Back to Journals » International Medical Case Reports Journal » Volume 12
Corneal deposition of fluoroquinolones after penetrating keratoplasty: case series
Received 12 December 2018
Accepted for publication 20 February 2019
Published 8 May 2019 Volume 2019:12 Pages 151—154
DOI https://doi.org/10.2147/IMCRJ.S198011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser

Sanjeeta Sitaula,1 Sanjay K Singh2
1B.P. Koirala Lions Centre for Ophthalmic Studies, Institute Of Medicine, Kathmandu, Nepal; 2Department of Cornea Clinic, Biratnagar Eye Hospital, Biratnagar, Nepal
Purpose: To report three cases of fluroquinolone deposition in the cornea after topical administration post-penetrating keratoplasty.
Case reports: Herein we report three patients ranging in age from 42–65 years who underwent keratoplasty with cataract extraction, with intraocular lens implantation in the first two cases and left aphakic due to a posterior capsular tear in the third case. The first two patients received ciprofloxacin-dexamethasone combination drops, and developed drug deposition, which was observed at the first follow-up after 7 and 10 days respectively. The third patient received prednisolone acetate and ofloxacin eyedrops postoperatively, and developed drug deposits in the cornea after 20 days. In all of the three patients, the fluroquinolone group of drugs was discontinued and the cornea cleared gradually over the next 3–4 weeks. Although the cornea cleared, the first two grafts failed due to recurrent viral infection in one case, and graft rejection in the other case.
Conclusion: Deposition of many different fluroquinolones in the cornea has been reported after a variety of surgeries, including penetrating keratoplasty. Drug deposition post-penetrating keratoplasty may seem innocuous due to self-resolution on cessation of the drugs, but it may have deleterious effects on graft survival. Hence, fluroquinolones, especially ciprofloxacin, should be cautiously used in patients undergoing penetrating keratoplasty if frequent dosing is prescribed or if used concurrently with other topical medications containing preservatives.
Keywords: ciprofloxacin, corneal deposits, fluroquinolones, ofloxacin, penetrating keratoplasty
Introduction
Fluoroquinolones are fluorinated derivatives of nalidixic acid and are broad-spectrum antimicrobial agents. Corneal deposits have been reported with topical administration of various fluoroquinolones including, ciprofloxacin,1–3 ofloxacin,4 gatifloxacin,5 sparfloxacin,6 moxifloxacin,7,8 and tosufloxacin.9,10 Herein, three cases of drug deposition in cornea post-penetrating keratoplasty (PK) have been reported. Written informed consent was provided by the patients to have the case details and any accompanying images published. Institutional approval was obtained for the publication of the case details.
Case reports
Case 1
The first case study was a 65 year old male with diminution of vision in the right eye (RE). The patient had a history of viral keratits in his RE within the past year. Upon examination, it was found that the patient had visual acuity of perception of light and an adherent leukoma with dry ocular surface in the RE. PK was performed in the RE with cataract extraction and posterior chamber intraocular lens (IOL) implantation under peribulbar anesthesia by using an 8 mm donor graft.
On the first postoperative day, the graft had mild edema with subtotal epithelial defect. The anterior chamber was formed with mild cellular reaction and IOL was in place. The patient was started on ciprofloxacin-dexamethasone combination drops (Ciplox-D, Cipla Ltd) six times a day, carboxymethyl cellulose 1% (Relub-DS with stabilized Oxychloro Complex as preservative from Centaur pharmaceuticals) drops ten times a day, and hypromellose 2% (Lacrigel from Sunways India Pvt. Ltd.) gel at bedtime. During the first weeks follow up, the patient complained of blurred vision and white discoloration of the cornea. On examination, there was a whitish granular deposition in the subepithelial region in the inferior half of the cornea corresponding to the site of the previously present epithelial defect (Figure 1). The patient did not complain of pain or irritation. However, it was suspected that ciprofloxacin deposition was present, and the patient’s treatment was therefore switched to prednisolone acetate 1% (Predmet, Sun Pharmaceuticals) drops four times and tobramycin 0.3% (Toba, Sun Pharmaceuticals) drops four times a day, along with lubrication. The graft cleared leaving a persistent epithelial defect (PED) after 4 weeks of treatment. After 2 weeks the graft developed recurrent viral keratitis and the graft eventually failed despite all efforts.
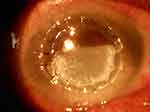 | Figure 1 White granular deposits in the inferior cornea in the area of the epithelial defect at day 7 after corneal transplant. |
Case 2
A 45 year old male with bullous keratopathy in the RE in a case of presumed posterior polymorphous corneal dystrophy was scheduled to undergo PK. The patient suffered from intermittent pain and redness of his eye in the past 4 years. On examination, it was found that his RE had visual acuity of hand movement and mild circumciliary congestion with hazy cornea. There was epithelial micro- and macrobullae with diffuse stromal edema. The anterior chamber had an irregular depth and 360° posterior synechiae with a cataract. Posterior segment could not be visualized. IOP was 12 mmHg in the RE, as measured by an airpuff tonometer.
