Back to Journals » International Medical Case Reports Journal » Volume 16
Corneal Perforation Associated with Lacrimal Canaliculitis: A Case Series
Authors Minezaki T, Hattori T, Shibata M, Nakagawa H, Kumakura S, Goto H
Received 2 November 2022
Accepted for publication 16 January 2023
Published 31 January 2023 Volume 2023:16 Pages 83—89
DOI https://doi.org/10.2147/IMCRJ.S394715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Teruumi Minezaki, Takaaki Hattori, Motoko Shibata, Hayate Nakagawa, Shigeto Kumakura, Hiroshi Goto
Department of Ophthalmology, Tokyo Medical University, Tokyo, Japan
Correspondence: Takaaki Hattori, Department of Ophthalmology, Tokyo Medical University, 6-7-1 Nishishinjuku, Shinjuku-ku, Tokyo, 160-0023, Japan, Tel +81-3-3342-6111, Fax +81-3-3342-9170, Email [email protected]
Purpose: To report seven eyes of six patients diagnosed with corneal perforation and lacrimal canaliculitis in a single facility.
Methods: Clinical records of patients with corneal perforation accompanied by lacrimal canaliculitis seen by the authors were reviewed.
Results: Six patients (7 eyes) with corneal perforation accompanied by lacrimal canaliculitis were identified. All patients were female, and all were treated with topical antibiotics while five were receiving topical corticosteroids. Two patients had a history of dacryocystitis and three had systemic immune diseases. The corneal lesions did not respond to topical antibiotics but were effectively treated by removal of concretions in the lacrimal canaliculi and lacrimal duct drainage together with conjunctival autograft or corneal transplantation.
Conclusion: Lacrimal canaliculitis is a risk factor for corneal perforation. When corneal perforation does not respond to antibiotics, lacrimal canaliculitis should be considered.
Keywords: corneal perforation, lacrimal canaliculitis, concretions
Introduction
Canaliculitis is a lacrimal duct disease that may present as longstanding conjunctivitis refractory to general treatment, and patients may have used antibiotics or corticosteroid eye drops for a long period. Canaliculitis has an incidence of 2–4% among lacrimal duct diseases.1 It can occur at any age, and various studies have shown a female preponderance.2–4 Concretions are found in the canaliculi, resulting in symptoms such as inflammation of the punctum, epiphora, and discharge. Symptoms persist unless canalicular concretions are completely removed.5,6 Actinomyces israelii is the most well known pathogen responsible for canaliculitis. However, recent studies have shown an increased incidence of the involvement of many other microorganisms, including Gram-positive bacteria and fungi. Conservative medical therapy is often prescribed, but removal of all concretions in the lacrimal canaliculus is essential for symptom resolution.2,4,5 Recently, lacrimal endoscopy has been reported to be useful in the diagnosis and treatment of canaliculitis.7
Corneal perforation is occasionally caused by infectious ulcers due to bacterial, viral, or fungal infection, trauma, and autoimmune etiologies such as rheumatoid arthritis.8 Severe dry eye may also result in corneal perforation. The common etiologies of dry eye leading to corneal perforation are systemic autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, and Sjögren’s syndrome.9–12 Generally, treatment for corneal perforation is challenging, and blindness may result. Therefore, it is necessary to differentiate the etiology of the perforation before making treatment decisions. Based on the cause, corneal perforation is treated with a combination of medical and surgical interventions. In recent years, corneal perforation in patients with canaliculitis or chronic dacryocystitis has been reported13–16 suggesting an association between corneal perforation and lacrimal duct disease.
We report seven eyes of six patients with corneal perforation associated with canaliculitis. These patients had corneal perforation that did not respond to topical and systemic antibiotics, but was resolved by treatment of canaliculitis.
Materials and Methods
Study Design and Subjects
This retrospective interventional case series included all patients with corneal perforation who were diagnosed with lacrimal canaliculitis at the Tokyo Medical University Hospital, Tokyo, Japan, between July 2011 and March 2021.
An institutional review board approval was not required to publish data of patients. Written informed consent was obtained from all patients for publication of this case series prior to all investigations and surgical procedures.
Data Collection
Clinical and microbiological data were retrieved from medical records. Detailed history was obtained based on the medical records, which included the patient’s age; sex; prior medications; history of systemic autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, and Sjögren’s syndrome; and history of ocular diseases associated with autoimmune diseases. The clinical data recorded included best-corrected Snellen visual acuity and slit-lamp examination findings.
Bacteriological Culture
Microbiological investigations, including Gram staining and culture, were performed on corneal scraping and lacrimal duct discharge samples. The samples were inoculated onto blood agar, chocolate agar, Sabouraud dextrose agar, and non-nutrient agar plates for bacterial and fungal isolation.
