous Transvascular Implantation of a Coronary Sinus Reducing Stent. Systematic Review. Decision Support Document No. 121; 2020. Vienna: Ludwig Boltzmann Institute for Health Technology Assessment. Available from: https://eprints.aihta.at/1256/1/DSD_121.pdf . Accessed August 18, 2020
11. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi:10.1016/j.jclinepi.2010.07.015
12. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011.
13. Verheye S, Jolicoeur EM, Behan MW, et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N Engl J Med. 2015;372(6):519–527. doi:10.1056/NEJMoa1402556
14. Tzanis G, Palmisano A, Gallone G, et al. The impact of the coronary sinus reducer upon left ventricular function in patients with refractory angina pectoris. Catheter Cardiovasc Interv. 2019; 95(6):1104–1108.
15. Ponticelli F, Tzanis G, Gallone G, et al. Safety and efficacy of coronary sinus reducer implantation at 2-year follow-up. Int J Cardiol. 2019;292:87–90. doi:10.1016/j.ijcard.2019.05.026
16. Konigstein M, Meyten N, Verheye S, Schwartz M, Banai S. Transcatheter treatment for refractory angina with the coronary sinus reducer. EuroIntervention. 2014;9(10):1158–1164. doi:10.4244/EIJV9I10A196
17. Konigstein M, Bazan S, Revivo M, Banai S. Coronary sinus reducer implantation improves symptoms, ischaemia and physical capacity in patients with refractory angina unsuitable for myocardial revascularisation: a single-centre experience. EuroIntervention. 2018;14(4):e452–e8. doi:10.4244/EIJ-D-18-00102
18. Giannini F, Baldetti L, Ponticelli F, et al. Coronary Sinus Reducer Implantation for the Treatment of Chronic Refractory Angina: A Single-Center Experience. JACC Cardiovasc Interv. 2018;11(8):784–792.
19. Banai S, Ben Muvhar S, Parikh KH, et al. Coronary sinus reducer stent for the treatment of chronic refractory angina pectoris: a prospective, open-label, multicenter, safety feasibility first-in-man study. J Am Coll Cardiol. 2007;49(17):1783–1789. doi:10.1016/j.jacc.2007.01.061
20. Giannini F, Baldetti L, Konigstein M, et al. Safety and efficacy of the reducer: A multi-center clinical registry – REDUCE study. Int J Cardiol. 2018;269:40–44.
21. Institute of Health Economics (IHE). Quality Appraisal of Case Series Studies Checklist. Edmonton (AB): Institute of Health Economics; 2014. Available from: http://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about.
22. De Maria GL, Kassimis G, Raina T, Banning AP. Reconsidering the back door approach by targeting the coronary sinus in ischaemic heart disease. Heart. 2016;102(16):1263. doi:10.1136/heartjnl-2016-309642
23. Mohl W, Punzengruber C, Moser M, et al. Effects of pressure-controlled intermittent coronary sinus occlusion on regional ischemic myocardial function. J Am Coll Cardiol. 1985;5(4):939–947. doi:10.1016/S0735-1097(85)80437-X
24. Kato S, Saito N, Nakachi T, et al. Stress perfusion coronary flow reserve versus cardiac magnetic resonance for known or suspected CAD. J Am Coll Cardiol. 2017;70(7):869–879. doi:10.1016/j.jacc.2017.06.028
25. Giannini F, Gallone G, Baldetti L, et al. Reply to: “Coronary sinus reducer for the treatment of refractory angina”. Int J Cardiol. 2019;276:42. doi:10.1016/j.ijcard.2018.11.088
26. Zivelonghi C, Vermeersch G, Verheye S, Agostoni P. Incomplete coronary sinus reducer endothelialization as potential mechanism of clinical failure. Catheter Cardiovasc Interv. 2019;94(1):120–122.
27. Konigstein M, Giannini F, Banai S. The reducer device in patients with angina pectoris: mechanisms, indications, and perspectives. Eur Heart J. 2018;39(11):925–933. doi:10.1093/eurheartj/ehx486
28. Banai S, Verheye S, Jolicoeur EM. A device to narrow the coronary sinus for angina. N Engl J Med. 2015;372(20):1967–1968.
29. Pizarro G, Sánchez-Quintana D, Cabrera JA. A device to narrow the coronary sinus for angina. N Engl J Med. 2015;372(20):1965–1966.
30. Gallone G, Armeni P, Verheye S, et al. Cost-effectiveness of the coronary sinus reducer and its impact on the healthcare burden of refractory angina patients. Eur Heart J Qual Care Clin Outcomes. 2019. doi:10.1093/ehjqcco/qcz027
31. Ludwig Boltzmann Institut für Health Technology Assessment (LBI-HTA). Internes manual abläufe und methoden Vienna; 2020. Available from: http://hta.lbg.ac.at/uploads/tableTool/UllCmsPage/gallery/InternesManual_2.Aufl.pdf.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