The patient underwent PK in the RE with cataract extraction and posterior chamber IOL implantation under peribulbar anesthesia using an 8 mm donor graft. Postoperatively, the graft had mild edema with Descemet folds and a central epithelial defect. The anterior chamber was formed with moderate cellular reaction and fibrinous exudates. The patient was started on ciprofloxacin-dexamethasone combination drops (Ciplox-D, Cipla Ltd) eight times a day, carboxymethyl cellulose 1% drops (Relub-DS with stabilized Oxychloro Complex as preservative from Centaur pharmaceuticals) six times a day, and atropine 1% (N-pin, National Healthcare) drops three times a day, and followed-up after 7 days.
A presence of whitish granular subepithelial deposition in the central cornea (Figure 2) was found when the patient presented on the 10th postoperative day. Suspecting ciprofloxacin deposition, the medications were changed and the patient was started on prednisolone acetate 1% (Predmet, Sun Pharmaceuticals) eyedrops six times a day, tobramycin 0.3% (Toba, Sun Pharmaceuticals) drops four times a day, and carboxymethylcellulose (Relub-DS with stabilized Oxychloro Complex as preservative from Centaur pharmaceuticals) drops eight times a day.
 | Figure 2 Whitish granular deposition in the central cornea on the 10th postoperative day after penetrating keratoplasty with cataract extraction and posterior chamber intraocular lens implantation. |
The deposits completely cleared after 20 days and the epithelium healed leaving behind a faint opacity in the area of the drug deposition, probably due to delayed epithelialization of the cornea. On the next follow-up after a month, the graft had developed features of acute graft reaction and the graft failed despite medical management.
Case 3
A 42 year old male was scheduled for a triple procedure for adherent leukoma following perforated corneal ulcer in the RE. Visual acuity was hand motion in the RE and 6/6 in the left eye. The IOP was 31.8 mmHg in RE and 12.2 mmHg measured with a Schiotz tonometer. After intravenous mannitol (5 mL/kg), the patient underwent PK with lens extraction under peribulbar anesthesia. Intraoperatively, there was high vitreous pressure, and the posterior chamber IOL was not stable. While removing the IOL, there was a tear in the posterior capsule, and an anterior vitrectomy was performed. Postoperatively, the graft host junction was well apposed and an epithelial defect was present. The graft had moderate edema with descemet folds and there was a fibrinous exudate in the anterior chamber. The patient was started on prednisolone acetate 1% (Predmet, Sun Pharmaceuticals) eyedrops ten times a day, ofloxacin 0.3% (Ocucin, Sun Pharmaceuticals) drops eight times a day, carboxymethylcellulose drops (Relub-DS with stabilized Oxychloro Complex as preservative from Centaur pharmaceuticals), six times a day, and timolol-brimonidine combination drops (Brimopress-T, Centaur pharmaceuticals) 2 twice a day. The patient was followed-up after 20 days. The epithelial defect was healing but there was whitish granular deposition in the subepithelial layer beneath the healed epithelium with central epithelial defect (Figure 3). Ofloxacin eyedrops were stopped and the frequency of tear substitutes was increased. The cornea cleared gradually over the next 4 weeks.
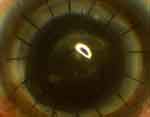 | Figure 3 Whitish deposition over the graft on the 20th postoperative day with healing epithelial defect under the bandage contact lens. |
Discussion and conclusion
Topical fluroquinolones are widely used in ophthalmology because of their broad spectrum of action and are readily available and cost effective. Corneal deposition of these drugs has been reported following treatment of bacterial keratitis,2 corneal transplant,10 cataract surgery,1,8 and LASEK.3 Ciprofloxacin is frequently associated with drug deposition in the cornea, however, in recent times, newer drugs like ofloxacin, gatifloxacin, moxifloxacin, and tosufloxacin have been found to cause similar depositions.