Pathological Examination
Sections were prepared from concretions removed from the canaliculi, and Gram, PAS, and Grocott staining were performed. The slides were examined for microorganisms within the concretions using an optical microscope.
Results
The clinical profiles of the patients are summarized in Table 1. All six patients were female and were diagnosed with lacrimal canaliculitis. Two patients had a history of dacryocystitis, while the other four had an unknown history of lacrimal duct disease. Regarding systemic disease, two patients had rheumatoid arthritis and one patient had lymphoma and graft versus host disease (GVHD) after bone marrow transplantation for lymphoma. The diagnosis at the previous clinic was corneal ulcer in two cases and corneal epithelial defect in one case. Before visiting our hospital, all patients were treated with topical antibiotics, and five patients were treated with topical corticosteroids.
 |
Table 1 Demographic and Clinical Data of the Patients Studied |
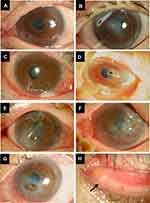
The ophthalmological findings of the patients at presentation are summarized in Table 2. Corneal perforation affected the left eye only in five eyes, the right eye only in two eyes, and both eyes in Case 6. The perforation was on the nasal side in three eyes and on the temporal side in four eyes. In addition, perforation was observed in the peripheral cornea in three eyes and in the central cornea in four eyes (Figure 1A–G). Lacrimal endoscopy revealed concretions in the upper canaliculi in only one eye, lower canaliculi in only two eyes, and both upper and lower canaliculi in four eyes. In addition, four eyes of three patients had concurrent nasolacrimal duct obstruction. All cases had minimal corneal infiltration around the perforation site, and there was little corneal opacity, except in Case 5 (Figure 1A–G).
 |
Table 2 Clinical Findings of Corneal Perforation |
Microbiological culture of corneal scrape samples was performed in three cases (four eyes), and all eyes were culture-negative (Table 3). In contrast, almost all microbiological cultures of the lacrimal duct discharge were positive (Table 3). Pathological examination of the concretions removed from the canaliculi was performed in four eyes of three cases, yielding findings of actinomycetes in two eyes of two cases, and Candida in two eyes of one case.
 |
Table 3 Results of the Bacterial Culture and Pathological Examination |
All patients received corneal treatment, including corneal transplantation or conjunctival autograft, as well as lacrimal duct drainage once or twice a day, removal of concretions in the lacrimal canaliculi, and topical and systemic antibiotics. In five cases, silicone tube intubation of the lacrimal duct was performed. Corneal perforations were successfully treated with these interventions in all patients. There was no recurrence of corneal perforation in any of the patients during follow-up.
Case Presentation: Case 5
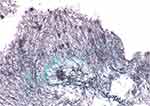
A 49-year-old woman was referred to our hospital for the treatment of corneal perforation. She had a history of lymphoma and GVHD after bone marrow transplantation, resulting in dry eye, which was treated with hyaluronic acid eye drops. At the previous hospital, she was diagnosed with a corneal ulcer in the left eye and was treated with antibiotics and corticosteroid eye drops. She was referred to our hospital because of corneal perforation. At presentation, slit-lamp examination of the left eye revealed paracentral corneal perforation on the lower nasal side with abundant discharge, and the anterior chamber was absent due to leakage of aqueous humor from the perforated cornea (Figure 1G). Concretions were observed in the left lower punctum (Figure 1H). Her visual acuity in the left eye was hand movement at 30 cm. We performed conjunctival autograft transplantation, dilated the punctum to expel the concretion and inserted a lacrimal duct tube. Pathological examination of the concretion revealed small aggregates of Gram-positive cocci and filamentous bodies by Gram staining, and filamentous bodies over a wide area by PAS and Grocott staining (Figure 2). Three months later, we performed corneal transplantation, and her visual acuity improved to 0.1; these treatments succeeded in stabilizing the cornea. There was no recurrence of corneal ulcers or perforation during follow-up of 11 months.
Discussion
We report seven eyes of six patients with corneal perforation and concurrent lacrimal canaliculitis. All patients were female, and half of the patients had local or systemic immune disorders. Corneal perforations occurred in all parts of the cornea with limited stromal infiltration and no hypopyon. The cultures of almost all canalicular discharge samples were positive, whereas those of corneal scrapings were negative. All cases were successfully treated surgically for corneal perforation with additional lacrimal drainage, removal of concretions in the lacrimal duct, and systemic and topical antibiotics.
Corneal perforation may lead to blindness; thus, it is important to understand the patient characteristics and pathogenesis of the diseases that cause corneal perforation. In this report, all six patients with corneal perforation and lacrimal canaliculitis were female, and most of them were aged 70 years or above. Previous studies have reported two male cases and six female cases of corneal perforation associated with canalliculitis13,14,16,17 It is possible that corneal perforation caused by lacrimal canaliculitis is much more common in females. A possible explanation is the preponderance of lacrimal duct diseases, including canaliculitis, in older females.4,18–20 Patient characteristics revealed that half of the patients had systemic immunological disorders such as rheumatoid arthritis and GVHD. A patient with ocular cicatricial pemphigoid who also had corneal perforation caused by lacrimal canaliculitis has been reported.14 Immune disorders are known to induce corneal perforation. In our cases, however, corneal perforations occurred even though the patients were treated with corticosteroids. Moreover, the treatment of lacrimal canaliculitis led to stabilization of the ocular surface without the use of corticosteroids. Thus, our cases suggest that even in inpatients with underlying immunological disorders, corneal perforation appears to be caused by lacrimal canaliculitis, rather than by immunological pathogenesis, although these cases may be rare. Corneal perforation associated with lacrimal canaliculitis may tend to occur more commonly on the nasal side because of its proximity to the punctum. In our cases, corneal perforation occurred on the nasal side in three eyes and on the temporal side in four eyes, not only in the peripheral cornea but also in the central cornea. In previous reports of six cases in which the corneal perforation site was known, perforations were observed on the nasal side.13,14,16 However, our case series shows that corneal perforation with lacrimal canaliculitis can occur in any part of the cornea.
The pathogenesis of corneal ulcer and perforation associated with lacrimal canaliculitis remains unclear. Bacteria causing lacrimal canaliculitis may infect the cornea and directly cause corneal ulceration and perforation. Previous reports have shown that 20–35% of patients with bacterial keratitis had lacrimal duct obstruction and that dacryocystitis was highly predictive of a corneal ulcer.21,22 In two cases of corneal ulcer associated with lacrimal canaliculitis, the same organism was isolated from the cultures of corneal scraping and canalicular discharge.17,23 In another study of 35 patients with keratitis and nasolacrimal duct obstruction, the same organism was cultured from corneal scraping and mucopurulent material in the lacrimal sac.24 In our case series, cultures of corneal scrapings at the perforation sites yielded no microorganisms, although most cultures of canalicular discharge were positive for bacteria or fungi. Furthermore, slit-lamp examination of seven eyes showed mild stromal infiltration and mild anterior chamber inflammation without hypopyon, despite the presence of corneal perforation, which was clearly different from typical corneal perforation caused by bacterial keratitis. Yokogawa et al13 speculated that allergies against toxins produced by some bacteria may be involved in the mechanism underlying corneal perforation associated with canaliculitis. Ishikawa et al14 hypothesized that corneal melting is due to enzymes released by bacteria. It is possible that there are two types of corneal perforation caused by lacrimal canaliculitis. First, bacteria from lacrimal canaliculitis infect the cornea, directly causing corneal ulceration in the presence of immunosuppression induced by corticosteroid eye drops, and finally leading to corneal perforation. Second, enzymes and toxins from bacteria in the canaliculi cause corneal melting, as observed in our cases.
In the present cases, bacteria infecting the lacrimal duct caused corneal perforation, even though the patients were treated with antibiotics. Corneal ulcer associated with lacrimal canaliculitis may be a rare cause of corneal perforation, but our cases and previous reports suggest that lacrimal duct examination should be conducted in patients with antibiotic-resistant corneal ulcer.
A limitation of the present study was the retrospective design with a small number of cases. The present findings need to be verified by a prospective study with a larger sample size.