Ciprofloxacin is soluble at a pH of 4.5 and stromal crystallization is common at neutral pH of the tearfilm (pH 7.2).5 The corneal epithelium may play an important role as a barrier that prevents drug particle deposition in the stroma based on various cases in the literature.2,9 Deposition in the cornea is facilitated in the presence of an epithelial defect or severe ocular surface disorder and also in polypharmacy, probably due to the change in ocular pH. However, there are cases where drug depositions are seen despite intact corneal epithelium, and especially when associated with frequent dosing or polypharmacy.1,8 Ofloxacin is soluble at a pH of 6.4 and rarely precipitates, but there are reports in the literature where ofloxacin depositions have been documented.4,11,12
All of our three cases of PK were combined with cataract surgery, and there was a presence of an epithelial defect at the time of discharge. The first patient had dry eye as a compounding factor leading to deposition of ciprofloxacin. In the second case, there was severe inflammation with fibrinous reaction in the anterior chamber. The frequent application of ciprofloxacin-dexamethasone may have contributed to drug deposition in this case. Similarly, in the third case, ofloxacin was prescribed to be used eight times a day along with prednisolone acetate drops and timolol-brimonidine combination drugs. This polypharmacy and the preservative in them may have been a factor in promoting deposition of ofloxacin in the third case.
In all of the three cases, the fluroquinolones were discontinued and the cornea cleared on its own over the next 3–4 weeks. The deposits were not scraped and biochemical analysis was not done, but since the deposits cleared after cessation of fluroquinolones, it can be inferred that fluroquinolones were the problem. Other reports in literature have reported spontaneous resolution of deposits within 2–5 months.1,9 The deposition of the drug led to a prolonged epithelial healing time with a PED. In the first case there was recurrence of viral keratitis, and the graft eventually failed. In the second case, the PED eventually healed but was followed by features of graft rejection, probably due to the inflammatory reaction triggered by the PED. Although drug deposition post-PK may seem innocuous due to self-resolution on cessation of the drugs, it may have a deleterious effect on graft survival. A similar problem of acute graft rejection following resolution of corneal fluroquinolone deposits was also noted in another study.9
Hence, fluroquinolones, especially ciprofloxacin, should be cautiously used in patients undergoing PK if frequent dosing is prescribed, or if concurrently used with other topical medications containing preservatives.
Abbreviation list
IOL, intraocular lens; IOP, intraocular pressure; LE, left eye; PED, persistent epithelial defect; PK, penetrating keratoplasty; RE, right eye
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zawar SV, Mahadik S. Corneal deposit after topical ciprofloxacin as postoperative medication after cataract surgery. Can J Ophthalmol. 2014;49(4):392–394. doi:10.1016/j.jcjo.2014.04.013
2. Wilhelmus KR, Abshire RL. Corneal ciprofloxacin precipitation during bacterial keratitis. Am J Ophthalmol. 2003;136(6):1032–1037.
3. De Benedetti G, Brancaccio A. Corneal deposit of ciprofloxacin after laser assisted subepithelial keratomileusis procedure: a case report. J Ophthalmol. 2010;2010. doi:10.1155/2010/296034
4. Mitra A, Tsesmetzoglou E, McElvanney A. Corneal deposits and topical ofloxacin—the effect of polypharmacy in the management of microbial keratitis. Eye. 2007;21(3):410–412. doi:10.1038/sj.eye.6702303
5. Elia M, Khodadadeh S, Chow J. Corneal crystalline deposits associated with topically applied gatifloxacin. Cornea. 2014;33(6):638–639. doi:10.1097/ICO.0000000000000101
6. Agarwal AK, Ram J, Singh R. Case report: sparfloxacin-associated corneal epithelial toxicity. BMJ Case Rep. 2014;2014. doi:10.1136/bcr-2014-203786
7. Alshamrani AA, Alharbi SS. Corneal deposits following topical moxifloxacin use. Saudi J Ophthalmol. 2018. doi:10.1016/j.sjopt.2018.04.006
8. Babitha V, Prasannakumary C, Fathima Z, Raju KV. Postoperative corneal deposits the following polypharmacy. J Clin Ophthalmol Res. 2016;4(3):155. doi:10.4103/2320-3897.190789
9. Kim YD, Kim MK, Wee WR, Choi HJ. Tosufloxacin deposits in compromised corneas. Optom Vis Sci. 2014;91(9):e241–e244. doi:10.1097/OPX.0000000000000348
10. Katahira H, Kumakura S, Hattori T, Goto H. Corneal deposits associated with topical tosufloxacin following penetrating keratoplasty: a case report. Int Med Case Rep J. 2017;10:239. doi:10.2147/IMCRJ.S132531
11. Claerhout I, Kestelyn P, Meire F, Remon J-P, Decaestecker T, Van Bocxlaer J. Corneal deposits after the topical use of ofloxacin in two children with vernal keratoconjunctivitis. Br J ophthalmol. 2003;87(5):646.
12. Sinnaeve BA, Decaestecker TN, Claerhout IJ, Kestelyn P, Remon J-P, Van Bocxlaer JF. Confirmation of ofloxacin precipitation in corneal deposits by microbore liquid chromatography–quadrupole time-of-flight tandem mass spectrometry. J Chromatog B. 2003;785(1):193–196. doi:10.1016/S1570-0232(02)00854-1
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