In conclusion, we reported a case series of corneal perforations associated with lacrimal canaliculitis. These patients were elderly female, and half of them had systemic immune disorders. Corneal perforations occur in any part of the cornea. Lacrimal duct disease should be suspected when corneal ulcers or perforations do not respond to antibiotic treatment. Additional treatments for lacrimal canaliculitis, including lacrimal drainage, are required in these patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kim UR, Wadwekar B, Prajna L. Primary canaliculitis: the incidence, clinical features, outcome and long-term epiphora after snip-punctoplasty and curettage. Saudi J Ophthalmol. 2015;29(4):274–277. doi:10.1016/j.sjopt.2015.08.004
2. Xiang S, Lin B, Pan Q, et al. Clinical features and surgical outcomes of primary canaliculitis with concretions. Medicine. 2017;96(9):e6188. doi:10.1097/MD.0000000000006188
3. Balıkoğlu Yılmaz M, Şen E, Evren E, Elgin U, Yılmazbaş P. Canaliculitis Awareness. Turkish J Ophthalmol. 2016;46(1):25–29. doi:10.4274/tjo.68916
4. Lin SC, Kao SC, Tsai CC, et al. Clinical characteristics and factors associated the outcome of lacrimal canaliculitis. Acta Ophthalmol. 2011;89(8):759–763. doi:10.1111/j.1755-3768.2009.01827.x
5. Zaveri J, Cohen AJ. Lacrimal canaliculitis. Saudi J Ophthalmol. 2014;28(1):3–5. doi:10.1016/j.sjopt.2013.11.003
6. Kelly M, Kelly G. Actinomycosis canaliculitis. Pathology. 2022;54(4):497–499. doi:10.1016/j.pathol.2021.07.005
7. Zheng Q, Shen T, Luo H, et al. Application of lacrimal endoscopy in the diagnosis and treatment of primary canaliculitis: practical technique and graphic presentation. Medicine. 2019;98(33):e16789. doi:10.1097/MD.0000000000016789
8. Cohn H, Mondino BJ, Brown SI, Hall GD. Marginal corneal ulcers with acute beta streptococcal conjunctivitis and chronic dacryocystitis. Am J Ophthalmol. 1979;87(4):541–543. doi:10.1016/0002-9394(79)90246-0
9. Usuba FS, de Medeiros-Ribeiro AC, Novaes P, et al. Dry eye in rheumatoid arthritis patients under TNF-inhibitors: conjunctival goblet cell as an early ocular biomarker. Sci Rep. 2020;10(1):14054. doi:10.1038/s41598-020-70944-9
10. Wang L, Xie Y, Deng Y. Prevalence of dry eye in patients with systemic lupus erythematosus: a meta-analysis. BMJ open. 2021;11(9):e047081. doi:10.1136/bmjopen-2020-047081
11. Akpek EK, Bunya VY, Saldanha IJ. Sjögren’s syndrome: more than just dry eye. Cornea. 2019;38(5):658–661. doi:10.1097/ICO.0000000000001865
12. Generali E, Cantarini L, Selmi C. Ocular involvement in systemic autoimmune diseases. Clin Rev Allergy Immunol. 2015;49(3):263–270. doi:10.1007/s12016-015-8518-3
13. Yokogawa H, Kobayashi A, Yamazaki N, Masaki T, Sugiyama K. Surgical therapies for corneal perforations: 10 years of cases in a tertiary referral hospital. Clin Ophthalmol. 2014;8:2165–2170. doi:10.2147/OPTH.S71102
14. Ishikawa S, Kato N. A case with corneal perforation due to bacterial concretion derived from lacrimal canaliculitis. Am J Ophthalmol Case Rep. 2018;9:116–118. doi:10.1016/j.ajoc.2018.01.004
15. Nagasato D, Tabuchi H, Yamauchi T, Imamura H, Shimizu Y. Severe corneal melting and perforation secondary to chronic dacryocystitis due to delayed ophthalmology consultation. Oxford Med Case Rep. 2021;2021(4):omab009. doi:10.1093/omcr/omab009
16. Inoue H, Toriyama K, Ikegawa W, et al. Clinical characteristics of lacrimal drainage pathway disease-associated keratopathy. BMC Ophthalmol. 2022;22(1):353. doi:10.1186/s12886-022-02580-y
17. Chou YP, Yeh PH, Tsai YJ, Yen CH, Hsiao CH. Infectious keratitis secondary to canaliculitis with concretions: a case report. Medicine. 2019;98(40):e17444. doi:10.1097/MD.0000000000017444
18. Pinar-Sueiro S, Sota M, Lerchundi TX, et al. Dacryocystitis: systematic approach to diagnosis and therapy. Curr Infect Dis Rep. 2012;14(2):137–146. doi:10.1007/s11908-012-0238-8
19. Hussain I, Bonshek RE, Loudon K, Armstrong M, Tullo AB. Canalicular infection caused by Actinomyces. Eye. 1993;7(Pt 4)):542–544. doi:10.1038/eye.1993.118
20. Pavilack MA, Frueh BR. Through curettage in the treatment of chronic canaliculitis. Arch Ophthalmol. 1992;110(2):200–202. doi:10.1001/archopht.1992.01080140056026
21. Aasuri MK, Reddy MK, Sharma S, Rao GN. Co-occurrence of pneumococcal keratitis and dacryocystitis. Cornea. 1999;18(3):273–276. doi:10.1097/00003226-199905000-00005
22. Li G, Guo J, Liu R, et al. Lacrimal duct occlusion is associated with infectious keratitis. Int J Med Sci. 2016;13(10):800–805. doi:10.7150/ijms.16515
23. Feder RS, Rao RR, Lissner GS, Bryar PJ, Szatkowski M. Atypical mycobacterial keratitis and canaliculitis in a patient with an indwelling SmartPLUG. Br J Ophthalmol. 2010;94(3):383–384. doi:10.1136/bjo.2009.160853
24. Nayak A, Mitra Basu S, De A, Mallick A, Das S, Rath S. Concurrent microbial keratitis and nasolacrimal duct obstruction: concordance, etiopathogenesis, and outcome. Cornea. 2019;38(1):84–88. doi:10.1097/ICO.0000000000001767
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.